Cocaine Effects on Reproductive Behavior and Fertility: An Overview
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Cocaine Production and Use
1.2. Cocaine Pharmacokinetics and Pharmacodynamics
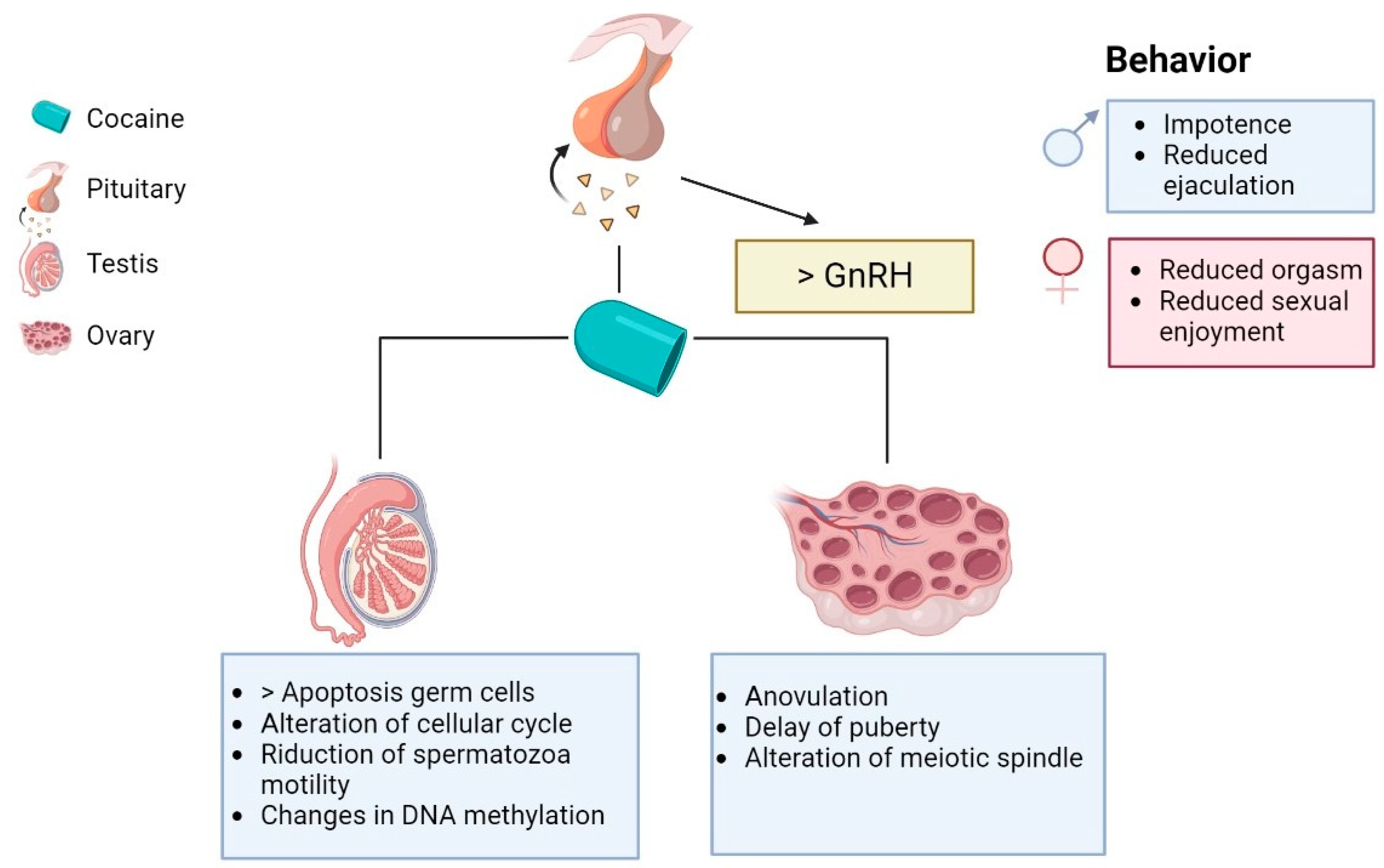
2. Cocaine and Hypothalamus–Pituitary–Gonadal (HPG) Axis
3. Cocaine and Alterations of Reproductive Behavior
Cocaine and the Sexual Dysfunction
4. Cocaine and Changes in Fertility
4.1. Males
4.1.1. Effect of Cocaine on Hormones
4.1.2. Morpho Functional Effects of Cocaine on Germ Cells
4.1.3. Effect of Cocaine on Fertilization
4.2. Females
4.2.1. Effect of Cocaine on Ovary Function
4.2.2. Effect of Cocaine on Oocytes Function
4.3. No Mammalian Species
Effect of Cocaine on Ovary Function
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNODC. World Drug Report (United Nations Publication, Sales, No. E.22.XI.8). Book 2: Global Overview of Drug Demand and Drug Supply; UNODC: Vienna, Austria, 2022. [Google Scholar]
- UNODC. World Drug Report (United Nations Publication, Sales, No. E.22.XI.8). Book 4: Drug Market Trends of Cocaine, Ampheta-Mine-Type Stimulants, and New Psychoactive Substances; UNODC: Vienna, Austria, 2022. [Google Scholar]
- UNODC. World Drug Report (United Nations Publication, Sales, No. E.22.XI.8). Book 5: Drugs and the Environment; UNODC: Vienna, Austria, 2022. [Google Scholar]
- Casale, J.F.; Klein, R.F.X. Illicit Production of Cocaine. Forensic Sci. Rev. 1993, 5, 95–107. [Google Scholar]
- UNODC. Cocaine-a Spectrum of Products, Cocaine Insights 2; UNODC: Vienna, Austria, 2021. [Google Scholar]
- Grilly, M.G.; Salamone, J.D. Drugs, Brain and Behavior, 6th ed.; Pearson: Boston, MA, USA, 2012; pp. 132–178. [Google Scholar]
- Roque Bravo, R.; Faria, A.C.; Brito-Da-Costa, A.M.; Carmo, H.; Mladěnka, P.; Dias Da Silva, D.; Remião, F.; On Behalf of The OEMONOM Researchers. Cocaine: An Updated Overview on Chemistry, Detection, Biokinetics, and Pharmacotoxicological Aspects Including Abuse Pattern. Toxins 2022, 14, 278. [Google Scholar] [CrossRef]
- Heard, K.; Palmer, R.; Zahniser, N.R. Mechanisms of Acute Cocaine Toxicity. Open Pharmacol. J. 2008, 2, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Manetti, L.; Cavagnini, F.; Martino, E.; Ambrogio, A. Effects of Cocaine on the Hypothalamic-Pituitary-Adrenal Axis. J. Endocrinol. Investig. 2014, 37, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Gay, F.; Maddaloni, M.; Valiante, S.; Laforgia, V.; Capaldo, A. Endocrine Disruption in the European Eel, Anguilla anguilla, Exposed to an Environmental Cocaine Concentration. Water Air Soil Pollut. 2013, 224, 1579. [Google Scholar] [CrossRef]
- Pinto, A.M.; Carvalho, D. Human Infertility: Are Endocrine Disruptors to Blame? Endocr. Connect. 2013, 2, R15–R29. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Rodriguez, A.; Kauffman, A.S.; Cherrington, B.D.; Borges, C.S.; Roepke, T.A.; Laconi, M. Emerging Insights into Hypothalamic-Pituitary-Gonadal Axis Regulation and Interaction with Stress Signalling. J. Neuroendocrinol. 2018, 30, E12590. [Google Scholar] [CrossRef]
