Antibiotic-Resistant Escherichia coli Strains Isolated from Captive Giant Pandas: A Reservoir of Antibiotic Resistance Genes and Virulence-Associated Genes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Antibiotic Sensitivity and Resistance
2.3. DNA Extraction and Screening for ARGs and VAGs
2.4. Association Analysis between ARGs and AMR/VAGs/MGEs
3. Results
3.1. Distribution of ARGs, VAGs and Phylogenetic Grouping
3.2. Associations between ARGs and AMR
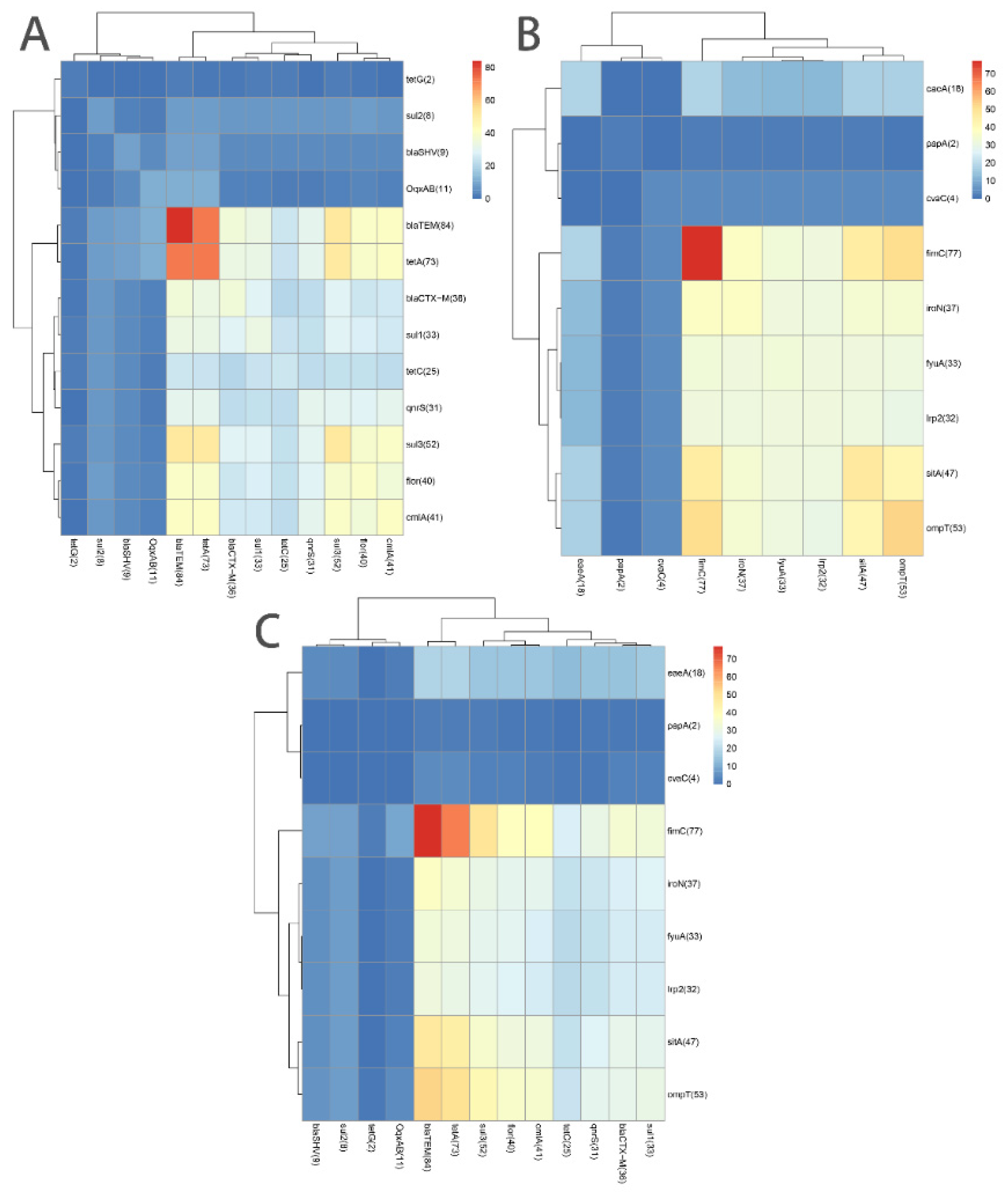
3.3. Associations among 13 ARGs and among 9 VAGs
3.4. Association of ARGs with VAGs or MGEs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vila, J.; Sáez-López, E.; Johnson, J.R.; Römling, U.; Dobrindt, U.; Cantón, R.; Giske, C.G.; Naas, T.; Carattoli, A.; Martínez-Medina, M.; et al. Escherichia coli: An old friend with new tidings. FEMS Microbiol. Rev. 2016, 40, 437–463. [Google Scholar] [CrossRef] [PubMed]
- Foster-Nyarko, E.; Pallen, M.J. The microbial ecology of Escherichia coli in the vertebrate gut. FEMS Microbiol. Rev. 2022, 46, fuac008. [Google Scholar] [CrossRef] [PubMed]
- Arbab, S.; Ullah, H.; Wang, W.; Zhang, J. Antimicrobial drug resistance against Escherichia coli and its harmful effect on animal health. Vet. Med. Sci. 2022, 8, 1780–1786. [Google Scholar] [CrossRef]
- Pakbin, B.; Bruck, W.M.; Rossen, J.W.A. Virulence Factors of Enteric Pathogenic Escherichia coli: A Review. Int. J. Mol. Sci. 2021, 22, 9922. [Google Scholar] [CrossRef]
- Anand, U.; Reddy, B.; Singh, V.K.; Singh, A.K.; Kesari, K.K.; Tripathi, P.; Kumar, P.; Tripathi, V.; Simal-Gandara, J. Potential Environmental and Human Health Risks Caused by Antibiotic-Resistant Bacteria (ARB), Antibiotic Resistance Genes (ARGs) and Emerging Contaminants (ECs) from Municipal Solid Waste (MSW) Landfill. Antibiotics 2021, 10, 374. [Google Scholar] [CrossRef]
- Song, J.; Jongmans-Hochschulz, E.; Mauder, N.; Imirzalioglu, C.; Wichels, A.; Gerdts, G. The Travelling Particles: Investigating microplastics as possible transport vectors for multidrug resistant E. coli in the Weser estuary (Germany). Sci. Total Environ. 2020, 720, 137603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Abbas, M.; Rehman, M.U.; Huang, Y.; Zhou, R.; Gong, S.; Yang, H.; Chen, S.; Wang, M.; Cheng, A. Dissemination of antibiotic resistance genes (ARGs) via integrons in Escherichia coli: A risk to human health. Environ. Pollut. 2020, 266, 115260. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Huang, H.; Chen, Y. Effects of emerging pollutants on the occurrence and transfer of antibiotic resistance genes: A review. J. Hazard. Mater. 2021, 420, 126602. [Google Scholar] [CrossRef]
- Li, L.G.; Huang, Q.; Yin, X.; Zhang, T. Source tracking of antibiotic resistance genes in the environment—Challenges, progress, and prospects. Water Res. 2020, 185, 116127. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, S.; Qi, M.; Liu, H.; Zhang, S.; Liu, H.; Zhou, Z.; Wang, L.; Wang, C.; Luo, Y.; et al. Prevalence and characterization of antibiotic resistance genes and integrons in Escherichia coli isolates from captive non-human primates of 13 zoos in China. Sci. Total Environ. 2021, 798, 149268. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Pan, S.; Wei, B.; Liu, H.; Zhou, Z.; Huang, X.; Luo, Y.; Zhou, L.; Zhang, S.; Ma, X.; et al. High prevalence of multi-drug resistances and diversity of mobile genetic elements in Escherichia coli isolates from captive giant pandas. Ecotoxicol. Environ. Saf. 2020, 198, 110681. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zou, W.; Xie, S.; Kong, L.; Chen, Y.; Zhang, X.; Li, J.; Wang, H.; Cheng, G.; Qin, Y.; et al. Serotype and Antimicrobial Resistance of Escherichia coli Isolated from Feces of Wild Giant Pandas (Ailuropoda melanoleuca) in Sichuan Province, China. J. Wildl. Dis. 2018, 54, 691–699. [Google Scholar] [CrossRef]
- Dolejska, M.; Papagiannitsis, C.C. Plasmid-mediated resistance is going wild. Plasmid 2018, 99, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Guyomard-Rabenirina, S.; Reynaud, Y.; Pot, M.; Albina, E.; Couvin, D.; Ducat, C.; Gruel, G.; Ferdinand, S.; Legreneur, P.; Le Hello, S.; et al. Antimicrobial Resistance in Wildlife in Guadeloupe (French West Indies): Distribution of a Single bla (CTX-M-1)/IncI1/ST3 Plasmid Among Humans and Wild Animals. Front. Microbiol. 2020, 11, 1524. [Google Scholar] [CrossRef]
- Bungau, S.; Tit, D.M.; Behl, T.; Aleya, L.; Zaha, D.C. Aspects of excessive antibiotic consumption and environmental influences correlated with the occurrence of resistance to antimicrobial agents. Curr. Opin. Environ. Sci. Health 2020, 19, 100224. [Google Scholar] [CrossRef]
- Chandler, J.C.; Aljasir, S.F.; Hamidi, A.; Sylejmani, D.; Gerow, K.G.; Bisha, B. Short communication: A countrywide survey of antimicrobial-resistant indicator bacteria in Kosovo’s dairy farms. J. Dairy Sci. 2018, 101, 6982–6989. [Google Scholar] [CrossRef]
- Guo, L.; Long, M.; Huang, Y.; Wu, G.; Deng, W.; Yang, X.; Li, B.; Meng, Y.; Cheng, L.; Fan, L.; et al. Antimicrobial and disinfectant resistance of Escherichia coli isolated from giant pandas. J. Appl. Microbiol. 2015, 119, 55–64. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Q.; Xia, X.; Zhang, Y.; Li, D.; Wang, C.; Chen, S.; Hou, R. Serotypes, virulence factors, and antimicrobial susceptibilities of vaginal and fecal isolates of Escherichia coli from giant pandas. Appl. Environ. Microbiol. 2013, 79, 5146–5150. [Google Scholar] [CrossRef]
- Rehman, M.U.; Zhang, H.; Iqbal, M.K.; Mehmood, K.; Huang, S.; Nabi, F.; Luo, H.; Lan, Y.; Li, J. Antibiotic resistance, serogroups, virulence genes, and phylogenetic groups of Escherichia coli isolated from yaks with diarrhea in Qinghai Plateau, China. Gut Pathog. 2017, 9, 24. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, S.; Rehman, M.U.; Yang, H.; Yang, Z.; Wang, M.; Jia, R.; Chen, S.; Liu, M.; Zhu, D.; et al. Distribution and association of antimicrobial resistance and virulence traits in Escherichia coli isolates from healthy waterfowls in Hainan, China. Ecotoxicol. Environ. Saf. 2021, 220, 112317. [Google Scholar] [CrossRef] [PubMed]
- Do, K.H.; Seo, K.W.; Byun, J.W.; Lee, W.K. Relationship between virulence factors and antimicrobial resistance genes of pathogenic Escherichia coli from diarrheic weaned piglets. Res. Vet. Sci. 2022, 150, 137–143. [Google Scholar] [CrossRef]
