The Role and Application of Exosomes and Their Cargos in Reproductive Diseases: A Systematic Review
Abstract
Simple Summary
Abstract
1. Search Strategy
2. Exosomes
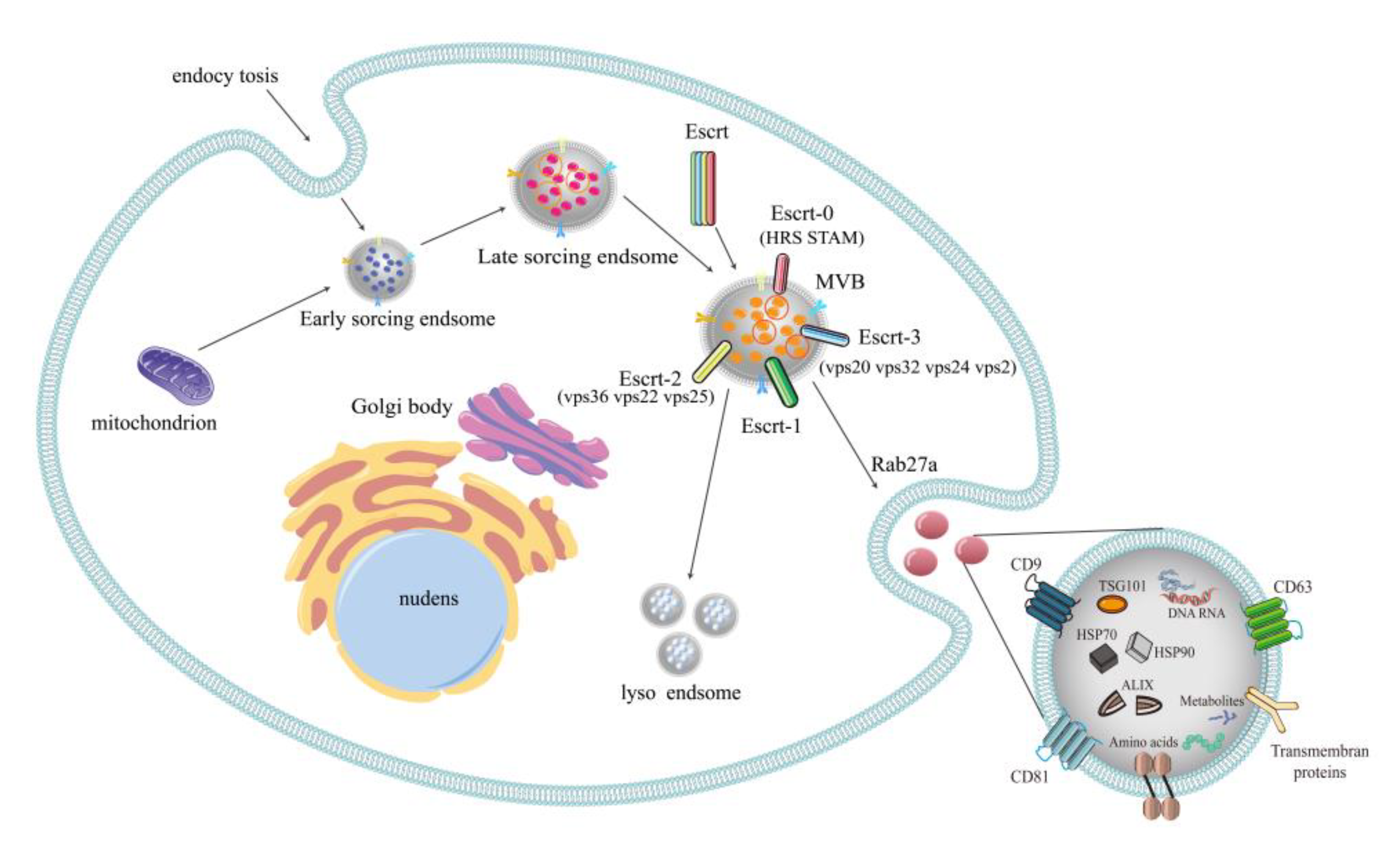
2.1. Formation of Exosomes
2.2. Multilaterality of Exosomal Contents
3. Exosomes in Reproductive Diseases
3.1. Premature Ovarian Failure
3.2. Polycystic Ovarian Syndrome
3.3. Preeclampsia
3.4. Endometriosis
3.5. Reproductive Cancers
| Diseases | Biomarkers | Variation Trend | Related Function | Reference |
|---|---|---|---|---|
| Polycystic ovarian syndrome | miR-25-3p | Increased | Amino acid metabolic pathway | [20,21] |
| miR-141-3p, miR-200a-3p, miR-200c-3p, miR-483-3p, miR-3911, miR-199a-5p, miR-199a-3p, miR-199b-3p, miR-629-5p, miR-193b-3p, miR-6087, miR-10a-5p, miR-23b-3p, miR-98-5p, miR-382-5p, miR-483-5p, miR-4532, miR-4745-3p, miR-143-3p | Decreased | |||
| miR-126-3p, miR-146a-5p | Increased | Endocytosis tumorigenesis pathways, axon guidance, circadian rhythm and MAPK signaling pathway | [17] | |
| miR-106a-5p, miR-20b-5p | Decreased | |||
| S100-A9 | Increased | Enhances inflammation and disrupts steroid production by activating NF-κB pathway | [25] | |
| miR-424-5p | Decreased | Blocks CDCA4-mediated Rb/E2F1 signaling and inhibits the proliferation of primary granulosa cells (GC) and induces cell senescence in PCOS | [23] | |
| lncRNA-H19, lncRNA-POP4, lncRNA-AKT3, lncRNA-HDAC6, lncRNA-NF1, lncRNAMUM1, lncRNA-LINC00173, lncRNA-DICER1, lncRNA-PTEN | Increased | Endocytosis, hippo signaling pathway, MAPK signaling pathway and HTLV-1 infection | [18] | |
| miR-27a-5p | Increased | Promotes cell migration and invasion | [100] | |
| Preeclampsia | has-miR-525-5p | Increased | Inhibits vasoactive intestinal peptide | [29,32,101] |
| has-miR-526b-5p | Increased | MMP-1 and HIF-1A | ||
| miR-192-5p, miR-205-5p, miR-208a-3p, miR-335-5p, miR-451a, miR-518a-3p, miR-542-3p, miR-23a-3p, miR-125b-2-3p, miR-144-3p, | Decreased | Inhibits TC migration and proliferation by targeting VEGFA | ||
| miR-141-3p, miR-199a-3p, miR-221-3p, miR-584-5p, miR-744-5p, miR-6724-5p let-7a-5p, miR-17-5p, miR-26a-5p, miR-30c-5p | Increased | Down-regulated inflammation by targeting PTPRO | ||
| has-miR-370-3p | Increased | Inhibits the proliferation, migration and invasion and promotes the apoptosis of trophoblast cells | [102] | |
| miR-15a-5p | Increased | Inhibits proliferation, invasion and apoptosis of trophoblast cells by targeting CDK1 | [103] | |
| miR-19a-3p, miR-19b-3p, miR-376c-3p | Decreased | unknown | [104] | |
| miR-885-5p | Increased | Related to the liver enzyme aspartate aminotransferase | ||
| miR-141, miR-133 | Increased | Regulation of trophoblast invasion and intercellular communication | [28,105] | |
| PP13 | Early decline, late rise | Increased prostacyclin release for remodeling of maternal spiral arteries in early placenta | [35] | |
| Endometriosis | miR-22-3p, miR-320a | Increased | TNF signaling, thyroid cancer, terpenoid skeleton biosynthesis, regulating stem cell pluripotency | [50] |
| miR-214 | Increased | Inhibit fibrosis and regulate the development of endometriosis lesions | [52,53] | |
| Thrombospondin-1 (TSP1/THBS1), TSP2/THBS2, Pigment epithelium-derived factor (SERPINF1), angiopoietin-related protein 6 | Increased | Angiogenesis, immune system regulation and metabolic process pathways | [46] | |
| PRDX1 | Increased | Protooncogene | [41] | |
| H2A type 2-C | Unknown | |||
| ANXA2 | Promote tumor metastasis | |||
| ITIH4 | It was expressed during the surgical trauma period | |||
| Tubulin α-chain | Unknown | |||
| miR-26b-5p, miR-215-5p | Decreased | Involves in MAPK and PI3K-Akt signaling pathways | [106] | |
| miR-6795-3p | Increased | |||
| lncRNA aHIF | Increased | Promotes angiogenesis in endometriosis | [47,48] | |
| lncRNA CHL1-AS1 | Increased | Promotes the migration, invasion and proliferation of endometrial stromal cells (eESC) and inhibits apoptosis | [54] | |
| tRF-Leu-AAG-001 | Increased | Promotes inflammation and angiogenesis | [56] | |
| miR-30c | Decreased | Reduces the invasion and migration of ectodermal endometrial epithelial cells (EECs) by targeting BCL9 | [107] | |
| B-cell lymphoma 9 (BCL9) | Increased | |||
| Breast cancer | miR-372, miR-101 | Increased | Distinguishs between BC and benign tumors | [59] |
| miR-24, miR-206, miR-1246, miR-373 | Increased | BC detection | [60] | |
| miR-340-5p | Increased | Predicting recurrence | [61] | |
| miR-17-5p, miR-93-5p, miR-130a-3p | Decreased | |||
| miR-421, miR-128-2, miR-128-1 | Increased | Predicts risks and adverse outcomes | [62] | |
| miR-30b | Decreased | Predicting recurrence | [63] | |
| miR-93 | Increased | Ductal carcinoma in situ diagnosis | ||
| miR-16 | Increased | Distinguishs ductal carcinoma in situ from health | ||
