Simple Summary
Bovine mastitis is a persistent and inflammatory reaction of the udder tissue that is usually caused by microbial infection, which can result in substantial losses due to reduced milk yield. Escherichia coli is considered a causative environmental pathogen and has been reported as a common cause of bovine mastitis worldwide. Because of its pathogenicity, Escherichia coli is always an important problem to the dairy industry worldwide and also poses a threat to food safety and public health, and with the widespread use of antibiotics, the resistance of Escherichia coli is increasing. Despite considerable research on bovine mastitis, the disease still remains one of the most prevalent and costly diseases of the dairy industry. The need to control mastitis is driven by multiple considerations, including milk quality, reductions in antimicrobial use, and animal welfare. Artemisinin is an antimalarial drug that was developed from a Chinese traditional herb, Qinghao. In recent years, other effects of artemisinin (including antitumor, anti-inflammatory, antifungal, etc.) have been increasingly discovered and applied. In this study, we demonstrated that artemisinin possesses a protective effect toward Escherichia coli–induced mastitis, thus providing a practical approach for the clinical control of mastitis.
Abstract
Bovine mastitis is an important disease affecting dairy farming, and it causes large economic losses to the dairy industry. Escherichia coli (E. coli) is considered to be a causative environmental pathogen and frequently enters into mammary glands, causing inflammation. Artemisinin is a highly effective malaria remedy and is not easy to develop drug resistance to. In recent years, other effects of artemisinin (including antitumor, anti-inflammatory, antifungal, etc.) have been increasingly discovered and applied. The current study aimed to investigate whether artemisinin could attenuate E. coli–induced inflammation. Through the E. coli mastitis model in MAC-T cells and mice, the protective effects of artemisinin were analyzed by CCK-8 (Cell Counting Kit-8), Western blot, and RT-qPCR. The results showed that artemisinin reversed the decrease of cell viability and upregulated TLR4 (toll-like receptor 4)/NF-κB (nuclear factor κB) and MAPK (mitogen activated protein kinase)/p38 signaling pathways, as well as restrained the expression of TNF-α, IL-6, and IL-1β mRNA caused by E. coli. Meanwhile, artemisinin also alleviated mammary tissue damage, reduced inflammatory cells’ infiltration, and decreased the levels of inflammatory factors in a mice mastitis model. This study demonstrated that artemisinin alleviated the inflammatory response of mouse mastitis and MAC-T cells induced by E. coli, thus providing a practical approach for the clinical control of mastitis.
1. Introduction
Bovine mastitis is a mammary tissue inflammatory disease that is caused by mechanical irritation, pathogenic microorganisms, and chemical and physical damage, and it is among the most common diseases in dairy farms. It not only reduces milk production but increases treatment costs, results in a loss of feed utilization, and creates milk waste [1]. The clinical manifestations of bovine mastitis are usually redness, swelling, fever, and pain in the mammary glands [2].
The defense machinery of mammary glands can be classified into nonspecific and specific immunity. Nonspecific immunity, also called innate immunity, is the main defense mechanism in the early phases of infection [3]. The specific immune system can facilitate or selectively eliminate pathogens by specifically recognizing their pathogenic factors through antibody molecules, macrophages, and lymphocytes [3,4,5,6]. In the mammary gland, innate and specific immunity coordinate with each other to protect against diseases. If the treatment is not timely or thorough, it is difficult to recover, and there is a risk of recurrence [7,8].
E. coli is widespread in the natural environment and does not cause infection in humans and animals under normal circumstances, but results in illness when the immunity of humans and animals is poor [9]. In dairy farms, E. coli is the most frequently occurring etiologic agent of environmental mastitis, and its incidence is closely related to the cows’ age, lactation period, and immune status, and other factors [10]. The pathogenicity of E. coli is determined by a variety of virulence factors, including pilin, adhesins, and lipopolysaccharides. To date, no specific virulence factors involving only bovine mastitis caused by E. coli have been identified [11]. After invading the host, E. coli will bind to TLR4 to activate the host’s innate immune system, thus inducing the activation of the MAPK and NF-κB signaling pathways [12]. The sensing of E. coli in bovine mammary glands involves epithelial cells that trigger a cascade of immunity-related processes [13]. Furthermore, a recent study suggested that the pathogenicity of E. coli in bovine mammary glands is associated with the presence of a new pathogenic phenotype known as mammary pathogenic Escherichia coli (MPEC) [14]. Meanwhile, the increasing antibiotic resistance of bacteria is one of the reasons for the low cure rate of mastitis in dairy cows and has attracted wide attention and intensive research in the livestock and public health industries [15,16,17,18].
Artemisinin is an endoperoxide terpene lactone compound that is found mainly in the Chinese medicine Artemisia annua [19,20]. Its derivatives include dihydroartemisinin, artesunate, artemisinin methyl ether, and others. In addition to its anti-malarial effects, artemisinin also has various biological functions in terms of antioxidant, anti-inflammatory, and vascular protection [21,22,23,24]. Wang et al. found that artemisinin treatment inhibited the expression of NF-κB-pathway-related proteins and the release of inflammatory factors such as IL-6 induced by LPS or E. coli, thus reducing the mortality of mice infected with E. coli [25]. These findings support the therapeutic potential of artemisinin for mastitis.