- Wahab, F.; Shahab, M.; Behr, R. The Involvement of Gonadotropin Inhibitory Hormone and Kisspeptin in the Metabolic Regulation of Reproduction. J. Endocrinol. 2015, 225, R49–R66. [Google Scholar] [CrossRef]
- King, T.S.; Kang, I.S.; Javors, M.A.; Schenken, R.S. Phorbol Esters Stimulate in Vitro GnRH Release from the Hypothalamus of the Estrogen-Primed, Ovariectomized Rat: Inhibitory Effects of Cocaine. Neuroendocrinology 1992, 56, 526–532. [Google Scholar] [CrossRef]
- King, T.S.; Canez, M.S.; Gaskill, S.; Javors, M.A.; Schenken, R.S. Chronic Cocaine Disruption of Estrous Cyclicity in the Rat: Dose-Dependent Effects. J. Pharmacol. Exp. Ther. 1993, 264, 29–34. [Google Scholar]
- Evans, S.M.; Foltin, R.W. Pharmacokinetics of Repeated Doses of Intravenous Cocaine across the Menstrual Cycle in Rhesus Monkeys. Pharmacol. Biochem. Behav. 2006, 83, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, J.H.; Mello, N.K.; Sholar, M.B.; Siegel, A.J.; Mutschler, N.; Halpern, J. Temporal Concordance of Cocaine Effects on Mood States and Neuroendocrine Hormones. Psychoneuroendocrinology 2002, 27, 71–82. [Google Scholar] [CrossRef]
- Mello, N.K. Hormones, Nicotine, and Cocaine: Clinical Studies. Horm. Behav. 2010, 58, 57–71. [Google Scholar] [CrossRef]
- Mello, N.K.; Mendelson, J.H.; Negus, S.S.; Kelly, M.; Knudson, I.; Roth, M.E. The Effects of Cocaine on Gonadal Steroid Hormones and LH in Male and Female Rhesus Monkeys. Neuropsychopharmacology 2004, 29, 2024–2034. [Google Scholar] [CrossRef]
- Heesch, C.M.; Negus, B.H.; Bost, J.E.; Keffer, J.H.; Snyder, R.W.; Eichhornm, E.J. Effects of Cocaine on Anterior Pituitary and Gonadal Hormones. J. Pharmacol. Exp. Ther. 1996, 278, 1195–1200. [Google Scholar]
- King, T.S.; McNichol, M.; Canez, M.S.; Javors, M.A.; Schenken, R.S. Effect of Acute Administration of Cocaine on Pituitary Gonadotrophin Secretion in Female Rats. Reproduction 2001, 122, 723–729. [Google Scholar] [CrossRef]
- Fontes, M.K.; Rosati, L.; Di Lorenzo, M.; Pereira, C.D.S.; Maranho, L.A.; Laforgia, V.; Capaldo, A. Aquatic Pollution and Risks to Biodiversity: The Example of Cocaine Effects on the Ovaries of Anguilla anguilla. Animals 2022, 12, 1766. [Google Scholar] [CrossRef]
- Dufour, S.; Sebert, M.E.; Weltzien, F.A.; Rousseau, E.; Pasqualini, C. Neudoendocrine Control by Dopamine Teleost Reproduction. J. Fish Biol. 2010, 76, 129–160. [Google Scholar] [CrossRef] [PubMed]
- Mello, N.K.; Mendelson, J.H.; Kelly, M.; Bowen, C.A. The Effects of Cocaine on Basal and Human Chorionic Gonadotropin-Stimulated Ovarian Steroid Hormones in Female Rhesus Monkeys. J. Pharmacol. Exp. Ther. 2004, 294, 1137–1145. [Google Scholar]