| Ovarian cancer | hsa_circ_0061140 | Increased | Promotes cell proliferation, invasion, apoptosis and EMT | [68] |
| circRNA CDR1as | Decreased | Inhibits cell proliferation, migration and invasion | [69] | |
| circRNA CDR1as | Increased | Inhibits migration and invasion, promote cell proliferation and stagnation of G0/G1 cell cycle | [70] | |
| circ-SMAD7 | Increased | Promotes cell proliferation, migration and invasion | [71] | |
| circHIPK3 | Increased | Predicts risks and prognosis | [72] | |
| circLARP4 | Decreased | Predicts risks and prognosis | [73] | |
| miR-34a | Increased | Predicts recurrence risks | [74] | |
| miR-21-5p | Increased | Diagnostic sign | [75] | |
| miR-29a-3P | Increased | Promotes the proliferation and metastasis of OC cells | ||
| miR-30a-5p | Increased | Diagnostic and therapeutic targets | ||
| Collagen type V alpha 2 chain (COL5A2) | Increased | Multiple diagnostic and prognostic markers of cancer | [64] | |
| lipoprotein lipase (LPL) | Unknown | |||
| Cervical cancer | miR-221-3p | — | Promotes local angiogenesis | [78,79] |
| miR-30d-5p, let-7d-3p | Decreased | Noninvasive screening diagnostic biomarkers for CC and its precursors | [80] | |
| miR-223 | Increased | A key factor of the STAT3-miR-223-HMGCS1/TGFBR3 axis regulating the progression of CC | [81] | |
| LncRNA-EXOC7 | — | Participates in cancer development and early diagnosis | [82] | |
| lncRNA HNF1A-AS1 | — | [83] | ||
| Endometrial cancer | miR-148b | Decreased | Induction of endometrial cancer progression | [85] |
| miR-320a | Decreased | Inhibits VEGFA expression and cell proliferation by targeting HIF1α | [86] | |
| Endothelial (CD144+), Monocytic (CD14+) | Increased | Related to histological grade and clinical stage of cancer | [91] | |
| miR-15a-5p, miR-106b-5p, miR107 | Increased | correlates with tumor size and invasion depthEarly detection marker | [87] | |
| miR-423-3p, miR-195-5p, miR-20b-5p, miR-204-5p, miR-484, miR-143-3p | Increased | Early detection marker | [88] | |
| LGALS3BP | Increased | Endometrial carcinoma (EC) growth and angiogenesis | [84] | |
| ANXA2 | Increased | Correlates with high grade, non-endometrioid subtype, advanced stage, and increased risks of recurrence | [92] | |
| miR-192-5p | Decreased | EC cell proliferationEMT | [89] | |
| miR-133a | Increased | EC and stromal cells communicate | [90] | |
| Prostate cancer | miR-196a-5p, miR-501-3p | Decreased | Diagnostic marker | [93] |
| miR-2909 | Increased | Diagnostic markers, disease risk classification, Involved in metabolic and immune regulation by targeting MALT1, KLF4 and UPC2 | [94] | |
| miR-1290, miR-375 | Increased | Associated with cancer treatment and indicative of prognosis | [95] | |
| circ_0044516 | Increased | PC cell survival and metastasis | [96] | |
| miR-1246 | Increased | Associated with cell proliferation and EMT | [97] | |
| miR-940 | Increased | Promotes osteogenic differentiation by targeting ARHGAP1 and FAM134A | [98] | |
| exosomal ephrinA2 and serum ephrinA2 | Increased | Distinguishes PC patients from benign prostatic hyperplasia (BPH) patents | [99] |
4. Application of Exosomes in the Treatment of Reproductive Diseases
4.1. The Function of MSC-Exos
4.2. The Application of MSC-Exos to Animal Models of Reproductive Diseases
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta. 2012, 1820, 940–948. [Google Scholar] [CrossRef]
- Wortzel, I.; Dror, S.; Kenific, C.M.; Lyden, D. Exosome-Mediated Metastasis: Communication from a Distance. Dev. Cell 2019, 49, 347–360. [Google Scholar] [CrossRef]
- Stoorvogel, W. Resolving sorting mechanisms into exosomes. Cell Res. 2015, 25, 531–532. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT pathway. Dev. Cell 2011, 21, 77–91. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Hu, Q.; Su, H.; Li, J.; Lyon, C.; Tang, W.; Wan, M.; Hu, T.Y. Clinical applications of exosome membrane proteins. Precis. Clin. Med. 2020, 3, 54–66. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Exosome Biochemistry and Advanced Nanotechnology for Next-Generation Theranostic Platforms. Adv. Mater. 2019, 31, e1802896. [Google Scholar] [CrossRef]
- Hoek, A.; Schoemaker, J.; Drexhage, H.A. Premature ovarian failure and ovarian autoimmunity. Endocr. Rev. 1997, 18, 107–134. [Google Scholar] [PubMed]