Despite accumulating evidence that artemisinin is effective in suppressing inflammation-related diseases, studies related to the treatment of bovine mastitis are lacking. Whether artemisinin could alleviate E. coli–induced bovine mastitis is uncertain. Therefore, this study established a mouse mastitis model by injecting E. coli into mammary glands and infecting MAC-T cells with E. coli to investigate whether artemisinin exerts anti-inflammatory defense effects by regulating the expression of inflammatory-pathway-related proteins, as well as inflammatory factors.
2. Materials and Methods
2.1. Reagents and Antibodies
Artemisinin was provided by Solarbio (Beijing, China). DMEM/high-glucose medium was obtained from Servicebio (Wuhan, China). The following primary antibodies were used: NF-κB p-65 (1:1000) and phosphorylated NF-κB p-65 (1:700) were procured from ABclonal (Wuhan, China); p-38 (1:1000), phosphorylated p38 (1:700), IKK (1:1000), and phosphorylated IKK (1:700) were purchased from CST (Boston, MA, USA); and TLR4 (1:1000), Myd88 (1:1000), β-actin (1:1000), GAPDH (1:80000), and Tubulin (1:50000) were purchased from Proteintech (Wuhan, China). The secondary antibodies, HRP-conjugated Affinipure Goat Anti-Rabbit IgG (1:5000) and HRP-conjugated Affinipure Goat Anti-Mouse IgG (1:5000), were obtained by Proteintech (Wuhan, China). The RT-qPCR-related reagents were provided by Accurate Biotechnology (Hunan, China).
2.2. Bacteria Strains and Culture
E. coli (ATCC25922) was inoculated on LB agar and incubated in an incubator at 37 °C. A randomly selected single colony was added to the LB broth and placed in a shaker at 37 °C and 200 rpm. After 12 h, the OD600nm values were measured.
2.3. Cell Culture
MAC-T (bovine mammary alveolar cell-T) cells were cultured in DMEM/high-glycemic medium containing 5% fetal bovine serum and grown in a sterile incubator at 37 °C, containing 5% CO2. When the cell fusion reached 90%, the following experiments were performed.
2.4. Mastitis Mouse Model and Sample Collection
Compared with other experimental animals, the mouse mastitis model is considered to be a straightforward and suitable model to study bovine mastitis because of its ease of manipulation and lower cost; it provides valuable information about the pathogenic mechanisms of bovine mastitis [26]. SPF Kunming mice were housed at 25 °C, 50% humidity, with 12 h of light and 12 h of darkness, in an experimental animal housing, and provided with food and water. The mice used in this experiment met the requirements of animal care and use suggested by the Committee of Shandong Agricultural University (SDAUA-2021-008). The model of mouse mastitis was established according to the previously described methods [27,28]. Female mice with similar delivery time were distributed to four groups randomly: control group, artemisinin control group, E. coli group, and artemisinin treatment group, with ten mice in each group. The female mice were slowly injected with 10 μL of 1 × 107 CFU/mL E. coli solution into the fourth pair of mammary glands, using a microsyringe once daily for three days. The female mice in the artemisinin-treatment group were the same as the E. coli group and treated with 50 mg/kg of artemisinin by oral gavage once daily for three days after the onset of mastitis was induced in the mice. Serum and mammary tissue were collected from the mice at the end of treatment.
Mice were anesthetized, and the fourth pair of mammary tissues was collected to make pathological sections and observed for pathological damage. The collected blood of mice was left for 2 h and then centrifuged at 3000 rpm for 5 min to obtain the serum, which was then kept at −80 °C for backup.
2.5. Cell Viability Assay
MAC-T cells were cultured in 96-well plates at a density of 1 × 104 cells per well in an incubator. MAC-T cells were infected with different concentrations (105, 106, 107, and 108 CFU/mL) of E. coli for 4, 6, and 8 h, with or without artemisinin (100 μg/mL) treatment. Cell viability was measured by using the CCK-8 (Cell Counting Kit-8) method. Briefly, 10 μL CCK-8 reagent was added to cells and incubated for 2 h. The absorbance was detected at 450 nm. Cell viability = (experimental group − blank group)/(control group − blank group) × 100%; the blank group contained medium and CCK-8 reagent but did not contain cells and E. coli, and the control group contained cells, medium, and CCK-8 reagent but did not contain E. coli. The results were obtained from three independent experiments. After combining the results of this part, MAC-T cells infected with E. coli of 107 CFU/mL for 4 h were selected to perform the subsequent experiments.
2.6. Quantitative Real-Time PCR (RT-qPCR)
MAC-T cells were infected with 107 CFU/mL of E. coli for 4 h, with or without artemisinin (100 or 200 μg/mL) treatment. The cells in each group were lysed with pre-cooled TRIzol and chloroform and left to stand for 5 min. The cell lysate was centrifuged at 12,000 g for 10 min. The supernatant was obtained and added to isopropanol and centrifuged again. Then the RNA precipitate was solubilized by RNA-free water. The cDNA was synthesized according to the kit operation, and IL-6, IL-1β, and TNF-α mRNA expressions were assayed by qPCR. The primers used in this study are shown in Table 1.

Table 1.
Primers used in this study.