- Malekzadegan, Y.; Khashei, R.; Sedigh Ebrahim-Saraie, H.; Jahanabadi, Z. Distribution of virulence genes and their association with antimicrobial resistance among uropathogenic Escherichia coli isolates from Iranian patients. BMC Infect. Dis. 2018, 18, 572. [Google Scholar] [CrossRef]
- Dai, Q.L.; Li, J.W.; Yang, Y.; Li, M.; Zhang, K.; He, L.Y.; Zhang, J.; Tang, B.; Liu, H.P.; Li, Y.X.; et al. Genetic Diversity and Prediction Analysis of Small Isolated Giant Panda Populations After Release of Individuals. Evol. Bioinform. Online 2020, 16, 1176934320939945. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Li, J. Role of nature reserves in giant panda protection. Environ. Sci. Pollut. Res. Int. 2018, 25, 4474–4478. [Google Scholar] [CrossRef]
- Seghal Kiran, G.; Anto Thomas, T.; Selvin, J.; Sabarathnam, B.; Lipton, A.P. Optimization and characterization of a new lipopeptide biosurfactant produced by marine Brevibacterium aureum MSA13 in solid state culture. Bioresour. Technol. 2010, 101, 2389–2396. [Google Scholar] [CrossRef] [PubMed]
- Vale, A.P.; Shubin, L.; Cummins, J.; Leonard, F.C.; Barry, G. Detection of bla (OXA-1), bla (TEM-1), and Virulence Factors in E. coli Isolated From Seals. Front. Vet. Sci. 2021, 8, 583759. [Google Scholar] [CrossRef] [PubMed]
- Paixão, A.C.; Ferreira, A.C.; Fontes, M.; Themudo, P.; Albuquerque, T.; Soares, M.C.; Fevereiro, M.; Martins, L.; de Sá, M.I.C. Detection of virulence-associated genes in pathogenic and commensal avian Escherichia coli isolates. Poult. Sci. 2016, 95, 1646–1652. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Inoue, H.; Kanda, T.; Ijiri, M.; Uemura, R. Virulence-Associated Gene Profiles of Escherichia coli Isolated from Chickens with Colibacillosis in Japan and Their Correlation with Pathogenicity in Chicken Embryos. Avian Dis. 2021, 65, 401–405. [Google Scholar] [CrossRef]
- Algammal, A.M.; Hetta, H.F.; Batiha, G.E.; Hozzein, W.N.; El Kazzaz, W.M.; Hashem, H.R.; Tawfik, A.M.; El-Tarabili, R.M. Virulence-determinants and antibiotic-resistance genes of MDR-E. coli isolated from secondary infections following FMD-outbreak in cattle. Sci. Rep. 2020, 10, 19779. [Google Scholar] [CrossRef] [PubMed]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef] [PubMed]
- Abia, A.L.; Schaefer, L.; Ubomba-Jaswa, E.; Le Roux, W. Abundance of Pathogenic Escherichia coli Virulence-Associated Genes in Well and Borehole Water Used for Domestic Purposes in a Peri-Urban Community of South Africa. Int. J. Environ. Res. Public Health 2017, 14, 320. [Google Scholar] [CrossRef] [PubMed]
- Badura, A.; Luxner, J.; Feierl, G.; Reinthaler, F.F.; Zarfel, G.; Galler, H.; Pregartner, G.; Riedl, R.; Grisold, A.J. Prevalence, antibiotic resistance patterns and molecular characterization of Escherichia coli from Austrian sandpits. Environ. Pollut. 2014, 194, 24–30. [Google Scholar] [CrossRef]
- Di Cesare, A.; Pinnell, L.J.; Brambilla, D.; Elli, G.; Sabatino, R.; Sathicq, M.B.; Corno, G.; O'Donnell, C.; Turner, J.W. Bioplastic accumulates antibiotic and metal resistance genes in coastal marine sediments. Environ. Pollut. 2021, 291, 118161. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Li, C.; Yang, X.; Wang, Y.; Cheng, G.; Zeng, J.; Zhang, X.; Chen, Y.; Cai, R.; Huang, Q.; et al. Frequency of antimicrobial resistance and integron gene cassettes in Escherichia coli isolated from giant pandas (Ailuropoda melanoleuca) in China. Microb Pathog. 2018, 116, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Poeta, P.; Sáenz, Y.; Vinué, L.; Coelho, A.C.; Matos, M.; Rojo-Bezares, B.; Rodrigues, J.; Torres, C. Mechanisms of antibiotic resistance in Escherichia coli isolates recovered from wild animals. Microb Drug Resist. 2008, 14, 71–77. [Google Scholar] [CrossRef]