- Yang, M.; Lin, L.; Sha, C.; Li, T.; Zhao, D.; Wei, H.; Chen, Q.; Liu, Y.; Chen, X.; Xu, W.; et al. Bone marrow mesenchymal stem cell-derived exosomal miR-144-5p improves rat ovarian function after chemotherapy-induced ovarian failure by targeting PTEN. Lab. Invest. 2020, 100, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Ma, Y.; Wang, F.; Hu, L.; Sun, Y. miR-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of p53 to inhibit ovarian granulosa cell apoptosis. Stem Cell Res. Ther. 2019, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, J.; Huang, Y.; Bu, S.; Guo, Y.; Gu, T.; Li, B.; Wang, C.; Lai, D. Human Amniotic Epithelial Cell-Derived Exosomes Restore Ovarian Function by Transferring MicroRNAs against Apoptosis. Mol. Ther. Nucleic Acids 2019, 16, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.Y.; Cheng, C.C.; Chiang, Y.S.; Cheng, W.T.; Liu, I.H.; Wu, S.C. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci. Rep. 2016, 6, 23120. [Google Scholar] [CrossRef]
- Azziz, R. Polycystic Ovary Syndrome. Obstet. Gynecol. 2018, 132, 321–336. [Google Scholar] [CrossRef]
- Jiang, X.; Li, J.; Zhang, B.; Hu, J.; Ma, J.; Cui, L.; Chen, Z.J. Differential expression profile of plasma exosomal microRNAs in women with polycystic ovary syndrome. Fertil. Steril. 2021, 115, 782–792. [Google Scholar] [CrossRef]
- Wang, L.; Fan, H.; Zou, Y.; Yuan, Q.; Hu, X.; Chen, X.; Zhu, C.; Zhang, X.; Cui, H. Aberrant Expression of Long Non-coding RNAs in Exosomes in Follicle Fluid From PCOS Patients. Front. Genet. 2021, 11, 608178. [Google Scholar] [CrossRef]
- Zhou, Z.; Tu, Z.; Zhang, J.; Tan, C.; Shen, X.; Wan, B.; Li, Y.; Wang, A.; Zhao, L.; Hu, J.; et al. Follicular Fluid-Derived Exosomal MicroRNA-18b-5p Regulates PTEN-Mediated PI3K/Akt/mTOR Signaling Pathway to Inhibit Polycystic Ovary Syndrome Development. Mol. Neurobiol. 2022, 59, 2520–2531. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, S.; Li, Y.; Wu, X. Exosomal miR-143-3p derived from follicular fluid promotes granulosa cell apoptosis by targeting BMPR1A in polycystic ovary syndrome. Sci. Rep. 2022, 12, 4359. [Google Scholar] [CrossRef]
- Hu, J.; Tang, T.; Zeng, Z.; Wu, J.; Tan, X.; Yan, J. The expression of small RNAs in exosomes of follicular fluid altered in human polycystic ovarian syndrome. PeerJ 2020, 8, e8640. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Huo, P.; Cui, K.; Wei, H.; Cao, J.; Wang, J.; Liu, Q.; Lei, X.; Zhang, S. Follicular fluid-derived exosomal miR-143-3p/miR-155-5p regulate follicular dysplasia by modulating glycolysis in granulosa cells in polycystic ovary syndrome. Cell Commun. Signal. 2022, 20, 61. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Luo, J.; Sun, Y.; Hao, L.; Zheng, J.; Yang, Z. PCOS follicular fluid derived exosomal miR-424-5p induces granulosa cells senescence by targeting CDCA4 expression. Cell. Signal. 2021, 85, 110030. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tao, M.; Wei, M.; Du, S.; Wang, H.; Wang, X. Mesenchymal stem cells derived exosomal miR-323-3p promotes proliferation and inhibits apoptosis of cumulus cells in polycystic ovary syndrome (PCOS). Artif. Cells Nanomed. Biotechnol. 2019, 47, 3804–3813. [Google Scholar] [CrossRef]
- Li, H.; Huang, X.; Chang, X.; Yao, J.; He, Q.; Shen, Z.; Ji, Y.; Wang, K. S100-A9 protein in exosomes derived from follicular fluid promotes inflammation via activation of NF-κB pathway in polycystic ovary syndrome. J. Cell. Mol. Med. 2020, 24, 114–125. [Google Scholar] [CrossRef]
- Wallis, A.B.; Saftlas, A.F.; Hsia, J.; Atrash, H.K. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987-2004. Am. J. Hypertens. 2008, 21, 521–526. [Google Scholar] [CrossRef]
- Abalos, E.; Cuesta, C.; Grosso, A.L.; Chou, D.; Say, L. Global and regional estimates of preeclampsia and eclampsia: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 1–7. [Google Scholar] [CrossRef]
- Wang, D.; Na, Q.; Song, G.Y.; Wang, L. Human umbilical cord mesenchymal stem cell-derived exosome-mediated transfer of microRNA-133b boosts trophoblast cell proliferation, migration and invasion in preeclampsia by restricting SGK1. Cell Cycle 2020, 19, 1869–1883. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, P.; Wang, Z.; Qin, Z.; Xiu, X.; Xu, D.; Zhang, X.; Wang, Y. MiRNA-548c-5p downregulates inflammatory response in preeclampsia via targeting PTPRO. J. Cell. Physiol. 2019, 234, 11149–11155. [Google Scholar] [CrossRef]
- Ma, H.Y.; Cu, W.; Sun, Y.H.; Chen, X. MiRNA-203a-3p inhibits inflammatory response in preeclampsia through regulating IL24. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5223–5230. [Google Scholar]
- Huang, Q.; Gong, M.; Tan, T.; Lin, Y.; Bao, Y.; Fan, C. Human Umbilical Cord Mesenchymal Stem Cells-Derived Exosomal MicroRNA-18b-3p Inhibits the Occurrence of Preeclampsia by Targeting LEP. Nanoscale Res. Lett. 2021, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Xueya, Z.; Yamei, L.; Sha, C.; Dan, C.; Hong, S.; Xingyu, Y.; Weiwei, C. Exosomal encapsulation of miR-125a-5p inhibited trophoblast cell migration and proliferation by regulating the expression of VEGFA in preeclampsia. Biochem. Biophys. Res. Commun. 2020, 525, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Liang, Z.; Shi, X.; Xu, L.; Li, X.; Wu, J.; Zhao, L.; Liu, G. Exosomal miR-486-5p derived from human placental microvascular endothelial cells regulates proliferation and invasion of trophoblasts via targeting IGF1. Hum. Cell 2021, 34, 1310–1323. [Google Scholar] [CrossRef] [PubMed]