2.7. Western Blot
MAC-T cells were infected with 107 CFU/mL of E. coli for 4 h, with or without artemisinin (100 or 200 μg/mL) treatment. The protein lysis solution and protease inhibitor were added to the cells for lysis. The lysed cells were collected into a 1.5 mL centrifuge tube, using a cell scraper. After centrifugation, the supernatant was obtained and transferred to a new centrifuge tube. The protein concentration was measured by using the BCA method, run with 10% sodium dodecyl sulfate polyacrylamide gels, and then electo-transferred to PVDF membranes. After PVDF membranes were blocked with 5% BSA solution at room temperature for 2 h, the membranes were incubated with the primary antibody overnight at 4 °C. After that, the membranes were incubated with secondary antibody at room temperature for 1 h. After washing with TBST, the aim target proteins were visualized by using ECL Western Detection Reagent and analyzed by using ImageJ software.
2.8. Histopathological Examination
The fresh tissues were soaked in 4% formalin fixative; after 72 h, the tissues were soaked in gradient alcohol for dehydration. Paraffin sections were prepared for HE (hematoxylin–eosin) staining, and once stained, the stained sections were dehydrated with anhydrous ethanol, washed with xylene, and placed in a ventilation cabinet. Finally, the slices were covered with neutral resin and observed under an optical microscope.
2.9. Statistical Analysis
The results of the presented experiments were obtained from three independent experiments and presented as mean ± standard deviation (mean ± SD). The statistical significance of differences between groups was analyzed by One-Way ANOVA, followed by Tukey’s post hoc test. Experimental data were analyzed by using SPSS biostatistics software Version 24.0 and charted by using Graph Pad Prism. The p-values less than 0.05 were regarded as significant.
3. Results
3.1. Artemisinin Reverses the Decrease of Cell Viability in E. coli–Infected MAC-T Cells
Firstly, no effect on cell viability and E. coli proliferation was detected that when MAC-T cells and E. coli exposed to artemisinin alone. To further explore the effect of artemisinin on cells challenged by E. coli, MAC-T cells were exposed to different concentrations of E. coli for 4, 6, and 8 h, with or without artemisinin treatment. As shown in Figure 1, the viability of MAC-T cells was significantly reduced in the E. coli–infected group as compared with the control group. Artemisinin treatment markedly inhibited the decrease in cell viability caused by 105 CFU/mL E. coli infection for 4, 6, and 8 h; and 106 and 107 CFU/mL E. coli infection for 4 and 6 h. After treating the MAC-T cells for 4 h with 107 CFU/mL E. coli, cell viability decreased by about 50%. In this study, we focused on the immunostimulatory effect on host cells at the early stage of E. coli infection, and, therefore, the MAC-T cells infected with E. coli of 107 CFU/mL for 4 h were selected to perform the subsequent experiments.
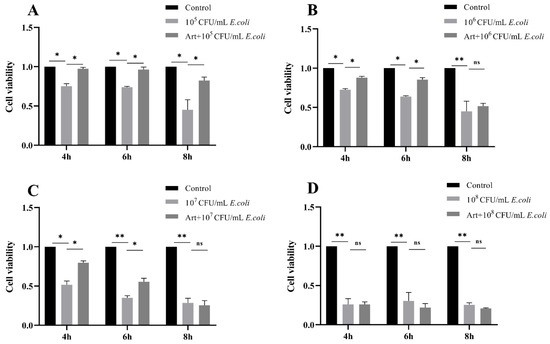
Figure 1.
(A–D) Effects of different concentrations of E. coli on MAC-T cells’ activity after 4, 6, and 8 h of infection and effect of artemisinin on the activity of E. coli–infected MAC-T cells. * p < 0.05, and ** p < 0.01.
3.2. Artemisinin Inhibits the Expression of TLR4/NF-κB Inflammatory Pathway
In the present study, E. coli dramatically induced the expression of TLR4 and the adaptor protein Myd88 in MAC-T cells, whereas artemisinin treatment significantly reversed this change. Next, the phosphorylation of IKK and NF-κB/p65 was examined, and the findings indicate that artemisinin can significantly reduce E. coli–induced phosphorylation of IKK and NF-κB/p65 (Figure 2). These results indicate that artemisinin inhibited E. coli–induced upregulation of the TLR4/NF-κB signaling pathway.
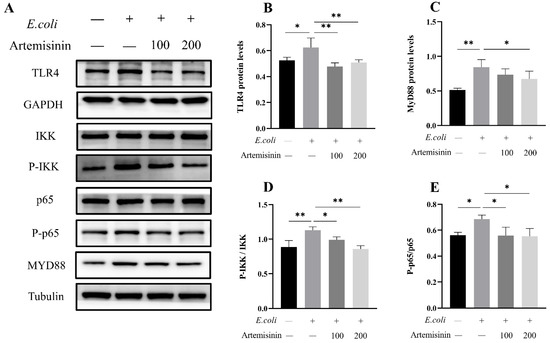
Figure 2.
(A–E) Protein levels of TLR4, MyD88, p65, p-p65, IKK, and p-IKK were determined by Western blot (the original figures were provided as File S1 of supplementary materials). GAPDH and Tubulin were used as loading controls. Note that “−” and “+” indicate not added and added, respectively. * p < 0.05, and ** p < 0.01.
3.3. Artemisinin Inhibits the Activation of MAPK/p38 Inflammatory Pathway
It is well-known that MAPK/p38 is also an important inflammatory pathway. Therefore, in this study, the changes of p38 protein were examined in E. coli–infected MAC-T cells. As shown in Figure 3, E. coli infection significantly promoted the phosphorylation of p38 in MAC-T cells, while artemisinin remarkably inhibited this effect. This suggests that artemisinin may inhibit the inflammatory response through MAPK/p38.
