Simple Summary
Activins and inhibins are closely related protein heterodimers with opposing functions in follicular development. The increased circulating follicle-stimulating hormone (FSH) levels and strengthened estrus behavior may result from the immune neutralization of the inhibin bioactivity, which might improve ovarian follicle formation. However, the direct effect of activins, or immunization against inhibin, on the granulosa cells (GCs) functions remains largely unknown. We aimed to examine the effects of activin A (ACT-A) on the function of porcine ovarian GCs. The results showed that ACT-A could suppress ROS accumulation through the upregulation of the expression of estrogen receptor-β (ERβ), thus attenuating apoptosis in the porcine granulosa cells and promoting estradiol synthesis. These results identified a novel protective role of ACT-A in the regulation of the follicle functions, which revealed the mechanism of improvement locally in the ovary caused by immunization against inhibin.
Abstract
Unfavorable conditions compromise animal reproduction by altering the ovarian granulosa cells’ follicular dynamics and normal physiological function (GCs), eventually resulting in oxidative damage and cell apoptosis. Activin is produced in the GCs and plays a vital role in folliculogenesis. This study investigated the effects of activin A (ACT-A) treatment in vitro on the apoptosis of porcine GCs and the underlying molecular mechanism. We found that ACT-A could attenuate the apoptosis of the GCs and enhance the synthesis of estrogen (E2). ACT-A also enhanced FSH-induced estrogen receptor-β (ERβ) expression, inhibiting ERβ aggravated intracellular accumulation of the reactive oxygen species (ROS) and apoptosis. The E2 levels in the culture medium, the mRNA expression pattern of the apoptosis-related genes (CASPASE 3, BCL2, and BAX), steroidogenesis-related gene (CYP19A1), and cell viability were analyzed to confirm the results. In summary, this study indicated the protective role of ACT-A in apoptosis by attenuating the ROS accumulation through ERβ. These results aim to enhance the follicular functions and improve animal reproductive performance.
1. Introduction
Researchers and practitioners are becoming more concerned about the growing infertility issue in people and animals, which may be brought on by stress and hyperandrogenism-prompted ovarian follicular maldevelopments [1,2,3]. More than 99% of the follicles in mammalian ovaries have atretic degeneration before ovulation [4]. During the past decades, several endocrine manipulating reproductive protocols have been developed to enhance the ovarian functions and improve reproductive performance [5,6], but the current protocols are far from satisfactory. The three interrelated characteristics that impact an animal’s ability to reproduce soundly are a sound-growing ovarian follicle that may create a high-quality egg, a high-quality embryo consequently, and a high-quality corpus luteum [7,8]. By increasing the circulating follicle-stimulating hormone (FSH) concentrations, the bolstering estrus behavior, and enhancing the oocyte and early embryo development competence in dairy cows [9], water buffaloes [10], and pigs [11], a method of immunoneutralization of inhibin bioactivity has been developed in recent decades to effectively stimulate or enhance the granulosa cells and ovarian follicle development. In addition to increasing the conception rate, immunization against inhibin, when combined with the OvSynch protocol, also increased the plasma concentrations of the interferon tau (IFN-tau) in dairy cows around the time of pregnancy recognition, further demonstrating its efficacy in enhancing early embryo development and oocyte maturation [12,13].
Activins and inhibins are closely related protein heterodimers [14], belonging to the transforming growth factor-beta (TGF-β) superfamily, however, these two complexes have opposing functions in follicular development [15,16]. The hormone inhibin is a dimeric glycoprotein which is composed of an α-subunit and either a βA or a βB-subunit (inhibin A and inhibin B, respectively). It is primarily secreted by the gonads and inhibits the pituitary secretion of the FSH through negative feedback regulation [17,18]. Activins are composed of homodimers of β subunits, namely activin A (βA βA), activin AB (βA βB), and activin B (βB βB). In the pituitary, the activin increased the synthesis and secretion of FSH, and this process could be counter regulated by inhibin. The inhibin antagonizes the activin signaling by competitively binding to the activin type 2 receptors (ActRII). It has been hypothesized that, in addition to the stimulation brought on by the increased FSH secretion, the immunoneutralization of the inhibin bioactivity may also directly boost the granulosa or follicular cell function [19]. Additionally, Cai [20] cultivated porcine granulosa cells using an anti-inhibin-subunit antibody and found that, through enhancing activin signaling pathways, the immunoneutralization of inhibin bioactivity allowed the formation of healthy and viable granulosa cells. Currently, the reported effects of activins on the proliferation in the ovary are conflicting. Some groups reported that activin A (ACT-A) played a role in the oocyte maturation and the proliferation of the granulosa cells and pre-antral follicles in mice, as well as increased the FSH receptor (FSHR) expression in vitro. In cattle ovary research, ACT-A was reported to attenuate the apoptosis of the bovine ovarian granulosa cell in the atretic follicles [21]. However, the mechanism underlying the enhanced growth and development of the ovarian follicles by activin remains to be further understood. Others observed the opposite outcome, which hindered human follicular development and lowered the swine granulosa cells in vitro production of estradiol (E2) and progesterone (P4).