- Taga, S.; Hayashi, M.; Nunode, M.; Nakamura, N.; Ohmichi, M. miR-486-5p inhibits invasion and migration of HTR8/SVneo trophoblast cells by down-regulating ARHGAP5. Placenta 2022, 123, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Gadde, R.; Cd, D.; Sheela, S.R. Placental protein 13: An important biological protein in preeclampsia. J. Circ. Biomark. 2018, 7, 1849454418786159. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Vigano, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef]
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef]
- Klemmt, P.A.B.; Starzinski-Powitz, A. Molecular and Cellular Pathogenesis of Endometriosis. Curr. Womens Health Rev. 2018, 14, 106–116. [Google Scholar] [CrossRef]
- Wu, D.; Lu, P.; Mi, X.; Miao, J. Exosomal miR-214 from endometrial stromal cells inhibits endometriosis fibrosis. Mol. Hum. Reprod. 2018, 24, 357–365. [Google Scholar] [CrossRef]
- Lobb, R.J.; Lima, L.G.; Moller, A. Exosomes: Key mediators of metastasis and pre-metastatic niche formation. Semin. Cell Dev. Biol. 2017, 67, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Nazri, H.M.; Imran, M.; Fischer, R.; Heilig, R.; Manek, S.; Dragovic, R.A.; Kessler, B.M.; Zondervan, K.T.; Tapmeier, T.T.; Becker, C.M. Characterization of exosomes in peritoneal fluid of endometriosis patients. Fertil. Steril. 2020, 113, 364–373.e2. [Google Scholar] [CrossRef] [PubMed]
- Asante, A.; Taylor, R.N. Endometriosis: The role of neuroangiogenesis. Annu. Rev. Physiol. 2011, 73, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, D.; Yuan, M.; Li, Q.; Li, N.; Wang, G. Eutopic stromal cells of endometriosis promote neuroangiogenesis via exosome pathwaydagger. Biol. Reprod. 2019, 100, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Wu, P.; Xiao, N.; Qiu, F.; Zeng, Q.P. Nitric oxide-driven hypoxia initiates synovial angiogenesis, hyperplasia and inflammatory lesions in mice. PLoS ONE 2012, 7, e34494. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Luo, F.; Liu, Y.; Shi, L.; Lu, X.; Xu, W.; Liu, Q. Exosomal miR-21 derived from arsenite-transformed human bronchial epithelial cells promotes cell proliferation associated with arsenite carcinogenesis. Arch. Toxicol. 2015, 89, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Freger, S.; Leonardi, M.; Foster, W.G. Exosomes and their cargo are important regulators of cell function in endometriosis. Reprod. Biomed. Online 2021, 43, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Poh, Q.H.; Fatmous, M.; Fang, H.; Gurung, S.; Vollenhoven, B.; Salamonsen, L.A.; Greening, D.W. Proteomic profiling of human uterine extracellular vesicles reveal dynamic regulation of key players of embryo implantation and fertility during menstrual cycle. Proteomics 2021, 21, e2000211. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.J.; Lin, X.J.; Zheng, T.T.; Tang, X.Y.; Zhang, Y.; Hua, K.Q. The Exosomal Long Noncoding RNA aHIF is Upregulated in Serum From Patients With Endometriosis and Promotes Angiogenesis in Endometriosis. Reprod. Sci. 2019, 26, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, Z.; Qu, Q.; Li, X.; Lu, X.; Zhang, H. Exosomal lncRNA HOTAIR Promotes the Progression and Angiogenesis of Endometriosis via the miR-761/HDAC1 Axis and Activation of STAT3-Mediated Inflammation. Int. J. Nanomed. 2022, 17, 1155–1170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, H.; Yuan, M.; Li, D.; Sun, C.; Wang, G. Serum Exosomal MicroRNAs as Potential Circulating Biomarkers for Endometriosis. Dis. Markers 2020, 2020, 2456340. [Google Scholar] [CrossRef]
- Kharazi, U.; Badalzadeh, R. A review on the stem cell therapy and an introduction to exosomes as a new tool in reproductive medicine. Reprod. Biol. 2020, 20, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chang, X.; Wu, D.; Deng, M.; Miao, J.; Jin, Z. Down-regulation of Exosomal miR-214-3p Targeting CCN2 Contributes to Endometriosis Fibrosis and the Role of Exosomes in the Horizontal Transfer of miR-214-3p. Reprod. Sci. 2021, 28, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Schjenken, J.E.; Panir, K.; Robertson, S.A.; Hull, M.L. Exosome-mediated intracellular signalling impacts the development of endometriosis-new avenues for endometriosis research. Mol. Hum. Reprod. 2019, 25, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, M.; Zheng, C.; Zhang, D.; Li, M.; Zhang, L. Exosomal lncRNA CHL1-AS1 Derived from Peritoneal Macrophages Promotes the Progression of Endometriosis via the miR-610/MDM2 Axis. Int. J. Nanomed. 2021, 16, 5451–5464. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, L.; Li, H.; Ye, J.; Lin, N.; Chen, M.; Pan, D.; Chen, Z. Endometriosis derived exosomal miR-301a-3p mediates macrophage polarization via regulating PTEN-PI3K axis. Biomed. Pharmacother. 2022, 147, 112680. [Google Scholar] [CrossRef]