- Swift, B.M.C.; Bennett, M.; Waller, K.; Dodd, C.; Murray, A.; Gomes, R.L.; Humphreys, B.; Hobman, J.L.; Jones, M.A.; Whitlock, S.E.; et al. Anthropogenic environmental drivers of antimicrobial resistance in wildlife. Sci. Total Environ. 2019, 649, 12–20. [Google Scholar] [CrossRef]
- Sacristán, I.; Esperón, F.; Acuña, F.; Aguilar, E.; García, S.; López, M.J.; Cevidanes, A.; Neves, E.; Cabello, J.; Hidalgo-Hermoso, E.; et al. Antibiotic resistance genes as landscape anthropization indicators: Using a wild felid as sentinel in Chile. Sci. Total Environ. 2020, 703, 134900. [Google Scholar] [CrossRef]
- Yuan, Y.; Liang, B.; Jiang, B.W.; Zhu, L.W.; Wang, T.C.; Li, Y.G.; Liu, J.; Guo, X.J.; Ji, X.; Sun, Y. Migratory wild birds carrying multidrug-resistant Escherichia coli as potential transmitters of antimicrobial resistance in China. PLoS ONE 2021, 16, e0261444. [Google Scholar] [CrossRef]
- Chen, H.; Bai, X.; Jing, L.; Chen, R.; Teng, Y. Characterization of antibiotic resistance genes in the sediments of an urban river revealed by comparative metagenomics analysis. Sci. Total Environ. 2019, 653, 1513–1521. [Google Scholar] [CrossRef]
- Huijbers, P.M.; Graat, E.A.; Haenen, A.P.; van Santen, M.G.; van Essen-Zandbergen, A.; Mevius, D.J.; van Duijkeren, E.; van Hoek, A.H. Extended-spectrum and AmpC β-lactamase-producing Escherichia coli in broilers and people living and/or working on broiler farms: Prevalence, risk factors and molecular characteristics. J. Antimicrob Chemother. 2014, 69, 2669–2675. [Google Scholar] [CrossRef]
- Nnadozie, C.F.; Odume, O.N. Freshwater environments as reservoirs of antibiotic resistant bacteria and their role in the dissemination of antibiotic resistance genes. Environ. Pollut. 2019, 254, 113067. [Google Scholar] [CrossRef] [PubMed]
- Atterby, C.; Börjesson, S.; Ny, S.; Järhult, J.D.; Byfors, S.; Bonnedahl, J. ESBL-producing Escherichia coli in Swedish gulls-A case of environmental pollution from humans? PLoS ONE 2017, 12, e0190380. [Google Scholar] [CrossRef] [PubMed]
- Bujňáková, D.; Karahutová, L.; Kmeť, V. Escherichia coli Specific Virulence-Gene Markers Analysis for Quality Control of Ovine Cheese in Slovakia. Microorganisms 2021, 9, 1808. [Google Scholar] [CrossRef] [PubMed]
- Rybak, B.; Krawczyk, B.; Furmanek-Blaszk, B.; Wysocka, M.; Fordon, M.; Ziolkowski, P.; Meissner, W.; Stepniewska, K.; Sikorska, K. Antibiotic resistance, virulence, and phylogenetic analysis of Escherichia coli strains isolated from free-living birds in human habitats. PLoS ONE 2022, 17, e0262236. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hu, B.; Fan, H.; Zhang, H.; Lian, S.; Li, H.; Li, S.; Yan, X.; Wang, S.; Bai, X. Molecular Epidemiology of Extraintestinal Pathogenic Escherichia coli Causing Hemorrhagic Pneumonia in Mink in Northern China. Front. Cell Infect. Microbiol. 2021, 11, 781068. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Li, C.; Zhao, S.; Li, R.; He, Y.; Wu, D.; Yang, S.; Huang, Y.; Zhang, H.; Zou, L. Whole genome sequencing reveals the distribution of resistance and virulence genes of pathogenic Escherichia coli CCHTP from giant panda (in Chinese with English abstract). Hereditas 2019, 41, 1138–1147. [Google Scholar] [CrossRef]