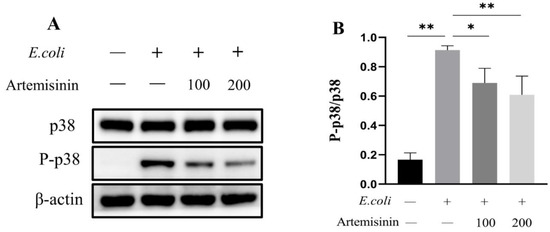
Figure 3.
The changes of MAPK/p38. (A) Western blot (the original figures were provided as File S1 of supplementary materials). β-actin served as the control. (B) Statistical analysis of MAPK/p38 changes. * p < 0.05, and ** p < 0.01.
3.4. Artemisinin Reduces IL-1β, IL-6, and TNF-α mRNA Expression in E. coli–Induced MAC-T Cells
Inflammatory factors could contribute to the incidence and progression of inflammation, such as IL-1β, IL-6, and TNF-α. As seen in Figure 4, the expression of mRNA for these three inflammatory factors was obviously raised after E. coli infection; meanwhile, artemisinin treatment effectively inhibited the expression of these inflammatory factors. This demonstrates that artemisinin could alleviate the MAC-T cells’ response to inflammation elicited by E. coli.
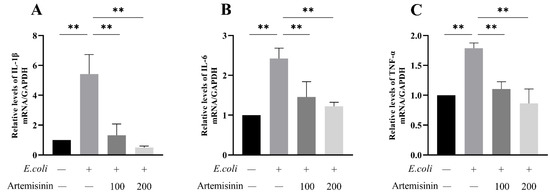
Figure 4.
RT-qPCR to measure inflammatory factor expression levels: (A) IL-1β, (B) IL-6, and (C) TNF-α. GAPDH as control. ** p < 0.01.
3.5. Artemisinin Reduces Serum Levels of IL-1β, IL-6 and TNF-α in Mice
The levels of IL-6, IL-1β, and TNF-α in mice serum were measured by using ELISA. The results shown in Figure 5 revealed that artemisinin suppressed the elevated levels of IL-1β, IL-6, and TNF-α in the serum of mice caused by E. coli.
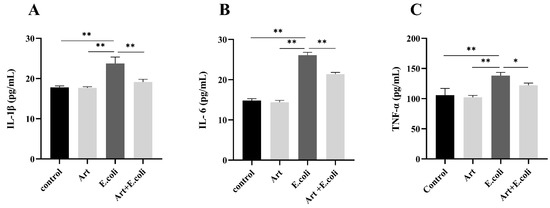
Figure 5.
ELISA to measure the level of inflammatory factors in mouse serum: (A) IL-1β, (B) IL-6, and (C) TNF-α. * p < 0.05, and ** p < 0.01.
3.6. Artemisinin Relieves the Pathological Damage of Mammary Gland in E. coli–Induced Mastitis Mice
To further evaluate the protective effect of artemisinin on mastitis, the mouse model was established by mammary duct infusion of E. coli. As illustrated in Figure 6, compared to the control group, the mice in the E. coli group had markedly more inflammatory cells that had infiltrated the mammary acini, thickened alveolar walls, and disrupted alveolar lumen. The artemisinin treatment significantly alleviated the tissue damage caused by E. coli, the inflammatory infiltrate was reduced, and the mammary acini tended to be intact.
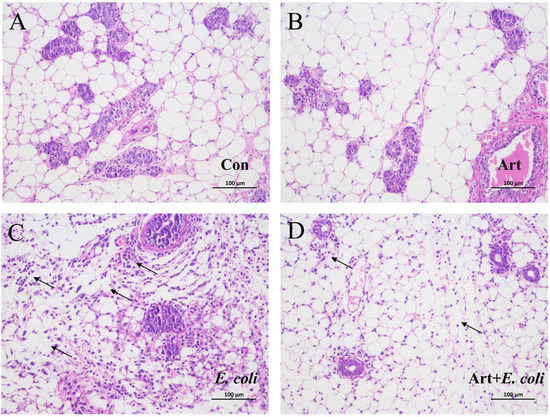
Figure 6.
Effects of artemisinin on the histology of E. coli–induced mammary gland pathology in mice (×200): (A) control group, (B) artemisinin control group, (C) E. coli group, and (D) artemisinin treatment group. Arrows indicates the infiltrated inflammatory cells.
4. Discussion
E. coli is widely distributed in the environment and often invades the udder tissues of dairy cows, causing inflammation. It is considered to be one of the primary causative agents of mastitis. Due to the widespread use of antibiotics, E. coli resistance has gradually increased [29]. E. coli–induced mastitis has not only seriously affected the sustainable development of dairy farming and the dairy industry but has also brought considerable hidden danger to public health safety and food safety [30,31]. Artemisinin is an important antimalarial drug that was developed from a Chinese traditional herb, Qinghao [19]. With the development of the research, other effects of artemisinin, which is very effective against malaria, such as anti-inflammatory and antiviral, are gradually being discovered [21]. Zhang et al. evaluated the anti-inflammatory effects of artemisinin in mouse models stimulated by LPS [32]. Qiao et al. showed that artemisinin could inhibit TLR4 signaling and inflammatory responses in LPS-induced BV2 microglial cells [33]. Kim et al. demonstrated that artemisinin has anti-inflammatory activities against periodontopathic bacteria [34]. Whether artemisinin could exert protective effects against E. coli–induced inflammation remains to be elucidated. In the present study, we explored the effect of artemisinin on E. coli–induced mastitis and studied the related inflammatory signaling pathways, and this helped us to further understand whether artemisinin could be an alternative to antibiotics for the prophylaxis and therapy of mastitis.