- Li, Y.; Cui, S.; Xu, Z.; Zhang, Y.; Wu, T.; Zhang, J.; Chen, Y. Exosomal tRF-Leu-AAG-001 derived from mast cell as a potential non-invasive diagnostic biomarker for endometriosis. BMC Womens Health 2022, 22, 253. [Google Scholar] [CrossRef]
- Cen, J.; Feng, L.; Ke, H.; Bao, L.; Li, L.Z.; Tanaka, Y.; Weng, J.; Su, L. Exosomal Thrombospondin-1 Disrupts the Integrity of Endothelial Intercellular Junctions to Facilitate Breast Cancer Cell Metastasis. Cancers 2019, 11, 1946. [Google Scholar] [CrossRef]
- Cui, Z.; Chen, Y.; Hu, M.; Lin, Y.; Zhang, S.; Kong, L.; Chen, Y. Diagnostic and prognostic value of the cancer-testis antigen lactate dehydrogenase C4 in breast cancer. Clin. Chim. Acta. 2020, 503, 203–209. [Google Scholar] [CrossRef]
- Eichelser, C.; Stückrath, I.; Müller, V.; Milde-Langosch, K.; Wikman, H.; Pantel, K.; Schwarzenbach, H. Increased serum levels of circulating exosomal microRNA-373 in receptor-negative breast cancer patients. Oncotarget 2014, 5, 9650–9663. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, Y.S.; Kang, K.N.; Kim, K.H.; Park, Y.J.; Kim, C.W. Multiple microRNAs as biomarkers for early breast cancer diagnosis. Mol. Clin. Oncol. 2021, 14, 31. [Google Scholar] [CrossRef]
- Sueta, A.; Yamamoto, Y.; Tomiguchi, M.; Takeshita, T.; Yamamoto-Ibusuki, M.; Iwase, H. Differential expression of exosomal miRNAs between breast cancer patients with and without recurrence. Oncotarget 2017, 8, 69934–69944. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Hu, T.; Liu, J.; Su, J.; Sun, J.; Ming, Y.; Li, J.; Wu, N.; Chen, H.; Zhou, M. Genomic instability-derived plasma extracellular vesicle-microRNA signature as a minimally invasive predictor of risk and unfavorable prognosis in breast cancer. J. Nanobiotechnol. 2021, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; Stevic, I.; Pan, C.; Müller, V.; Oliveira-Ferrer, L.; Pantel, K.; Schwarzenbach, H. Different signatures of miR-16, miR-30b and miR-93 in exosomes from breast cancer and DCIS patients. Sci. Rep. 2018, 8, 12974. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, K.; Qing, Y.; Li, D.; Cui, M.; Jin, P.; Xu, T. Proteomic and lipidomic analysis of exosomes derived from ovarian cancer cells and ovarian surface epithelial cells. J. Ovarian Res. 2020, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Sawada, K.; Miyamoto, M.; Shimizu, A.; Yamamoto, M.; Kinose, Y.; Nakamura, K.; Kawano, M.; Kodama, M.; Hashimoto, K.; et al. Exploring the potential of engineered exosomes as delivery systems for tumor-suppressor microRNA replacement therapy in ovarian cancer. Biochem. Biophys. Res. Commun. 2020, 527, 153–161. [Google Scholar] [CrossRef]
- Soltész, B.; Lukács, J.; Szilágyi, E.; Márton, É.; Szilágyi Bónizs, M.; Penyige, A.; Póka, R.; Nagy, B. Expression of CD24 in plasma, exosome and ovarian tissue samples of serous ovarian cancer patients. J. Biotechnol. 2019, 298, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Asare-Werehene, M.; Nakka, K.; Reunov, A.; Chiu, C.T.; Lee, W.T.; Abedini, M.R.; Wang, P.W.; Shieh, D.B.; Dilworth, F.J.; Carmona, E.; et al. The exosome-mediated autocrine and paracrine actions of plasma gelsolin in ovarian cancer chemoresistance. Oncogene 2020, 39, 1600–1616. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; He, Y.; Wang, Y. hsa_circ_0061140 Knockdown Reverses FOXM1-Mediated Cell Growth and Metastasis in Ovarian Cancer through miR-370 Sponge Activity. Mol. Ther. Nucleic Acids 2018, 13, 55–63. [Google Scholar] [CrossRef]
- Chen, H.; Mao, M.; Jiang, J.; Zhu, D.; Li, P. Circular RNA CDR1as acts as a sponge of miR-135b-5p to suppress ovarian cancer progression. OncoTargets Ther. 2019, 12, 3869–3879. [Google Scholar] [CrossRef]
- Karedath, T.; Ahmed, I.; Al Ameri, W.; Al-Dasim, F.M.; Andrews, S.S.; Samuel, S.; Al-Azwani, I.K.; Mohamoud, Y.A.; Rafii, A.; Malek, J.A. Silencing of ANKRD12 circRNA induces molecular and functional changes associated with invasive phenotypes. BMC Cancer 2019, 19, 565. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, X.P.; Lang, Y.P.; Kou, D.; Shao, Z.W. Circular RNA circ-SMAD7 promoted ovarian cancer cell proliferation and metastasis by suppressing KLF6. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5603–5610. [Google Scholar] [PubMed]
- Liu, N.; Zhang, J.; Zhang, L.Y.; Wang, L. CircHIPK3 is upregulated and predicts a poor prognosis in epithelial ovarian cancer. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3713–3718. [Google Scholar] [PubMed]