- Messaili, C.; Messai, Y.; Bakour, R. Virulence gene profiles, antimicrobial resistance and phylogenetic groups of fecal Escherichia coli strains isolated from broiler chickens in Algeria. Vet. Ital. 2019, 55, 35–46. [Google Scholar] [CrossRef]
- Hernández-Fillor, R.E.; Brilhante, M.; Marrero-Moreno, C.M.; Baez, M.; Espinosa, I.; Perreten, V. Characterization of Third-Generation Cephalosporin-Resistant Escherichia coli Isolated from Pigs in Cuba Using Next-Generation Sequencing. Microb. Drug Resist. 2021, 27, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Power, M.L.; Emery, S.; Gillings, M.R. Into the wild: Dissemination of antibiotic resistance determinants via a species recovery program. PLoS ONE 2013, 8, e63017. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Yarnall, B.; Koranteng, A.; Gibson, J.; Rahman, T.; Doyle, D.A. Protein over-expression in Escherichia coli triggers adaptation analogous to antimicrobial resistance. Microb. Cell Fact 2021, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; McAllister, T.A.; Guan, L.L. A review of the resistome within the digestive tract of livestock. J. Anim. Sci. Biotechnol. 2021, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Van Camp, P.J.; Haslam, D.B.; Porollo, A. Bioinformatics Approaches to the Understanding of Molecular Mechanisms in Antimicrobial Resistance. Int. J. Mol. Sci. 2020, 21, 1363. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.A.; Martiny, A.C. Gene Amplification Uncovers Large Previously Unrecognized Cryptic Antibiotic Resistance Potential in E. coli. Microbiol. Spectr. 2021, 9, e0028921. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Gillespie, B.E.; Lewis, M.J.; Nguyen, L.T.; Headrick, S.I.; Schukken, Y.H.; Oliver, S.P. Phenotypic and genotypic antimicrobial resistance patterns of Escherichia coli isolated from dairy cows with mastitis. Vet. Microbiol. 2007, 124, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Shabana, I.I.; Al-Enazi, A.T. Investigation of plasmid-mediated resistance in E. coli isolated from healthy and diarrheic sheep and goats. Saudi J. Biol. Sci. 2020, 27, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.H.; Qiao, M.; Chen, Z.; Su, J.Q.; Zhu, Y.G. Antibiotic resistance genes in manure-amended soil and vegetables at harvest. J. Hazard. Mater. 2015, 299, 215–221. [Google Scholar] [CrossRef]
- Cerqueira, F.; Matamoros, V.; Bayona, J.; Elsinga, G.; Hornstra, L.M.; Piña, B. Distribution of antibiotic resistance genes in soils and crops. A field study in legume plants (Vicia faba L.) grown under different watering regimes. Environ. Res. 2019, 170, 16–25. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, H.; Bi, D.; Khaledi, A.; Qiao, M. A systematic review and meta-analysis of antibiotic resistance patterns, and the correlation between biofilm formation with virulence factors in uropathogenic E. coli isolated from urinary tract infections. Microb Pathog. 2020, 144, 104196. [Google Scholar] [CrossRef]