E. coli is the environmental pathogen that causes bovine mastitis. After milking during lactation, when the sphincter around the teat duct is in a relaxed state, or when prolonged milking changes the teat state, E. coli enters the milk pool along the teat duct, and the milk provides an ideal environment for E. coli to multiply [35]. The pathogenicity of E. coli is closely related to its own adhesion and aggressiveness, as well as the age and immunity of the cow. It has previously been shown that it takes only 15.2 min for E. coli to multiply to 109 CFU/mL in milk, and the faster E. coli multiplies, the greater the number of virulence factors, such as LPS, in the mammary gland, and the greater the damage caused. Continuous infection with E. coli may be an essential reason for mammary gland damage in dairy cows [36,37].
Artemisinin and its derivatives have shown potent efficacy in malaria, but as research progresses, its anti-inflammatory aspects are also being reported. In this study, artemisinin inhibited the activation of TLR4/NF-κB pathways in E. coli–infected MAC-T cells, and this may be a potential mechanism for artemisinin treatment of mastitis. The effects of artemisinin on inflammatory diseases may be reflected in the modulation of inflammatory pathways. Zhang et al. found that artemisinin increased the activity of porcine mammary epithelial cells after LPS stimulation and attenuated LPS damage to porcine mammary glands by inhibiting NF-κB and MAPK inflammatory pathways [38]. Previous studies have shown that, when mice were attacked by heat-inactivated Staphylococcus aureus, artemisinin improved survival by suppressing TLR2 expression and activation of NF-κB, and exerted a protective effect in a dose-dependent manner. In an in vitro assay, artemisinin inhibited the release of TNF-α from S. aureus or peptidoglycan-induced mouse peritoneal macrophages [39]. Yuan et al. discovered that artemisinin inhibited neutrophil infiltration in rosacea-like mice and suppressed the activation of the NF-κB pathway, thus suggesting that artemisinin may improve chronic inflammatory skin diseases of the face by modulating immune response and angiogenesis [40]. A growing number of studies have indicated the potential of artemisinin in the treatment of inflammation [25,41,42].
The TLR4/NF-κB pathway has a critical function in the regulation of inflammatory responses. The cell membrane receptor TLR4 recognizes exogenous stimuli such as lipopolysaccharide from E. coli, and the signal is transmitted through MyD88, activates NF-κB, and then modulates many of the pro-inflammatory cytokines and chemokine transcripts that contribute to the development of mastitis. Previous studies have demonstrated that mRNA expression of TLR4 was upregulated and levels of inflammatory factors such as IL-8 and IL-6 were increased in MAC-T cells after E. coli infestation [43,44]. LPS stimulation of TLR4 induces activated NF-κB and MAPK inflammatory pathways [45]. These findings are similar to those of our study. Furthermore, it was demonstrated that E. coli infection mediated the inflammatory response through activation of NLRP3 and NLRC4 inflammasome in bovine mammary epithelial cells. The interaction of LPS with TLR4 resulted in the activation of NLRP3 and subsequent formation of NLRP3 inflammasome with ASC proteins. Inflammasome induced caspase-1 shearing and the production of active IL-1β and IL-18 [46,47]. It remains to be investigated whether artemisinin could alleviate E. coli–induced mastitis by inhibiting the activation of inflammasome in MAC-T cells.
In the present study, E. coli significantly upregulated the levels of inflammatory factors in MAC-T cells and mouse serum, but this was markedly reversed by artemisinin with a dose-dependent manner. The MAPK/p38 inflammatory pathway can be activated by many inflammation-related stimuli and has a key function in modulating the biological synthesis of pro-inflammatory cytokines [48]. It represents a therapeutic target for many inflammatory diseases [49]. In addition, pro-inflammatory cytokines serve as direct inflammatory indicators designed to reflect the severity of inflammation [50]. We examined the mRNA expression levels of IL-1β, IL-6, and TNF-α, using RT-qPCR, and found they were all remarkably elevated in cells infected by E. coli, whereas the intervention of artemisinin resulted in a considerable decrease in the expression of IL-1β, IL-6, and TNF-α that further suggests that artemisinin could alleviate E. coli–induced inflammation reactions.
The papillae and papillary ducts are the important line of defense for the mammary glands. When the natural barrier fails, the pathogens enter the milk pool along the nipple ducts. As the bacteria multiply, the inflammatory cells in the mammary tissue also gradually increase, further aggravating the damage and the loss of structural integrity of the mammary acini [35,51,52]. To further understand the protective effect of artemisinin on E. coli–induced bovine mastitis, an E. coli–induced mastitis model in mice was established. Our results showed that artemisinin treatment could obviously reduce inflammatory cells’ infiltration, alleviate mammary tissue damage, and inhibit the increase of inflammatory factors in the serum of E. coli–induced mice.
5. Conclusions
In conclusion, this study demonstrates that artemisinin possesses a protective effect toward E. coli–induced mastitis, thus providing an approach for treatment of bovine mastitis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vetsci9080381/s1, File S1: The original gel figures of the Western blot.