- Zou, T.; Wang, P.L.; Gao, Y.; Liang, W.T. Circular RNA_LARP4 is lower expressed and serves as a potential biomarker of ovarian cancer prognosis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7178–7182. [Google Scholar] [PubMed]
- Maeda, K.; Sasaki, H.; Ueda, S.; Miyamoto, S.; Terada, S.; Konishi, H.; Kogata, Y.; Ashihara, K.; Fujiwara, S.; Tanaka, Y.; et al. Serum exosomal microRNA-34a as a potential biomarker in epithelial ovarian cancer. J. Ovarian Res. 2020, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Horie, K.; Nanashima, N.; Yokoyama, Y.; Yoshioka, H.; Watanabe, J. Exosomal MicroRNA as Biomarkers for Diagnosing or Monitoring the Progression of Ovarian Clear Cell Carcinoma: A Pilot Study. Molecules 2022, 27, 3953. [Google Scholar] [CrossRef]
- Taylor, D.D.; Gercel-Taylor, C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008, 110, 13–21. [Google Scholar] [CrossRef]
- Bhat, A.; Yadav, J.; Thakur, K.; Aggarwal, N.; Tripathi, T.; Chhokar, A.; Singh, T.; Jadli, M.; Bharti, A.C. Exosomes from cervical cancer cells facilitate pro-angiogenic endothelial reconditioning through transfer of Hedgehog-GLI signaling components. Cancer Cell Int. 2021, 21, 319. [Google Scholar] [CrossRef]
- Hashemipour, M.; Boroumand, H.; Mollazadeh, S.; Tajiknia, V.; Nourollahzadeh, Z.; Rohani Borj, M.; Pourghadamyari, H.; Rahimian, N.; Hamblin, M.R.; Mirzaei, H. Exosomal microRNAs and exosomal long non-coding RNAs in gynecologic cancers. Gynecol. Oncol. 2021, 161, 314–327. [Google Scholar] [CrossRef]
- Wu, X.G.; Zhou, C.F.; Zhang, Y.M.; Yan, R.M.; Wei, W.F.; Chen, X.J.; Yi, H.Y.; Liang, L.J.; Fan, L.S.; Liang, L.; et al. Cancer-derived exosomal miR-221-3p promotes angiogenesis by targeting THBS2 in cervical squamous cell carcinoma. Angiogenesis 2019, 22, 397–410. [Google Scholar] [CrossRef]
- Zheng, M.; Hou, L.; Ma, Y.; Zhou, L.; Wang, F.; Cheng, B.; Wang, W.; Lu, B.; Liu, P.; Lu, W.; et al. Exosomal let-7d-3p and miR-30d-5p as diagnostic biomarkers for non-invasive screening of cervical cancer and its precursors. Mol. Cancer 2019, 18, 76. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, M.; Qian, L.; Lin, X.; Song, W.; Gao, Y.; Zhou, Y. The STAT3-miR-223-TGFBR3/HMGCS1 axis modulates the progression of cervical carcinoma. Mol. Oncol. 2020, 14, 2313–2331. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, X.; Wang, K.; He, Y. Appraising the Value of Serum and Serum-Derived Exosomal LncRNA-EXOC7 as a Promising Biomarker in Cervical Cancer. Clin. Lab. 2020, 66, 32658426. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wei, J.; Yang, F.L.; Pang, X.X.; Shi, F.; Wei, Y.X.; Liao, B.Y.; Wang, J.L. Exosomal lncRNA HNF1A-AS1 affects cisplatin resistance in cervical cancer cells through regulating microRNA-34b/TUFT1 axis. Cancer Cell Int. 2019, 19, 323. [Google Scholar] [CrossRef]
- Song, Y.; Wang, M.; Tong, H.; Tan, Y.; Hu, X.; Wang, K.; Wan, X. Plasma exosomes from endometrial cancer patients contain LGALS3BP to promote endometrial cancer progression. Oncogene 2021, 40, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Li, B.L.; Lu, W.; Qu, J.J.; Ye, L.; Du, G.Q.; Wan, X.P. Loss of exosomal miR-148b from cancer-associated fibroblasts promotes endometrial cancer cell invasion and cancer metastasis. J. Cell. Physiol. 2019, 234, 2943–2953. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Y.; Liu, H.; Shen, W. Extracellular vesicle encapsulated microRNA-320a inhibits endometrial cancer by suppression of the HIF1alpha/VEGFA axis. Exp. Cell Res. 2020, 394, 112113. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, W.; Wang, F.; Yang, S.; Hu, J.; Lu, B.; Pan, Z.; Ma, Y.; Zheng, M.; Zhou, L.; et al. Plasma-derived exosomal miR-15a-5p as a promising diagnostic biomarker for early detection of endometrial carcinoma. Mol. Cancer 2021, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zou, X.; Liu, C.; Cheng, W.; Zhang, S.; Geng, X.; Zhu, W. MicroRNA expression profile in serum reveals novel diagnostic biomarkers for endometrial cancer. Biosci. Rep. 2021, 41, BSR20210111. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, H.; Li, Y.; Su, R. MiR-192-5p-Modified Tumor-Associated Macrophages-Derived Exosome Suppressed Endometrial Cancer Progression Through Targeting IRAK1/NF-kappaB Signaling. Reprod. Sci. 2022, 29, 436–447. [Google Scholar] [CrossRef]
- Shi, S.; Tan, Q.; Feng, F.; Huang, H.; Liang, J.; Cao, D.; Wang, Z. Identification of core genes in the progression of endometrial cancer and cancer cell-derived exosomes by an integrative analysis. Sci. Rep. 2020, 10, 9862. [Google Scholar] [CrossRef] [PubMed]