Therefore, in this study, we investigated the effect of ACT-A from the perspectives of development and apoptosis on porcine granulosa cells, per se. Our findings revealed a primary protective role of ACT-A, with induced GC survival in an estradiol receptor beta (ERβ) dependent mode.
2. Materials and Methods
2.1. Granulosa Cell Isolation and Culture
The granulosa cells in the ovaries of prepubertal gilts aged 165–180 days were isolated and grown following the methods used in other investigations. In a nutshell, the follicles with a diameter of 3–6 mm were used to aspirate the granulosa cells using a syringe and sterile needles. The granulosa cells were then separated by centrifuging them for 5 min at 1000× g, rinsing them in a sterile F12 medium (Wisent Corporation, Nanjing, China), and resuspending them in the same medium with 10% fetal calf serum (10099141C, Gibco; Shanghai, China) and 1% antibiotic-antimycotic solution (Sigma, St. Louis, MO, USA) at a final density of 106 cells/mL. The cell suspension was then divided into aliquots and placed onto 6-well culture plates made by Nunc International (Naperville, IL, USA; 2 mL/well). The cells were incubated in humidified air with 5% CO2 at 37 °C. After 48 h of incubation, the wells were twice rinsed with PBS to remove the single cells and then refilled with 2 mL of brand-new F12 media with 2% fetal calf serum.
2.2. Cell Treatment
The unattached cells were eliminated and the cells were treated in a new cell culture media consisting of F12 medium supplemented with 2% FBS and 0.1 µM androstenedione. ACT-A (R&D systems, Minneapolis, MN, USA) was dissolved in the new media at 50 ng/mL. The H2O2 was diluted to 0.4 mM, as previously reported [22,23]. PHTPP (4-[2-Phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]-pyrimidin-3-yl] phenol, Sigma, Burlington, MA, USA) was used at the concentration of 10 µM, as previously described [24,25]. The cells were treated for 24 h and harvested for mRNA and protein detection.
2.3. Cell Viability Assay
The optical density of the yellow color was measured at 490 nm using a BioTek Eon microtiter plate reader. The GCs were cultured in 96-well plates, and their viability was assessed using the CCK-8 cell viability assay kit (Cell Counting Kit-8; Beyotime Co., Ltd., Shanghai, China), following the manufacturer’s instructions after the heat treatment. The percentage of the absorbance readings compared to the control was used to indicate the cell viability. Three distinct cultures were used in the tests, and three copies of each sample were analyzed.
2.4. Measurement of E2 Secretion
According to the manufacturer’s instructions, an ELISA kit (Beijing North Institute of Biological Technology, Beijing, China) was used to quantify E2 in the culture media. The kit’s standard curve covered the concentration range of 0 to 400 pg/mL, and the intra- and inter-assay coefficients of the variation were both less than 10%. Every sample was measured three times.
2.5. Gene Expression Analysis
The TRIzol Reagent (74104, Invitrogen, Shanghai, China) was used to separate the total RNA from the grown GCs, and the 1st-Strand cDNA Synthesis Kit (11119ES60, YEASEN, Shanghai, China) was used to reverse-transcribe the total RNA into cDNA, following the manufacturer’s instructions. In porcine granulosa cells, the mRNA expression levels of β-Actin, CYP19A1, FSHR, BAX, CASPASE 3, BCL2, and ERβ were quantified using a real-time quantitative polymerase chain reaction (the primer information is shown in Table 1). The PCRs were performed using a One-Step RT-qPCR SYBR Green Kit (11143ES850) on an ABI 7500 (Applied Biosystems; Foster City, CA, USA) with a 20 μL reaction volume. After the real-time qPCR was finished, the ABI 7500 software V.2.0.6 determined the threshold cycle (Ct) values (Applied Biosystems; Foster City, CA, USA). As reported in our earlier work, the 2−ΔΔCt technique was used to quantify the gene expression levels, which were then normalized to the expression levels of the internal housekeeping gene β-Actin. The triplicates of each sample were analyzed.

Table 1.
Primers utilized in this investigation.
2.6. Analysis and Detection of ROS
Using cell-permeant 2′, 7′-dichlorodihydrofluorescein diacetates (H2DCFDA; Beyotime Institute of Biotechnology, Shanghai, China), as previously reported [41], the intracellular ROS levels in the cells following the H2O2 treatment were measured. In a 24-well plate, the sterile coverslips were inserted in each well before the seeding of the granulosa cells. The cells were treated, as previously indicated, and incubated in H2DCFDA/PBS solutions (1:1000) at 37 °C for 30 min. The coverslips were placed on the glass slides after being thoroughly washed in DPBS (with the cell side laid face down to the glass slide). Finally, a confocal microscope was used to analyze the instantaneousness of the cells (Zeiss LSM700 META). The average pixel intensity of three distinct fields from each experiment was examined for the ROS level analysis (all the cells in each field were examined), and the areas adjacent to the cells that do not fluoresce were designated as the background.
2.7. Analytical Oxidative Stress-Associated Parameters
Using the xanthine oxidase technique, the activity of SOD in the granulosa cells was quantified and represented as units per mg of protein. Thiobarbituric acid was used to assess the MDA concentration in the granulosa cells, and the results were expressed as mol per mg of protein. The exact stages were carried out following the instructions included with the kits.