Author Contributions
Conceptualization, Y.L. and J.L.; methodology, Z.L. and J.H.; software, Z.L.; validation, X.W. and Y.D.; formal analysis, Z.L.; investigation, Z.L., J.H. and J.Y.; resources, J.H. and S.C.; data curation, Z.L. and J.G.; writing—original draft preparation, Z.L.; writing—review and editing, Y.L.; supervision, B.H.; project administration, J.L.; funding acquisition, Y.L. and J.L. All authors have read and agreed to the published version of the manuscript.
Funding
The project was supported by the National Natural Science Foundation of China (31802259, 31872535, and 31960721), Shandong Natural Science Foundation of China (ZR2018MC027), China Postdoctoral Science Foundation (2018M632704, 2019T120601), and Funds of Shandong “Double Tops” Program.
Institutional Review Board Statement
The mice used in this experiment met the requirements of animal care and use suggested by the Committee of Shandong Agricultural University (SDAUA-2021-008).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no competing interest.
References
- Gomes, F.; Henriques, M. Control of Bovine Mastitis: Old and Recent Therapeutic Approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riemann, T.; Bergmann, A. Epidemiology, pathogenesis, treatment and prevention of bovine acute Escherichia coli mastitis, a literature review. DTW. Dtsch. Tierarztl. Wochenschr. 2000, 107, 444–454. [Google Scholar] [PubMed]
- Sordillo, L.M.; Shafer-Weaver, K.; DeRosa, D. Immunobiology of the mammary gland. J. Dairy Sci. 1997, 80, 1851–1865. [Google Scholar] [CrossRef]
- Shimazaki, K.I.; Kawai, K. Advances in lactoferrin research concerning bovine mastitis. Biochem. Cell Biol. Biochim. Biol. Cell. 2017, 95, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Kehrli, M.E., Jr.; Harp, J.A. Immunity in the mammary gland. Vet. Clin. N. Am. Food Anim. Pract. 2001, 17, 495–516. [Google Scholar] [CrossRef]
- Chaneton, L.; Tirante, L.; Maito, J.; Chaves, J.; Bussmann, L.E. Relationship between milk lactoferrin and etiological agent in the mastitic bovine mammary gland. J. Dairy Sci. 2008, 91, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Iqbal Yatoo, M.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R.; et al. Advances in therapeutic and managemental approaches of bovine mastitis: A comprehensive review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.R.; Saeman, A.; Fox, L.K.; Lombard, J.; Hogan, J.S.; Smith, K.L. The National Mastitis Council: A Global Organization for Mastitis Control and Milk Quality, 50 Years and Beyond. J. Mammary Gland. Biol. Neoplasia 2014, 19, 241–251. [Google Scholar] [CrossRef]
- Ramos, S.; Silva, V.; Dapkevicius, M.L.E.; Caniça, M.; Tejedor-Junco, M.T.; Igrejas, G.; Poeta, P. Escherichia coli as Commensal and Pathogenic Bacteria Among Food-Producing Animals: Health Implications of Extended Spectrum β-lactamase (ESBL) Production. Animals 2020, 10, 2239. [Google Scholar] [CrossRef]
- Duse, A.; Persson-Waller, K.; Pedersen, K. Microbial Aetiology, Antibiotic Susceptibility and Pathogen-Specific Risk Factors for Udder Pathogens from Clinical Mastitis in Dairy Cows. Animals 2021, 11, 2113. [Google Scholar] [CrossRef]
- Croxen, M.A.; Finlay, B.B. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 2010, 8, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tang, R.; Zhao, C.; Mu, R.; Wang, Y.; Cao, Y.; Zhang, N.; Fu, Y. The Prevention Effect of Bacillus subtilis on Escherichia coli-Induced Mastitis in Mice by Suppressing the NF-kappaB and MAPK Signaling Pathways. Probiotics Antimicrob. Proteins, 2021; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kerro Dego, O.; Oliver, S.P.; Almeida, R.A. Host-pathogen gene expression profiles during infection of primary bovine mammary epithelial cells with Escherichia coli strains associated with acute or persistent bovine mastitis. Vet. Microbiol. 2012, 155, 291–297. [Google Scholar] [CrossRef]
- Leimbach, A.; Poehlein, A.; Vollmers, J.; Görlich, D.; Daniel, R.; Dobrindt, U. No evidence for a bovine mastitis Escherichia coli pathotype. BMC Genom. 2017, 18, 359. [Google Scholar] [CrossRef] [PubMed]
- Yair, Y.; Gophna, U. Pandemic Bacteremic Escherichia coli Strains: Evolution and Emergence of Drug-Resistant Pathogens. Curr. Top. Microbiol. Immunol. 2018, 416, 163–180. [Google Scholar] [CrossRef]
- Gao, J.; Duan, X.; Li, X.; Cao, H.; Wang, Y.; Zheng, S.J. Emerging of a highly pathogenic and multi-drug resistant strain of Escherichia coli causing an outbreak of colibacillosis in chickens. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2018, 65, 392–398. [Google Scholar] [CrossRef]