- Dziechciowski, M.; Zapala, B.; Skotniczny, K.; Gawlik, K.; Pawlica-Gosiewska, D.; Piwowar, M.; Balajewicz-Nowak, M.; Basta, P.; Solnica, B.; Pitynski, K. Diagnostic and prognostic relevance of microparticles in peripheral and uterine blood of patients with endometrial cancer. Ginekol. Pol. 2018, 89, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Herrero, C.; de la Fuente, A.; Casas-Arozamena, C.; Sebastian, V.; Prieto, M.; Arruebo, M.; Abalo, A.; Colás, E.; Moreno-Bueno, G.; Gil-Moreno, A.; et al. Extracellular Vesicles-Based Biomarkers Represent a Promising Liquid Biopsy in Endometrial Cancer. Cancers 2019, 11, 2000. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.; Bajo-Santos, C.; Hessvik, N.P.; Lorenz, S.; Fromm, B.; Berge, V.; Sandvig, K.; Linē, A.; Llorente, A. Identification of non-invasive miRNAs biomarkers for prostate cancer by deep sequencing analysis of urinary exosomes. Mol. Cancer 2017, 16, 156. [Google Scholar] [CrossRef]
- Wani, S.; Kaul, D.; Mavuduru, R.S.; Kakkar, N.; Bhatia, A. Urinary-exosomal miR-2909: A novel pathognomonic trait of prostate cancer severity. J. Biotechnol. 2017, 259, 135–139. [Google Scholar] [CrossRef]
- Huang, X.; Yuan, T.; Liang, M.; Du, M.; Xia, S.; Dittmar, R.; Wang, D.; See, W.; Costello, B.A.; Quevedo, F.; et al. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur. Urol. 2015, 67, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Sun, X.; Chen, L. Exosome circ_0044516 promotes prostate cancer cell proliferation and metastasis as a potential biomarker. J. Cell. Biochem. 2020, 121, 2118–2126. [Google Scholar] [CrossRef] [PubMed]
- Bhagirath, D.; Yang, T.L.; Bucay, N.; Sekhon, K.; Majid, S.; Shahryari, V.; Dahiya, R.; Tanaka, Y.; Saini, S. microRNA-1246 Is an Exosomal Biomarker for Aggressive Prostate Cancer. Cancer Res. 2018, 78, 1833–1844. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Ochi, H.; Sunamura, S.; Kosaka, N.; Mabuchi, Y.; Fukuda, T.; Yao, K.; Kanda, H.; Ae, K.; Okawa, A.; et al. Cancer-secreted hsa-miR-940 induces an osteoblastic phenotype in the bone metastatic microenvironment via targeting ARHGAP1 and FAM134A. Proc. Natl. Acad. Sci. USA 2018, 115, 2204–2209. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Y.; Chen, W.; Yin, L.; Zhu, J.; Zhang, H.; Cai, C.; Li, P.; Huang, L.; Ma, P. Exosomal ephrinA2 derived from serum as a potential biomarker for prostate cancer. J. Cancer 2018, 9, 2659–2665. [Google Scholar] [CrossRef]
- Che, X.; Jian, F.; Chen, C.; Liu, C.; Liu, G.; Feng, W. PCOS serum-derived exosomal miR-27a-5p stimulates endometrial cancer cells migration and invasion. J. Mol. Endocrinol. 2020, 64, 1–12. [Google Scholar] [CrossRef]
- Truong, G.; Guanzon, D.; Kinhal, V.; Elfeky, O.; Lai, A.; Longo, S.; Nuzhat, Z.; Palma, C.; Scholz-Romero, K.; Menon, R.; et al. Oxygen tension regulates the miRNA profile and bioactivity of exosomes released from extravillous trophoblast cells-Liquid biopsies for monitoring complications of pregnancy. PLoS ONE 2017, 12, e0174514. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Sun, Y.; Cao, Y.; Zhang, Y. Small RNA sequencing reveals placenta-derived exosomal microRNAs associated with preeclampsia. J. Hypertens. 2022, 40, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, X.; Wang, J. Transfer of miR-15a-5p by placental exosomes promotes pre-eclampsia progression by regulating PI3K/AKT signaling pathway via CDK1. Mol. Immunol. 2020, 128, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Sandrim, V.C.; Luizon, M.R.; Palei, A.C.; Tanus-Santos, J.E.; Cavalli, R.C. Circulating microRNA expression profiles in pre-eclampsia: Evidence of increased miR-885-5p levels. BJOG 2016, 123, 2120–2128. [Google Scholar] [CrossRef]
- Ospina-Prieto, S.; Chaiwangyen, W.; Herrmann, J.; Groten, T.; Schleussner, E.; Markert, U.R.; Morales-Prieto, D.M. MicroRNA-141 is upregulated in preeclamptic placentae and regulates trophoblast invasion and intercellular communication. Transl. Res. 2016, 172, 61–72. [Google Scholar] [CrossRef]
- Wu, Y.; Yuan, W.; Ding, H.; Wu, X. Serum exosomal miRNA from endometriosis patients correlates with disease severity. Arch. Gynecol. Obstet. 2022, 305, 117–127. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.; Xia, X.; Fang, X.; Zhang, T.; Huang, F. Endometrial epithelial cells-derived exosomes deliver microRNA-30c to block the BCL9/Wnt/CD44 signaling and inhibit cell invasion and migration in ovarian endometriosis. Cell Death Discov. 2022, 8, 151. [Google Scholar] [CrossRef]
- Bunggulawa, E.J.; Wang, W.; Yin, T.; Wang, N.; Durkan, C.; Wang, Y.; Wang, G. Recent advancements in the use of exosomes as drug delivery systems. J. Nanobiotechnology 2018, 16, 81. [Google Scholar] [CrossRef]
- Depuydt, E.; Chiers, K.; Van Hecke, L.; Saunders, J.; Martens, A.; Pille, F.; Spaas, J. Assessing the functional properties of tenogenic primed mesenchymal stem cells in ex vivo equine tendon and ligament explants: A preliminary study. Stem Cell Res. 2022, 65, 102963. [Google Scholar] [CrossRef]