2.8. Statistical Analysis
The student’s t-test was used to examine the data presented as the mean SD. SPSS Statistics version 25.0 was used to conduct all the statistical analyses (SPSS Inc., Chicago, IL, USA). The cutoff for the statistical significance was p < 0.05.
3. Results
3.1. ACT-A Enhances the Expression of FSHR and ERβ and Significantly Increases Granulosa Cells’ Sensitivity (GCs) Sensitivity to FSH Treatment
As shown in Figure 1A, ACT-A increased the gene expression of FSHR. As a result, we included FSH in the following treatment to explore the effect of ACT-A on the sensitivity of the GCs to the FSH. It was found that co-treatment of ACT-A and FSH dramatically enhanced the secretion of estrogen (E2, Figure 1B) and the gene expression of CYP19A1 (Figure 1C), suggesting that ACT-A demonstrates a synergetic effect in FSH-induced follicle development. As reported, ERβ expression contributes to E2 synthesis and plays a role in FSH-mediated follicle development. Therefore, the impact of ACT-A and co-treatment of ACT-A and FSH on ERβ abundance was investigated in this study. As shown in Figure 1D, the ERβ expression level was significantly increased under both conditions, suggesting a potential role of ERβ underlying ACT-A on the GC’s growth and survival.
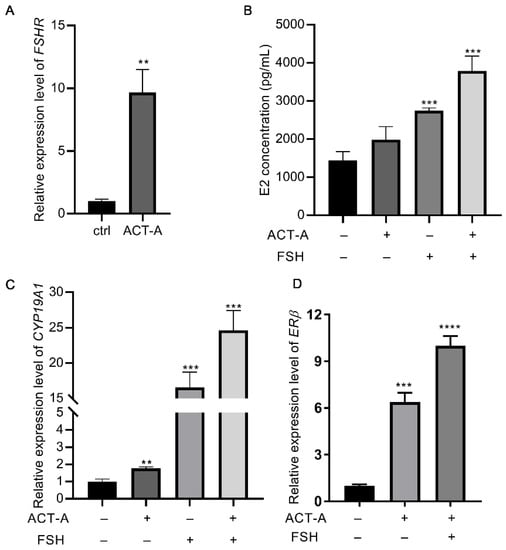
Figure 1.
Effect of ACT-A on ERβ and FSHR expression and GC sensitivity to FSH therapy. qRT-PCR was used to evaluate the gene expression level of FSHR (A), CYP19A1 (C), ERβ (D), and E2 concentration levels in GCs (B). The ctrl means control (untreated group). ** p < 0.01, *** p < 0.001, **** p < 0.0001.
3.2. ACT-A Attenuates Apoptosis of GCs
As observed in the CCK-8 assay (Figure 2A), ACT-A treatment inhibits the GC’s apoptosis. So, using the real-time PCR, we determined the prevalence of the genes BAX, BCL2, and CASPASE 3 associated with apoptosis. According to Figure 2B, ACT-A inhibits the apoptosis of the GCs, as shown by the significantly reduced relative expression of BAX and CASPASE 3, and the elevated expression of BCL2.
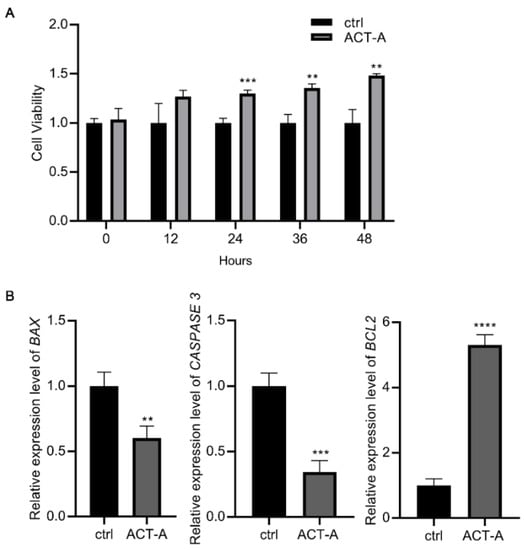
Figure 2.
Effect of ACT-A on apoptosis of GCs. (A). The CCK-8 test was used to determine how ACT-A affected the apoptosis of GCs. (B). Expression of apoptosis-related genes BAX, CASPASE 3, and BCL2 was assessed using qRT-PCR. ** p < 0.01, *** p < 0.001, **** p < 0.0001.