- Babakhani, S.; Oloomi, M. Transposons: The agents of antibiotic resistance in bacteria. J. Basic Microbiol. 2018, 58, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, C.; Hall, J.P.; Harrison, E.; Wood, A.; Brockhurst, M.A. Gene mobility promotes the spread of resistance in bacterial populations. ISME J. 2017, 11, 1930–1932. [Google Scholar] [CrossRef]
- Zhang, R.; Gu, J.; Wang, X.; Li, Y. Antibiotic resistance gene transfer during anaerobic digestion with added copper: Important roles of mobile genetic elements. Sci. Total Environ. 2020, 743, 140759. [Google Scholar] [CrossRef] [PubMed]
- Kayali, O.; Icgen, B. intI1 Type Mobile Genetic Elements Co-selected Antibiotic-Resistant Genes in Untreated Hospital Wastewaters. Bull. Environ. Contam. Toxicol. 2021, 106, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Feng, J.; Huang, J.; Li, X.; Li, B. Reponses of microbial community and antibiotic resistance genes to the selection pressures of ampicillin, cephalexin and chloramphenicol in activated sludge reactors. Sci. Total Environ. 2021, 755, 142632. [Google Scholar] [CrossRef]
| Category of Virulence | Genes | No. of Positive Isolates | Percentage of Positive Isolates (%) |
|---|---|---|---|
| Adhesion-related genes | tsh | 0 | 0.00 |
| eaeA | 18 | 21.43 | |
| papA | 2 | 2.38 | |
| fimC | 77 | 91.67 | |
| Hemolysin-related genes | hlyA | 0 | 0.00 |
| hlyF | 0 | 0.00 | |
| Invasion- and toxin-related genes | elt | 0 | 0.00 |
| estA | 0 | 0.00 | |
| estB | 0 | 0.00 | |
| astA | 0 | 0.00 | |
| Iron transport-related genes | fyuA | 33 | 39.29 |
| iroN | 37 | 44.05 | |
| irp2 | 32 | 38.10 | |
| sitA | 47 | 55.95 | |
| Antiserum survival factor | cvaC | 4 | 4.76 |
| ompT | 53 | 63.10 | |
| iss | 0 | 0.00 |
| Category of Antibiotic | Antibiotic | Results of the Antibiotic Sensitivity Test | Antibiotic Resistance Genes | No. of Positive Isolates (%) | ||
|---|---|---|---|---|---|---|
| No. of Resistant Ones (%) | No. of Intermediate Ones (%) | No. of Sensitive Ones (%) | ||||
| Tetracyclines | DOX | 52 (61.90) | 20 (23.81) | 12 (14.29) | tetA | 73 (86.90) |
| tetB | - | |||||
| tetC | 25 (29.76) | |||||
| TET | 41 (48.81) | 34 (40.48) | 9 (10.71) | tetD | - | |
| tetE | - | |||||
| tetG | 2 (2.38) | |||||
| Amide alcohols | CHL | 18 (21.43) | 10 (11.90) | 56 (66.67) | flor | 40 (47.62) |
| FFC | 18 (21.43) | 3 (3.57) | 63 (75.00) | cmlA | 41 (48.81) | |
| Aminoglycosides | AMK | 3 (3.57) | 4 (4.76) | 78 (92.86) | aacC2 aacC4 aadA1 aphA3 | 1 (1.19) - - - |
| KAN | 6 (7.14) | 4 (4.76) | 74 (88.10) | |||
| STR | 9 (10.71) | 4 (4.76) | 71 (84.52) | |||
| NEO | 3 (3.57) | 6 (7.14) | 75 (89.29) | |||
| GM | 5 (5.95) | 0 (0.00) | 79 (94.05) | |||
| β-lactams | AML | 39 (46.43) | 35 (41.67) | 10 (11.90) | blaCTX-M blaTEM blaSHV | 36 (42.86) 84 (100.00) 9 (10.71) |
| AMP | 58 (69.05) | 21 (25.00) | 5 (5.95) | |||
| SAM | 1(1.19) | 6 (7.14) | 77 (91.67) | |||
| AMC | 68 (80.95) | 7 (8.33) | 9 (10.71) | |||
| AZM | 6 (7.14) | 7 (7.14) | 73(86.90) | |||
| CRO | 6 (7.14) | 3 (3.57) | 75 (89.29) | |||
| CTX | 8 (9.52) | 9 (10.71) | 67 (79.76) | |||
| CEZ | 7 (8.33) | 4 (4.76) | 73 (86.90) | |||
| Quinolones | CIP | 6 (7.14) | 2 (2.38) | 76 (90.48) | qnrA | - |