- Anes, J.; Nguyen, S.V.; Eshwar, A.K.; McCabe, E.; Macori, G.; Hurley, D.; Lehner, A.; Fanning, S. Molecular characterisation of multi-drug resistant Escherichia coli of bovine origin. Vet. Microbiol. 2020, 242, 108566. [Google Scholar] [CrossRef] [PubMed]
- Brennan, E.; Martins, M.; McCusker, M.P.; Wang, J.; Alves, B.M.; Hurley, D.; El Garch, F.; Woehrlé, F.; Miossec, C.; McGrath, L.; et al. Multidrug-Resistant Escherichia coli in Bovine Animals, Europe. Emerg. Infect. Dis. 2016, 22, 1650–1652. [Google Scholar] [CrossRef] [Green Version]
- Ma, N.; Zhang, Z.; Liao, F.; Jiang, T.; Tu, Y. The birth of artemisinin. Pharmacol. Ther. 2020, 216, 107658. [Google Scholar] [CrossRef]
- Tu, Y. Artemisinin-A Gift from Traditional Chinese Medicine to the World (Nobel Lecture). Angew. Chem. Int. Ed. 2016, 55, 10210–10226. [Google Scholar] [CrossRef]
- Ho, W.E.; Peh, H.Y.; Chan, T.K.; Wong, W.S. Artemisinins: Pharmacological actions beyond anti-malarial. Pharmacol. Ther. 2014, 142, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Oesch, F. The immunosuppressive activity of artemisinin-type drugs towards inflammatory and autoimmune diseases. Med. Res. Rev. 2021, 41, 3023–3061. [Google Scholar] [CrossRef] [PubMed]
- Kiani, B.H.; Kayani, W.K.; Khayam, A.U.; Dilshad, E.; Ismail, H.; Mirza, B. Artemisinin and its derivatives: A promising cancer therapy. Mol. Biol. Rep. 2020, 47, 6321–6336. [Google Scholar] [CrossRef] [PubMed]
- Dolivo, D.; Weathers, P.; Dominko, T. Artemisinin and artemisinin derivatives as anti-fibrotic therapeutics. Acta Pharm. Sin. B 2021, 11, 322–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, H.; Zheng, J.; Cheng, J.; Liu, W.; Ding, G.; Wang, L.; Luo, P.; Lu, Y.; Cao, H.; et al. The antimalarial artemisinin synergizes with antibiotics to protect against lethal live Escherichia coli challenge by decreasing proinflammatory cytokine release. Antimicrob. Agents Chemother. 2006, 50, 2420–2427. [Google Scholar] [CrossRef] [Green Version]
- Notebaert, S.; Meyer, E. Mouse models to study the pathogenesis and control of bovine mastitis. A review. Vet. Q. 2006, 28, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Hu, X.; Bao, L.; Wu, K.; Feng, L.; Qiu, M.; Hao, H.; Fu, Y.; Zhang, N. Aryl hydrocarbon receptor activation by Lactobacillus reuteri tryptophan metabolism alleviates Escherichia coli-induced mastitis in mice. PLoS Pathog. 2021, 17, e1009774. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Chu, B.; Liu, N.; Chen, S.; Wang, J. Lactobacillus rhamnosus GR-1 Prevents Escherichia coli-Induced Apoptosis Through PINK1/Parkin-Mediated Mitophagy in Bovine Mastitis. Front. Immunol. 2021, 12, 715098. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Shang, X.; Wang, L.; Li, H.; Wang, X. Characteristics of quinolone-resistant Escherichia coli isolated from bovine mastitis in China. J. Dairy Sci. 2018, 101, 6244–6252. [Google Scholar] [CrossRef] [Green Version]
- Dias, R.S.; Eller, M.R.; Duarte, V.S.; Pereira, Â.L.; Silva, C.C.; Mantovani, H.C.; Oliveira, L.L.; Silva Ede, A.; De Paula, S.O. Use of phages against antibiotic-resistant Staphylococcus aureus isolated from bovine mastitis. J. Anim. Sci. 2013, 91, 3930–3939. [Google Scholar] [CrossRef]
- El-Sayed, A.; Kamel, M. Bovine mastitis prevention and control in the post-antibiotic era. Trop. Anim. Health Prod. 2021, 53, 236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, X.; Lin, C.; Wu, S.; Wang, F.; Wang, H.; Wang, Y.; Peng, Y.; Hutchinson, M.R.; Li, H.; et al. Artemisinin inhibits TLR4 signaling by targeting co-receptor MD2 in microglial BV-2 cells and prevents lipopolysaccharide-induced blood-brain barrier leakage in mice. J. Neurochem. 2021, 157, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Zhang, H.; Sun, F.; Jiang, Z. Molecular Basis of Artemisinin Derivatives Inhibition of Myeloid Differentiation Protein 2 by Combined in Silico and Experimental Study. Molecules 2021, 26, 5698. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Choi, W.J.; Lee, S.; Kim, W.J.; Lee, D.C.; Sohn, U.D.; Shin, H.S.; Kim, W. Anti-inflammatory, Antioxidant and Antimicrobial Effects of Artemisinin Extracts from Artemisia annua L. Korean J. Physiol. Pharmacol. 2015, 19, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Bramley, A.J.; Dodd, F.H. Reviews of the progress of dairy science: Mastitis control-progress and prospects. J. Dairy Res. 1984, 51, 481–512. [Google Scholar] [CrossRef]