- Walker, Q.D.; Francis, R.; Cabassa, J.; Kuhn, C.M. Effect of Ovarian Hormones and Estrous Cycle on Stimulation of the Hypothalamo-Pituitary-Adrenal Axis by Cocaine. J. Pharmacol. Exp. Ther. 2001, 297, 291–298. [Google Scholar]
- Mendelson, J.H.; Teoh, S.K.; Lange, U.; Mello, N.K.; Weiss, R.; Skupny, A. Anterior Pituitary, Adrenal, and Gonadal Hormones during Cocaine Withdrawal. Am. J. Psychiatry 1988, 145, 1094–1098. [Google Scholar] [PubMed]
- Mendelson, J.H.; Sholar, M.B.; Mutschler, N.H.; Jaszyna-Gasior, M.; Goletiani, N.V.; Siegel, A.J. Effects of Intravenous Cocaine and Cigarette Smoking on Luteinizing Hormone, Testosterone and Prolactin in Men. J. Pharmacol. Exp. Ther. 2003, 307, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, A.B.; Brown, T.T.; John, M.; Frankowicz, J.K.; Cofranceso, J.; Golub, E.T.; Ricketts, E.P.; Dobs, A.S. Hypothalamic-Pituitary-Gonadal Function in Men and Women Using Heroin and Cocaine, Stratified by HIV Status. Gend. Med. 2007, 4, 35–44. [Google Scholar] [CrossRef]
- Berul, C.I.; Harclerode, J.E. Effects of Cocaine Hydrochloride on the Male Reproductive System. Life Sci. 1989, 45, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Gawin, F.H.; EUinwood, E.H. Cocaine and Other Stimulants: Actions, Abuse and Treatments. N. Engl. J. Med. 1990, 318, 1173–1182. [Google Scholar] [CrossRef]
- Chitwood, D.D.; Comerford, M. Drugs, Sex, and AIDS Risk. Am. Behav. Sci. 1990, 33, 465–477. [Google Scholar] [CrossRef]
- Siegal, R.K. Cocaine and Sexual Dysfunction: The Curse of Mama Coca. J. Psychoact. Drugs 1982, 14, 71–74. [Google Scholar] [CrossRef]
- Meston, C.M.; Gorzalka, B.B. Psychoactive Drugs and Human Sexual Behavior: The Role of Serotonergic Activity. J. Psychoact. Drugs 1992, 24, 1–40. [Google Scholar] [CrossRef]
- Wesson, D.R. Cocaine Use by Masseuses. J. Psychoact. Drugs 1982, 4, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, T.; Stimson, G.V. What Is the Relationship between Drug Taking and Sexual Risk? Social Relations and Social Research. Sociol. Health Illn. 1994, 16, 209–228. [Google Scholar] [CrossRef]
- Dallelucci, C.C.; Bragiato, E.C.; Areco, K.C.N.; Fidalgo, T.M.; Da Silveira, D.X. Sexual Risky Behavior, Cocaine and Alcohol Use among Substance Users in an Outpatient Facility: A Cross Section Study. Subst. Abus. Treat. Prev. Policy 2019, 14, 46. [Google Scholar] [CrossRef]
- Agrati, D.; Machado, L.; Delgado, H.; Uriarte, N.; Zuluaga, M.J.; Ferreira, A. Sexual Behaviour of the Female Rat during Late Adolescence: Effect of Chronic Cocaine Treatment. Behav. Pharmacol. 2019, 30, 396–404. [Google Scholar] [CrossRef]
- Bitran, D.; Hull, E.M. Pharmacological Analysis of Male Rat Sexual Behavior. Neurosci. Biobehav. Rev. 1987, 11, 365–389. [Google Scholar] [CrossRef] [PubMed]
- Chambers, K.C.; Phoenix, C.H. Apomorphine, Deprenyl, and Yohimbine Fail to Increase Sexual Behavior in Rhesus Males. Behav. Neurosci. 1989, 103, 816–823. [Google Scholar] [CrossRef]