- Chang, C.L.; Sung, P.H.; Chen, K.H.; Shao, P.L.; Yang, C.C.; Cheng, B.C.; Lin, K.C.; Chen, C.H.; Chai, H.T.; Chang, H.W.; et al. Adipose-derived mesenchymal stem cell-derived exosomes alleviate overwhelming systemic inflammatory reaction and organ damage and improve outcome in rat sepsis syndrome. Am. J. Transl. Res. 2018, 10, 1053–1070. [Google Scholar]
- Mohammadzadeh, A.; Pourfathollah, A.A.; Shahrokhi, S.; Hashemi, S.M.; Moradi, S.L.; Soleimani, M. Immunomodulatory effects of adipose-derived mesenchymal stem cells on the gene expression of major transcription factors of T cell subsets. Int. Immunopharmacol. 2014, 20, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Lopatina, T.; Bruno, S.; Tetta, C.; Kalinina, N.; Porta, M.; Camussi, G. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun. Signal. 2014, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Song, Z.J.; Cai, Q.W.; Chen, R.X.; Zou, Y.; Fu, Q.; Ma, Y.Y. Adipose mesenchymal stem cell-derived exosomes ameliorate hypoxia/serum deprivation-induced osteocyte apoptosis and osteocyte-mediated osteoclastogenesis in vitro. Biochem. Biophys. Res. Commun. 2019, 508, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Han, Y.D.; Yan, X.L.; Ren, J.; Zeng, Q.; Li, X.D.; Pei, X.T.; Han, Y. Adipose mesenchymal stem cell-derived exosomes stimulated by hydrogen peroxide enhanced skin flap recovery in ischemia-reperfusion injury. Biochem. Biophys. Res. Commun. 2018, 500, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, Y.; Zhang, Y.; Zhang, H.; Liu, W.; Zhang, N.; Zhang, X.; Zhou, G.; Wu, L.; Hua, K.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells accelerate growth of VK2 vaginal epithelial cells through MicroRNAs in vitro. Hum. Reprod. 2019, 34, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Lu, J.; Ding, C.; Zou, Q.; Wang, W.; Li, H. Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. Stem Cell Res. Ther. 2018, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Faruk, E.M.; El-Desoky, R.E.; Al-Shazly, A.M.; Taha, N.M. Does exosomes derived bone marrow mesenchymal stem cells restore ovarian function by promoting stem cell survival on experimentally induced polycystic ovary in adult female albino rats? (histological and immunohistochemical study). Stem Cell Res. Ther. 2018, 8, 1000442. [Google Scholar] [CrossRef]
- Ebrahim, N.; Mostafa, O.; El Dosoky, R.E.; Ahmed, I.A.; Saad, A.S.; Mostafa, A.; Sabry, D.; Ibrahim, K.A.; Farid, A.S. Human mesenchymal stem cell-derived extracellular vesicles/estrogen combined therapy safely ameliorates experimentally induced intrauterine adhesions in a female rat model. Stem Cell Res. Ther. 2018, 9, 175. [Google Scholar] [CrossRef]
- Zhao, S.; Qi, W.; Zheng, J.; Tian, Y.; Qi, X.; Kong, D.; Zhang, J.; Huang, X. Exosomes Derived from Adipose Mesenchymal Stem Cells Restore Functional Endometrium in a Rat Model of Intrauterine Adhesions. Reprod. Sci. 2020, 27, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Reza, A.M.M.T.; Choi, Y.J.; Yasuda, H.; Kim, J.H. Human adipose mesenchymal stem cell-derived exosomal-miRNAs are critical factors for inducing anti-proliferation signalling to A2780 and SKOV-3 ovarian cancer cells. Sci. Rep. 2016, 6, 38498. [Google Scholar] [CrossRef]
- Blázquez, R.; Sánchez-Margallo, F.M.; Álvarez, V.; Matilla, E.; Hernández, N.; Marinaro, F.; Gómez-Serrano, M.; Jorge, I.; Casado, J.G.; Macías-García, B. Murine embryos exposed to human endometrial MSCs-derived extracellular vesicles exhibit higher VEGF/PDGF AA release, increased blastomere count and hatching rates. PLoS ONE 2018, 13, e0196080. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.S.; Nascimento, H.S.; Costa, M.P.; Costa, N.N.; Brito, K.N.; Lopes, C.T.; Santos, S.S.; Cordeiro, M.S.; Ohashi, O.M. Increasing of blastocyst rate and gene expression in co-culture of bovine embryos with adult adipose tissue-derived mesenchymal stem cells. J. Assist. Reprod. Genet. 2016, 33, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Wang, X. The Role and Application of Exosomes and Their Cargos in Reproductive Diseases: A Systematic Review. Vet. Sci. 2022, 9, 706. https://doi.org/10.3390/vetsci9120706
Chen Z, Wang X. The Role and Application of Exosomes and Their Cargos in Reproductive Diseases: A Systematic Review. Veterinary Sciences. 2022; 9(12):706. https://doi.org/10.3390/vetsci9120706
Chicago/Turabian StyleChen, Zhi, and Xiangguo Wang. 2022. "The Role and Application of Exosomes and Their Cargos in Reproductive Diseases: A Systematic Review" Veterinary Sciences 9, no. 12: 706. https://doi.org/10.3390/vetsci9120706
APA StyleChen, Z., & Wang, X. (2022). The Role and Application of Exosomes and Their Cargos in Reproductive Diseases: A Systematic Review. Veterinary Sciences, 9(12), 706. https://doi.org/10.3390/vetsci9120706