3.3. ACT-A Mediates GCs Apoptosis via Modulating ERβ Expression
Based on the findings above, we hypothesized that ERβ modulation could be the underlying mechanism for ACT-A effect on the GC’s apoptosis. As a result, we treated the GCs with the selective ERβ inhibitor PHTPP (10 μM), in combination with ACT-A, and then examined the gene expression of BAX, BCL2, and CASPASE 3. Firstly, the decreased CYP19A1 expression was used to confirm the effect of PHTPP on the E2 synthesis (data not shown). Next, as shown in Figure 3C, the BAX and CASPASE 3 expressions were significantly increased, while the BCL2 expression was downregulated with both the PHTPP treatment alone and the co-treatment of PHTPP and ACT-A, indicating an induction effect of ERβ inhibition on the cell apoptosis. Meantime, it is remarkable that the PHTPP also inhibits the ERβ expression in Figure 3B, further implying an important role of the ERβ expression in the observed ACT-A effect. Finally, the CCK-8 assay was utilized in the following to validate this finding. As shown in Figure 3A, the result demonstrated that the PHTPP treatment promotes the GC’s apoptosis and thus verified the hypothesis that ACT-A mediates the GC’s apoptosis via the modulation ERβ expression.
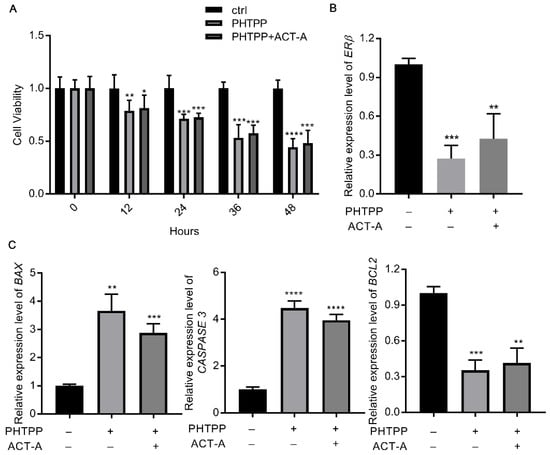
Figure 3.
ACT-A mediates GCs apoptosis via modulation ERβ expression. (A). The CCK-8 test was used to determine how PHTPP affected the apoptosis of GCs. B. C. Erβ. (B). BAX, CASPASE 3, and BCL2 (C). The gene expression levels were assessed by qRT-PCR. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
3.4. ACT-A Mediates Intracellular ROS Levels in GCs via ERβ
To further investigate the underlying mechanism by which ACT-A and ERβ regulate the GC’s apoptosis process, we drew attention to the cellular ROS level detection. As shown in Figure 4A, the ACT-A treatment could decrease the intracellular ROS levels of the granulosa cells, which might contribute to the inhibitory effect of ACT-A on apoptosis. Meanwhile, it was observed that the co-treatment of PHTPP with ACT-A attenuated the impact of ACT-A on ROS, further implying the role of ERβ in this process. Lipid peroxidation (MDA) detection (Figure 4B) and Superoxide Dismutase (SOD) measurement (Figure 4C) were thus performed, and the data indicated that ACT- A reduces ROS-mediated apoptosis through ERβ.
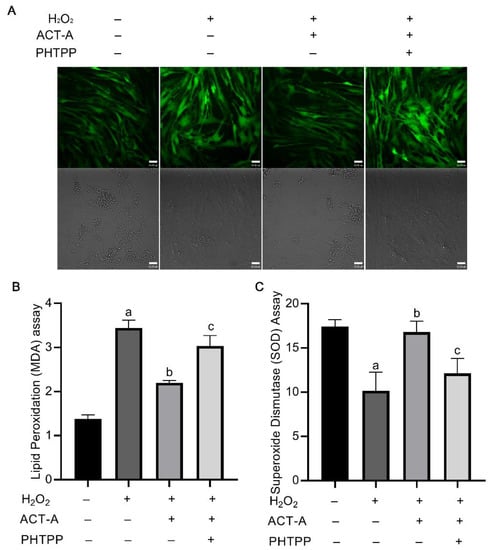
Figure 4.
ACT-A mediates intracellular ROS level of GCs in ERβ-dependent mode. (A). Measurement of cellular ROS level by treatment of ACT-A and PHTPP. (B). Lipid peroxidation assay was performed to detect MDA changes. (C). Superoxide dismutase assay was performed to detect SOD changes. “a” means a significant change compared with the control (untreated group), “b” means a substantial change to H2O2 treatment, and “c” means a considerable change to co-treatment of H2O2 and ACT-A.
4. Discussion
In this work, we demonstrated how ACT-A changed the E2 production and the cell death of granulosa cells. Substantial modulation of ROS by ERβ expression was found to contribute to these reactions. These results effectively combine the currently known effects of activin as a local factor on follicular growth with the impact of inhibin vaccination on the granulosa cell proliferation and steroid hormone release to enhance the follicle development.
GCs are steroidogenic cells surrounding the oocyte, which play an essential role in follicular development, oocyte maturation, and the subsequent embryo implantation [26]. The maintenance of the GCs contributes to normal follicular growth and, in particular, plays an important role in deciding the fate of the follicles. The apoptotic GCs may cause follicle development disturbance, poor quality of oocytes, and induce low reproductive performance [27]. As reported, multiple apoptotic signaling molecules in the GCs, such as hormones, growth factors, death ligand-receptor system, and Bcl-2 family members, affect each other, and activate Caspase 3 and the subsequent DNA fragmentation, and result in the GC’s apoptosis [28].