| NA | 9 (10.71) | 1 (1.19) | 74 (88.10) | qnrS | 31 (36.90) | |
| NOR | 7 (8.33) | 0 (0.00) | 77 (91.67) | cat1 | - | |
| OFX | 6 (7.14) | 1 (1.19) | 77 (91.67) | oqxAB | 11 (13.10) | |
| Sulfonamides | SXT | 15 (17.86) | 0 (0.00) | 69 (82.14) | sul1 | 33 (39.29) |
| sul2 | 8 (9.52) | |||||
| sul3 | 52 (61.90) | |||||
| Gene (No.) | OR (95% Confidence Interval) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| blaCTX-M (36) | blaSHV (9) | tetA (73) | tetC (25) | tetG (2) | sul3 (52) | sul1 (33) | sul2 (8) | qnrS (31) | flor (40) | oqxAB (11) | cmlA (41) | aacC2 (1) | |
| blaCTX-M (36) | NS | - | - | 15.40 (4.55–52.12) | - | 4.50 (1.66–12.25) | 45.57 (12.24–169.72) | 11.35 (1.33–96.99) | 6.81 (2.534–18.27) | 5.00 (1.96–12.74) | - | 5.72 (2.21–14.81) | - |
| blaSHV (9) | - | NS | - | - | - | - | - | 7.00 (1.34–36.69) | - | - | 14.38 (3.03–68.21) | - | - |
| tetA (73) | - | - | NS | - | - | 23.18 (2.80–192.27) | 7.81 (0.95–64.18) | - | NA | 11.47 (1.40–94.28) | - | NA | - |
| tetC (25) | 15.40 (4.55–52.12) | - | - | NS | - | 11.90 (2.57–55.07) | 133.33 (15.96–1113.64) | 22.56 (2.60–195.77) | 40.74 (10.05–165.14) | 28.41 (6.03–133.98) | - | 15.44 (4.11–58.03) | - |
| tetG (2) | - | - | - | - | NS | - | - | - | - | - | - | - | - |
| sul3 (52) | 4.50 (1.66–12.25) | - | 7.81 (0.95–64.18) | 11.90 (2.57–55.07) | - | NS | 8.83 (2.71–28.78) | - | 42.273 (5.36–333.64) | 40.714 (8.58–193.17) | 0.18 (0.05–0.76) | 103.33 (12.74–838.13) | - |
| sul1 (33) | 45.57 (12.24–169.72) | - | 7.81 (0.95–64.18) | 133.33 (15.96–1113.64) | - | 8.83 (2.71–28.78) | NS | 13.46 (1.57–115.36) | 12.36 (4.29–35.63) | 13.15 (4.44–38.96) | - | 8.91 (3.19–24.95) | - |
| sul2 (8) | 11.35 (1.33–96.99) | 7.00 (1.34–36.69) | - | 22.56 (2.60–195.77) | - | - | 13.46 (1.57–115.36) | NS | 15.17 (1.77–130.25) | NA | - | 8.65 (1.01–73.75) | - |
| qnrS (31) | 6.81 (2.534–18.27) | - | NA | 40.74 (10.05–165.14) | - | 42.273 (5.36–333.64) | 12.36 (4.29–35.63) | 15.17 (1.77–130.25) | NS | 55.36 (11.41–168.57) | - | NA | - |
| flor (40) | 5.00 (1.96–12.74) | - | 11.47 (1.40–94.28) | 28.41 (6.03–133.98) | - | 40.714 (8.58–193.17) | 13.15 (4.44–38.96) | NA | 55.36 (11.41–168.57) | NS | - | 70.20 (17.47–282.02) | - |
| oqxAB (11) | - | 14.38 (3.03–68.21) | - | - | - | 0.18 (0.05–0.76) | - | - | - | - | NS | - | - |
| cmlA (41) | 5.72 (2.21–14.81) | - | NA | 15.44 (4.11–58.03) | - | 103.33 (12.74–838.13) | 8.91 (3.19–24.95) | 8.65 (1.01–73.75) | NA | 70.20 (17.47–282.02) | - | NS | - |
| aacC2 (1) | - | - | - | - | - | - | - | - | - | - | - | - | NS |
| Gene (No.) | OR (95% Confidence Interval) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| fimC (77) | papA (2) | fyuA (33) | irp2 (32) | eaeA (18) | iroN (37) | sitA (47) | ompT (53) | cvaC (4) | |
| fimC (77) | NS | - | - | - | - | NA | - | - | - |
| papA (2) | - | NS | - | - | - | - | - | - | - |
| fyuA (33) | - | - | NS | NA | 4.29 (1.42–12.98) | 294.40 (32.82–2640.87) | 76.80 (9.60–614.43) | 20.43 (4.41–94.69) | NA |
| irp2 (32) | - | - | NA | NS | 4.60 (1.51–13.98) | 237.67 (27.26–2072.08) | 69.75 (8.74–556.40) | 18.91 (4.09–87.55) | NA |
| eaeA (18) | - | - | 4.29 (1.4215–12.98) | 4.60 (1.51–13.98) | NS | 4.55 (1.45–14.33) | 20.40 (2.56–162.34) | 14.17 (1.78–112.74) | - |