- Schukken, Y.H.; Günther, J.; Fitzpatrick, J.; Fontaine, M.C.; Goetze, L.; Holst, O.; Leigh, J.; Petzl, W.; Schuberth, H.J.; Sipka, A.; et al. Host-response patterns of intramammary infections in dairy cows. Vet. Immunol. Immunopathol. 2011, 144, 270–289. [Google Scholar] [CrossRef]
- Wellnitz, O.; Bruckmaier, R.M. The innate immune response of the bovine mammary gland to bacterial infection. Vet. J. 2012, 192, 148–152. [Google Scholar] [CrossRef]
- Zhang, W.; Xiong, L.; Chen, J.; Tian, Z.; Liu, J.; Chen, F.; Ren, M.; Guan, W.; Zhang, S. Artemisinin Protects Porcine Mammary Epithelial Cells against Lipopolysaccharide-Induced Inflammatory Injury by Regulating the NF-κB and MAPK Signaling Pathways. Animals 2021, 11, 1528. [Google Scholar] [CrossRef]
- Li, B.; Li, J.; Pan, X.; Ding, G.; Cao, H.; Jiang, W.; Zheng, J.; Zhou, H. Artesunate protects sepsis model mice challenged with Staphylococcus aureus by decreasing TNF-alpha release via inhibition TLR2 and Nod2 mRNA expressions and transcription factor NF-kappaB activation. Int. Immunopharmacol. 2010, 10, 344–350. [Google Scholar] [CrossRef]
- Yuan, X.; Li, J.; Li, Y.; Deng, Z.; Zhou, L.; Long, J.; Tang, Y.; Zuo, Z.; Zhang, Y.; Xie, H. Artemisinin, a potential option to inhibit inflammation and angiogenesis in rosacea. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 117, 109181. [Google Scholar] [CrossRef]
- Hou, L.F.; He, S.J.; Li, X.; Yang, Y.; He, P.L.; Zhou, Y.; Zhu, F.H.; Yang, Y.F.; Li, Y.; Tang, W.; et al. Oral administration of artemisinin analog SM934 ameliorates lupus syndromes in MRL/lpr mice by inhibiting Th1 and Th17 cell responses. Arthritis Rheum. 2011, 63, 2445–2455. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Tang, W.; Zhou, R.; Wan, J.; Shi, L.P.; Zhang, Y.; Yang, Y.F.; Li, Y.; Zuo, J.P. The new water-soluble artemisinin derivative SM905 ameliorates collagen-induced arthritis by suppression of inflammatory and Th17 responses. Br. J. Pharmacol. 2008, 153, 1303–1310. [Google Scholar] [CrossRef] [Green Version]
- Ma, N.; Chang, G.; Huang, J.; Wang, Y.; Gao, Q.; Cheng, X.; Liu, J.; Shen, X. cis-9, trans-11-Conjugated Linoleic Acid Exerts an Anti-inflammatory Effect in Bovine Mammary Epithelial Cells after Escherichia coli Stimulation through NF-κB Signaling Pathway. J. Agric. Food Chem. 2019, 67, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yue, Y.; Li, D.; Duan, C.; Qiu, X.; Zou, Y.; Zhu, Y.; Lauridsen, C.; Wang, J. Antibacterial and immunomodulatory effects of Pheromonicin-NM on Escherichia coli-challenged bovine mammary epithelial cells. Int. Immunopharmacol. 2020, 84, 106569. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, C.; Wei, Z.; He, X.; Kou, J.; Zhou, E.; Yang, Z.; Fu, Y. Morin suppresses inflammatory cytokine expression by downregulation of nuclear factor-κB and mitogen-activated protein kinase (MAPK) signaling pathways in lipopolysaccharide-stimulated primary bovine mammary epithelial cells. J. Dairy Sci. 2016, 99, 3016–3022. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Zhu, Y.H.; Xu, J.; Liu, X.; Duan, C.; Wang, M.J.; Wang, J.F. Lactobacillus rhamnosus GR-1 Ameliorates Escherichia coli-Induced Activation of NLRP3 and NLRC4 Inflammasomes With Differential Requirement for ASC. Front. Microbiol. 2018, 9, 1661. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.; Pei, Y.; Wang, X.; Feng, J.; Zhang, Y.; Gao, M.Q. LncRNA XIST mediates bovine mammary epithelial cell inflammatory response via NF-κB/NLRP3 inflammasome pathway. Cell Prolif. 2019, 52, e12525. [Google Scholar] [CrossRef] [Green Version]
- Machado, T.R.; Machado, T.R.; Pascutti, P.G. The p38 MAPK Inhibitors and Their Role in Inflammatory Diseases. Chem. Sel. 2021, 6, 5729–5742. [Google Scholar] [CrossRef]
- Yong, H.Y.; Koh, M.S.; Moon, A. The p38 MAPK inhibitors for the treatment of inflammatory diseases and cancer. Expert Opin. Investig. Drugs 2009, 18, 1893–1905. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [Green Version]
- Zecconi, A.; Hamann, J.; Bronzo, V.; Moroni, P.; Giovannini, G.; Piccinini, R. Relationship between teat tissue immune defences and intramammary infections. Adv. Exp. Med. Biol. 2000, 480, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Reyher, K.K.; Haine, D.; Dohoo, I.R.; Revie, C.W. Examining the effect of intramammary infections with minor mastitis pathogens on the acquisition of new intramammary infections with major mastitis pathogens--a systematic review and meta-analysis. J. Dairy Sci. 2012, 95, 6483–6502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).