From the earliest stages of follicle development, the granulosa cells begin to produce activin, and as follicle growth progresses, inhibin/follistatin, which inhibits activin’s actions, takes over in the granulosa cells [29]. The granulosa cells of dominant or prominent ovarian follicles are primarily responsible for the inhibin secretion [30]. Through a negative feedback loop [31], inhibin suppresses pituitary follicle-stimulating hormone (FSH) release. It limits the growth of subordinate follicles through the para/autocrine regulatory pathways, which adversely control ovarian follicular development [32]. Inhibin is known to act locally in the ovary, the most clearly defined paracrine function being to antagonize the effect of activin [33]. By passive or active immunization against the inhibin subunit peptide, the immuno-neutralization of inhibin’s ovarian follicle suppression activity improves the follicle development and hormone secretion capacity, significantly boosting ovulation rates and reproductive performance in various animal models.
According to reports, activin induces the expression of FSH-R and LH receptors, while also increasing the activity of the FSH-induced aromatase. In this study, after 24 h of culture, ACT-A was added to the culture medium. Compared with the cultured control, ACT-A promoted E2 secretion and caused an increase in FSHR mRNA levels and, as a result, increased GC sensitivity to FSH, which led to an increased CYP19A1 expression. Additionally, we discovered that ACT-A stimulates ERβ expression, and ERβ inhibition results in a deficit in E2 production. Our results align with the earlier research that suggests the GCs play a fundamental role in forming the ovarian follicles stimulated by FSH [21]. According to in vitro studies, the presence of ERβ in GCs is necessary to produce a subset of FSH-induced genes, including CYP19A1 [21]. In addition, previous studies conducted on ERβ-null mice reported that the knockout of ERβ significantly reduced the levels of FSH-induced estrogen synthesis [34,35]. In the meantime, the expression of ERβ is regulated by FSH through the PI3K/AKT pathway [35]. Our findings demonstrated that ACT-A increased the expression of ERβ caused by FSH. The underlying regulatory mechanism of the FSH, ERβ, and CYP19A1 network might explain the phenomenon that the elevation of E2 induced by ACT-A depends on FSH’s presence [36].
The cellular redox status is crucial to cell survival, growth, and death. The accelerated metabolic rates and the cumulative accumulation of the reactive oxygen species (ROS) are linked to higher demands for energy and nutrients throughout the reproductive process. Unfavorable environmental conditions, such as bacterial infection and heat stress, are well known to promote the accumulation of ROS [22,37]. Although ROS are by-products of aerobic metabolism that are naturally occurring, the excessive ROS production causes oxidative stress and cellular damage. In GCs, the excessive intracellular ROS could cause a series of damage, such as disruptive apoptosis, altered cell proliferation, and disordered E2 synthesis [23,38]. The death of GCs during follicular atresia, which may result in certain anovulatory illnesses, such as premature ovarian failure, is strongly supported by the data that oxidative stress plays a vital role in the process [39].
In this study, we demonstrated that ACT-A attenuated the GC’s apoptosis via mediating ROS production, and induced ERβ expression in the GCs. As observed in the rat ovary follicular growth and atresia development, the ERβ expression level decreases along with the apoptosis increasing in the follicular granulosa cells [40]. Moreover, ERβ was found to interact with vigilin to protect the ovarian granulosa cell-like human granulosa cells from the palmitic acid-induced apoptosis [41]. We thus hypothesized and validated that ERβ plays an essential role in the ACT-A action mode. Our data showed for the first time that the inhibition of ERβ significantly enhanced the intracellular ROS level of the GCs and resulted in a remarkable increase in cell apoptosis.
According to available research on ERβ, it may promote invasion, adhesion, inflammatory body activity, proliferation, and inflammatory signals of the ectopic lesions, while inhibiting apoptosis [42,43], which verifies the unveiled protective role of ERβ in our study. To prevent TNF-α induced apoptosis, it is well known that ERβ interacts with the cellular apoptotic machinery in the cytoplasm [44,45]. Meanwhile, synchronized changes in ERβ expression and ROS induction were detected in the human granulosa cells [46]. It is indicated in the human granulosa cell line KGN study that both the ERβ expression and the ROS-ASK1-JNK axis take part in Bisphenol AF-induced cell apoptosis [47]. A working model was also further proposed for the protecting action of ERβ against seminoma, in which ERβ regulated the gene expression of SIRT3, a major mitochondria nicotine adenine dinucleotide (NAD)+-dependent deacetylase, and resulted in ROS level reduction [46]. Therefore, our study proves that ACT-A could mediate the GC’s apoptosis in ERβ-dependent mode, and gene expression in mitochondrial adaptative responses to stress might be a potential mechanism for further exploration.
5. Conclusions
According to our results, ACT-A can suppress the ROS accumulation through the upregulation of the expression of ERβ, thus attenuating apoptosis in porcine granulosa cells. These results identified a novel protective role of ACT-A in the regulation of follicle functions, which revealed the mechanism of improvement locally in the ovary caused by the immunization against inhibin. This study also provides proof of principle for enhancing follicular functions and improving animal reproductive performance.
Author Contributions
F.C. and X.Z., Conceptualization; F.C., original draft writing; X.Z., review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge financial support from the Natural Science Foundation of Jiangsu Province (No. BK20190256), the Natural Science Foundation of Chongqing (No. cstc2020jcyj-msxmX0369), and the Grant of Chongqing Medical and Pharmaceutical College (No. ygz2021105).