| iroN (37) | NA | - | 294.40 (32.82–2640.87) | 237.67 (27.26–2072.08) | 4.55 (1.45–14.33) | NS | 29.64 (7.74–113.47) | 16.70 (4.48–62.30) | - |
| sitA (47) | - | - | 76.80 (9.60–614.43) | 69.75 (8.74–556.40) | 20.40 (2.56–162.34) | 29.6 (7.743–113.47) | NS | 19.86 (6.19–63.64) | - |
| ompT (53) | - | - | 20.43 (4.41–94.69) | 18.91 (4.09–87.55) | 14.17 (1.78–112.74) | 16.70 (4.48–62.30) | 19.86 (6.19–63.64) | NS | - |
| cvaC (4) | - | - | NA | NA | - | - | - | - | NS |
| Gene (No.) | OR (95% Confidence Interval) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| blaCTX-M (36) | tetA (73) | tetC (25) | sul3 (52) | sul1 (33) | sul2 (8) | qnrS (31) | flor (40) | cmlA (41) | |
| fyuA (33) | 8.67 (3.18–23.62) | - | 14.15 (4.45–45.03) | 6.30 (2.10–18.91) | 12.44 (4.35–35.61) | 13.46 (1.57–115.36) | 7.18 (2.67–19.32) | 13.15 (4.44–38.96) | 6.84 (2.54–18.43) |
| irp2 (32) | 10.00 (3.58–27.95) | - | 15.57 (4.88–50.33) | 5.83 (1.94–17.49) | 14.33 (4.89–42.01) | 14.28 (1.67–122.50) | 8.02 (2.94–21.88) | 11.76 (4.00–34.59) | 6.18 (2.30–16.59) |
| eaeA (18) | 7.00 (2.06–23.79) | - | 11.70 (3.50–39.09) | 3.92 (1.04–14.84) | 23.06 (4.80–110.85) | - | 10.09 (2.92–34.88) | 8.20 (2.16–31.18) | - |
| iroN (37) | 6.82 (2.60–17.89) | - | 14.11 (4.19–47.48) | 4.87 (1.79–13.27) | 13.51 (4.64–39.20) | 10.73 (1.26–91.70) | 8.01 (2.92–21.99) | 9.07 (3.35–24.59) | 8.14 (3.04–21.81) |
| sitA (47) | 6.91 (2.51–18.98) | 7.23 (1.46–35.93) | 0.25 (0.09–0.64) | 6.66 (2.04–21.82) | 3.43 (1.37–8.62) | - | 7.92 (2.63–23.90) | 7.73 (2.86–20.90) | 5.23 (2.04–13.44) |
| cvaC (4) | 4.14 (1.52–11.27) | 5.80 (1.41–23.88) | 6.62 (1.79–24.55) | 6.21 (2.34–16.51) | 12.17 (3.29–45.06) | - | 10.45 (2.83–38.63) | 14.29 (4.31–47.37) | 8.10 (2.82–23.31) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.; Jiang, S.; Luo, L.; Zhou, Z.; Wang, L.; Huang, X.; Liu, H.; Zhang, S.; Luo, Y.; Ren, Z.; et al. Antibiotic-Resistant Escherichia coli Strains Isolated from Captive Giant Pandas: A Reservoir of Antibiotic Resistance Genes and Virulence-Associated Genes. Vet. Sci. 2022, 9, 705. https://doi.org/10.3390/vetsci9120705
Fan S, Jiang S, Luo L, Zhou Z, Wang L, Huang X, Liu H, Zhang S, Luo Y, Ren Z, et al. Antibiotic-Resistant Escherichia coli Strains Isolated from Captive Giant Pandas: A Reservoir of Antibiotic Resistance Genes and Virulence-Associated Genes. Veterinary Sciences. 2022; 9(12):705. https://doi.org/10.3390/vetsci9120705
Chicago/Turabian StyleFan, Siping, Shaoqi Jiang, Lijun Luo, Ziyao Zhou, Liqin Wang, Xiangming Huang, Haifeng Liu, Shaqiu Zhang, Yan Luo, Zhihua Ren, and et al. 2022. "Antibiotic-Resistant Escherichia coli Strains Isolated from Captive Giant Pandas: A Reservoir of Antibiotic Resistance Genes and Virulence-Associated Genes" Veterinary Sciences 9, no. 12: 705. https://doi.org/10.3390/vetsci9120705
APA StyleFan, S., Jiang, S., Luo, L., Zhou, Z., Wang, L., Huang, X., Liu, H., Zhang, S., Luo, Y., Ren, Z., Ma, X., Cao, S., Shen, L., Wang, Y., Gou, L., Geng, Y., Peng, G., Zhu, Y., Li, W., ... Zhong, Z. (2022). Antibiotic-Resistant Escherichia coli Strains Isolated from Captive Giant Pandas: A Reservoir of Antibiotic Resistance Genes and Virulence-Associated Genes. Veterinary Sciences, 9(12), 705. https://doi.org/10.3390/vetsci9120705