Institutional Review Board Statement
Since no live animals were utilized in this investigation, no ethical review was necessary.
Informed Consent Statement
Not applicable.
Data Availability Statement
The corresponding author may provide the data used in this research upon request.
Conflicts of Interest
The authors say they have no competing interest. The study’s design, publication choice, and manuscript preparation were all made independently of the sponsors.
Abbreviations
ACT-A: activin A; ERβ: estrogen receptor β; ROS: reactive oxygen species; FSH: follicle-stimulating hormone; GCs: granulosa cells; E2: estrogen; IFN-tau: interferon tau; TGF-β: transforming growth factor β; ActRII: activin type 2 receptors; FSHR: FSH receptor; P4: progesterone; PHTPP: 4-[2-Phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]-pyrimidin-3-yl] phenol; CCK-8: Cell Counting Kit-8; ELISA: Enzyme-linked immunosorbent assay; PCR: polymerase chain reaction; RT-qPCR: reverse transcription-quantitative polymerase chain reaction; H2DCFDA: cell-permeant 2′, 7′-dichlorodihydrofluorescein diacetates; PBS: phosphate buffer saline; SOD: Superoxidedismutase; MDA: Malondialdehyde; SD: standard deviation.
References
- Azziz, R. Polycystic Ovary Syndrome. Obs. Gynecol. 2018, 132, 321–336. [Google Scholar] [CrossRef]
- Nakamura, K.; Sheps, S.; Arck, P.C. Stress and reproductive failure: Past notions, present insights and future directions. J. Assist. Reprod. Genet. 2008, 25, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [PubMed]
- Pettersen, K.; Andersen, S.; van der Veen, A.; Nonstad, U.; Hatakeyama, S.; Lambert, C.; Lach-Trifilieff, E.; Moestue, S.A.; Kim, J.; Grønberg, B.H.; et al. Autocrine activin A signalling in ovarian cancer cells regulates secretion of interleukin 6, autophagy, and cachexia. J. Cachexia Sarcopenia Muscle 2020, 11, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Pursley, J.R.; Mee, M.O.; Wiltbank, M.C. Synchronization of Ovulation in Dairy-Cows Using PGF2α and GnRH. Theriogenology 1995, 44, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Wiltbank, M.C.; Pursley, J.R. The cow as an induced ovulator: Timed AI after synchronization of ovulation. Theriogenology 2014, 81, 170–185. [Google Scholar] [CrossRef]
- Perry, G.A.; Smith, M.F.; Lucy, M.C.; Green, J.A.; Parks, T.E.; MacNeil, M.D.; Roberts, A.J.; Geary, T.W. Relationship between follicle size at insemination and pregnancy success. Proc. Natl. Acad. Sci. USA 2005, 102, 5268–5273. [Google Scholar] [CrossRef]
- Colazo, M.G.; Behrouzi, A.; Ambrose, D.J.; Mapletoft, R.J. Diameter of the ovulatory follicle at timed artificial insemination as a predictor of pregnancy status in lactating dairy cows subjected to GnRH-based protocols. Theriogenology 2015, 84, 377–383. [Google Scholar] [CrossRef]
- Liu, Y.P.; Mao, X.B.; Wei, Y.M.; Yu, J.N.; Li, H.; Chen, R.A.; Shi, Z.D. Studies on enhancing embryo quantity and quality by immunization against inhibin in repeatedly superovulated Holstein heifers and the associated endocrine mechanisms. Anim. Reprod. Sci. 2013, 142, 10–18. [Google Scholar] [CrossRef]
- Bahareldin-Ali, A.; Qin, G.-S.; Guo, R.-H.; Tsigkou, A.; Tan, Z.-Z.; Huang, J.; Li, H.; Shi, Z.-D. Endocrine and ovarian responses in water buffalo cows immunized against inhibin and subjected to the Ovsynch protocol. J. Integr. Agric. 2015, 14, 1827–1837. [Google Scholar] [CrossRef]
- Guo, R.-H.; He, P.-Y.; Mai, Y.-L.; Dai, Z.-C.; Chen, F.; Shi, Z.-D. A novel method to improve sow reproductive performance: Combination of pre-weaning immunization against inhibin and post-insemination hCG treatment. J. Integr. Agric. 2020, 19, 2286–2293. [Google Scholar] [CrossRef]
- Guo, R.; Chen, F.; Mei, C.; Dai, Z.; Yan, L.; Shi, Z. Conception Rate and Reproductive Hormone Secretion in Holstein Cows Immunized against Inhibin and Subjected to the Ovsynch Protocol. Animals 2020, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Lu, J.; Guo, R.; Mei, C.; Guo, B.; Li, W.; Tsigkou, A.; Shi, Z. Rectifying cow infertility under heat stress by immunization against inhibin and supplementation of progesterone. Domest. Anim. Endocrinol. 2022, 80, 106726. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, A.J.; Dahl, K.D.; Vaughan, J.; Tucker, E.; Rivier, J.; Bardin, C.W.; Vale, W. Heterodimers and Homodimers of Inhibin Subunits Have Different Paracrine Action in the Modulation of Luteinizing Hormone-Stimulated Androgen Biosynthesis. Proc. Natl. Acad. Sci. USA 1987, 84, 5082–5086. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.A.; Gray, P.C.; Blount, A.L.; MacConell, L.A.; Wiater, E.; Bilezikjian, L.M.; Vale, W. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature 2000, 404, 411–414. [Google Scholar] [CrossRef]
- Knight, P.G.; Glister, C. Potential local regulatory functions of inhibins, activins and follistatin in the ovary. Reproduction 2001, 121, 503–512. [Google Scholar] [CrossRef]
- Welt, C.; Sidis, Y.; Keutmann, H.; Schneyer, A. Activins, inhibins, and follistatins: From endocrinology to signaling. A paradigm for the new millennium. Exp. Biol. Med. 2002, 227, 724–752. [Google Scholar] [CrossRef]
- Luisi, S.; Palumbo, M.; Calonaci, G.; De Leo, V.; Razzi, S.; Inaudi, P.; Cobellis, G.; Petraglia, F. Serum inhibin B correlates with successful ovulation in infertile women. J. Assist. Reprod. Genet. 2003, 20, 241–247. [Google Scholar] [CrossRef]
- Jimenez-Krassel, F.; Winn, M.E.; Burns, D.; Ireland, J.L.H.; Ireland, J.J. Evidence for a negative intrafollicular role for inhibin in regulation of estradiol production by granulosa cells. Endocrinology 2003, 144, 1876–1886. [Google Scholar] [CrossRef]
- Cai, L.; Sun, A.; Li, H.; Tsinkgou, A.; Yu, J.; Ying, S.; Chen, Z.; Shi, Z. Molecular mechanisms of enhancing porcine granulosa cell proliferation and function by treatment in vitro with anti-inhibin alpha subunit antibody. Reprod. Biol. Endocrinol. 2015, 13, 26. [Google Scholar] [CrossRef]
- Liu, N.; Wang, S.; Yao, Q.; Li, Y.; Hu, H.; Tang, X.; Ran, H.; Price, C.A.; Jiang, Z. Activin A attenuates apoptosis of granulosa cells in atretic follicles through ERβ-induced autophagy. Reprod. Domest. Anim. 2022, 57, 625–634. [Google Scholar] [CrossRef]
- Azad, M.; Kikusato, M.; Sudo, S.; Amo, T.; Toyomizu, M. Time course of ROS production in skeletal muscle mitochondria from chronic heat-exposed broiler chicken. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 157, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Dou, J.; Wang, Y.; Jiang, X.; Khan, M.Z.; Luo, H.; Usman, T.; Zhu, H. Evaluation of heat stress effects on cellular and transcriptional adaptation of bovine granulosa cells. J. Anim. Sci. Biotechnol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, R.; Sekii, K.; Morohaku, K.; Li, J.; Pépin, D.; Obata, Y. Blocking estrogen-induced AMH expression is crucial for normal follicle formation. Development 2021, 148, dev197459. [Google Scholar] [CrossRef] [PubMed]
- Dilaver, N.; Pellatt, L.; Jameson, E.; Ogunjimi, M.; Bano, G.; Homburg, R.; Mason, H.D.; Rice, S. The regulation and signalling of anti-Müllerian hormone in human granulosa cells:relevance to polycystic ovary syndrome. Hum. Reprod. 2019, 34, 2467–2479. [Google Scholar]
- Anderson, E.; Albertini, D.F. Gap junctions between the oocyte and companion follicle cells in the mammalian ovary. J. Cell Biol. 1976, 71, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.E. TGFbeta signalling in the development of ovarian function. Cell Tissue Res. 2005, 322, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, F.; Inoue, N.; Manabe, N.; Ohkura, S. Follicular growth and atresia in mammalian ovaries: Regulation by survival and death of granulosa cells. J. Reprod. Dev. 2012, 58, 44–50. [Google Scholar] [CrossRef]
- Adu-Gyamfi, E.A.; Djankpa, F.T.; Nelson, W.; Czika, A.; Sah, S.K.; Lamptey, J.; Ding, Y.-B.; Wang, Y.-X. Activin and inhibin signaling: From regulation of physiology to involvement in the pathology of the female reproductive system. Cytokine 2020, 133, 155105. [Google Scholar] [CrossRef]
- Rahman, N.A.; Huhtaniemi, I. Hormonal regulation of proliferation of granulosa and Leydig cell lines derived from gonadal tumors of transgenic mice expressing the inhibin-alpha subunit promoter/simian virus 40 T-antigen fusion gene. Mol. Cell. Endocrinol. 1999, 149, 9–17. [Google Scholar]
- Lu, C.; Yang, W.; Chen, M.; Liu, T.; Yang, J.; Tan, P.; Li, L.; Hu, X.; Fan, C.; Hu, Z.; et al. Inhibin A inhibits follicle-stimulating hormone (FSH) action by suppressing its receptor expression in cultured rat granulosa cells. Mol. Cell. Endocrinol. 2009, 298, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Shi, F.; Watanabe, G.; Suzuki, A.K.; Taya, K. Regulatory role of inhibin in follicle-stimulating hormone secretion and folliculogenesis in the guinea pig. J. Vet. Med. Sci. 2001, 63, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.G. Regulatory functions for inhibin and activin in human ovaries. J. Endocrinol. 1991, 131, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.B.; Chakravarthi, V.P.; Wolfe, M.W.; Rumi, M.K. ERbeta Regulation of Gonadotropin Responses during Folliculogenesis. Int. J. Mol. Sci. 2021, 22, 10348. [Google Scholar] [CrossRef] [PubMed]
- Emmen, J.M.; Couse, J.F.; Elmore, S.A.; Yates, M.M.; Kissling, G.E.; Korach, K.S. In vitro growth and ovulation of follicles from ovaries of estrogen receptor (ER){alpha} and ER{beta} null mice indicate a role for ER{beta} in follicular maturation. Endocrinology 2005, 146, 2817–2826. [Google Scholar] [CrossRef]
- Couse, J.F.; Yates, M.M.; Deroo, B.J.; Korach, K.S. Estrogen receptor-beta is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology 2005, 146, 3247–3262. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, Y.; Ma, X.; Fu, B.; Li, N.; Zhang, C. Mechanisms of OCT4 on 3,5,3′-Tri-iodothyronine and FSH-induced Granulosa Cell Development in Female Mice. Endocrinology 2021, 162, bqab183. [Google Scholar] [CrossRef]
- Roth, Z.; Arav, A.; Bor, A.; Zeron, Y.; Braw-Tal, R.; Wolfenson, D. Improvement of quality of oocytes collected in the autumn by enhanced removal of impaired follicles from previously heat-stressed cows. Reproduction 2001, 122, 737–744. [Google Scholar] [CrossRef]
- Li, J.; Gao, H.; Tian, Z.; Wu, Y.; Wang, Y.; Fang, Y.; Lin, L.; Han, Y.; Wu, S.; Haq, I.; et al. Effects of chronic heat stress on granulosa cell apoptosis and follicular atresia in mouse ovary. J. Anim. Sci. Biotechnol. 2016, 7, 57. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Shen, M.; Wu, W.-J.; Li, B.-J.; Weng, Q.-N.; Li, M.; Liu, H.-L. Expression of PUMA in Follicular Granulosa Cells Regulated by FoxO1 Activation During Oxidative Stress. Reprod. Sci. 2015, 22, 696–705. [Google Scholar] [CrossRef]
- Takagi, K.; Yamada, T.; Miki, Y.; Umegaki, T.; Nishimura, M.; Sasaki, J. Makoto Nishimura, and Junzo Sasaki Histological Observation of the Development of Follicles and Follicular Atresia in Immature Rat Ovaries. Acta Med. Okayama 2007, 61, 283–298. [Google Scholar] [PubMed]
- Liao, H.-Q.; Zhou, J.; Cao, Y.; Nie, Y.-L.; Li, M.-Q.; Zhou, J. Vigilin interacts with ER-β to protect against palmitic acid-induced granulosa cells apoptosis via inhibiting calcineurin-mediated Drp1 signaling pathway. Steroids 2020, 163, 108699. [Google Scholar] [CrossRef] [PubMed]
- Regan, S.L.P.; Knight, P.; Yovich, J.L.; Leung, Y.; Arfuso, F.; Dharmarajan, A. Granulosa Cell Apoptosis in the Ovarian Follicle—A Changing view. Front. Endocrinol. 2018, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Jung, S.Y.; Wu, S.-P.; Hawkins, S.M.; Park, M.J.; Kyo, S.; Qin, J.; Lydon, J.P.; Tsai, S.Y.; Tsai, M.-J.; et al. Estrogen Receptor β Modulates Apoptosis Complexes and the Inflammasome to Drive the Pathogenesis of Endometriosis. Cell 2015, 163, 960–974. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.J.; Hussain, S.; Balanathan, P.; Hedwards, S.L.; Niranjan, B.; Grant, M.; Chandrasiri, U.P.; Toivanen, R.; Wang, Y.; Taylor, R.A.; et al. Estrogen receptor–β activated apoptosis in benign hyperplasia and cancer of the prostate is androgen independent and TNFα mediated. PNAS 2010, 107, 3123–3128. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Li, X.; Jia, S.; Liu, S.; Fu, L.; Jiang, X.; Yang, M. Bisphenol AF induces apoptosis via estrogen receptor beta (ERβ) and ROS-ASK1-JNK MAPK pathway in human granulosa cell line KGN. Environ. Pollut. 2021, 270, 116051. [Google Scholar] [CrossRef] [PubMed]
- Panza, S.; Santoro, M.; De Amicis, F.; Morelli, C.; Passarelli, V.; D’Aquila, P.; Giordano, F.; Cione, E.; Passarino, G.; Bellizzi, D.; et al. Estradiol via estrogen receptor beta influences ROS levels through the transcriptional regulation of SIRT3 in human seminoma TCam-2 cells. Tumor Biol. 2017, 39, 1–8. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).