- Pomeranz, S.M. Dopaminergic Influences on Male Sexual Behavior of Rhesus Monkeys: Effects of Dopamine Agonists. Eur. J. Pharmacol. 1992, 41, 511–517. [Google Scholar]
- Johanson, C.E.; Fischman, M.W. The Pharmacology of Cocaine Related to Its Abuse. Pharmacol. Rev. 1989, 41, 3–52. [Google Scholar]
- Linnankoski, I.; Grönroos, M.; Carlson, S.; Pertovaara, A. Effect of Cocaine on Sexual Behaviour in Male Stumptail Macaques (Macaca arctoides). Pharmacol. Biochem. Behav. 1995, 52, 211–216. [Google Scholar] [CrossRef]
- Andersen, M.L.; Tufik, S. Distinct Effects of Paradoxical Sleep Deprivation and Cocaine Administration on Sexual Behavior in Male Rats. Addict. Biol. 2002, 7, 251–253. [Google Scholar] [CrossRef]
- Andersen, M.L.; Tufik, S. Inhibitory Effect of GABAergic Drugs in Cocaine-Induced Genital Reflexes in Paradoxical Sleep-Deprived Male Rats. Pharmacol. Biochem. Behav. 2004, 78, 301–307. [Google Scholar] [CrossRef]
- Saso, L. Effects of Drug Abuse on Sexual Response. Ann. Dell’Istituto Super. Sanita 2002, 38, 289–296. [Google Scholar]
- De Angelis, C.; Nardone, A.; Garifalos, F.; Pivonello, C.; Sansone, A.; Conforti, A.; Di Dato, C.; Sirico, F.; Alviggi, C.; Isidori, A.; et al. Smoke, Alcohol and Drug Addiction and Female Fertility. Reprod. Biol. Endocrinol. 2020, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Rosati, L.; Agnese, M.; Di Lorenzo, M.; Barra, T.; Valiante, S.; Prisco, M. Spermatogenesis and Regulatory Factors in the Wall Lizard Podarcis sicula. Gen. Comp. Endocrinol. 2020, 298, 113579. [Google Scholar] [CrossRef]
- Li, M.; Arimura, A. Neuropeptides of the Pituitary Adenylate Cyclase-Activating Polypeptide/Vasoactive Intestinal Polypeptide/Growth Hormone-Releasing Hormone/Secretin Family in Testis. Endocrine 2003, 20, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Rosati, L.; Andreuccetti, P.; Prisco, M. Vasoactive intestinal peptide (VIP) localization in the epididymis of two vertebrate species. Comptes Rendus Biol. 2017, 340, 379–385. [Google Scholar] [CrossRef]
- Rosati, L.; Agnese, M.; Di Fiore, M.M.; Andreuccetti, P.; Prisco, M. P450 Aromatase: A Key Enzyme in the Spermatogenesis of the Italian Wall Lizard Podarcis sicula. J. Exp. Biol. 2016, 219, 2402–2408. [Google Scholar] [CrossRef]
- Rosati, L.; Di Fiore, M.M.; Prisco, M.; Di Giacomo Russo, F.; Venditti, M.; Andreuccetti, P.; Chieffi Baccari, G.; Santillo, A. Seasonal Expression and Cellular Distribution of Star and Steroidogenic Enzymes in Quail Testis. J. Exp. Zool. Part B Mol. Dev. Evol. 2019, 332, 198–209. [Google Scholar] [CrossRef]
- Rosati, L.; Falvo, S.; Chieffi Baccari, G.; Santillo, A.; Di Fiore, M.M. The Aromatase–Estrogen System in the Testes of Non-Mammalian Vertebrates. Animals 2021, 11, 1763. [Google Scholar] [CrossRef] [PubMed]
- Falvo, S.; Rosati, L.; Di Fiore, M.M.; Di Giacomo Russo, F.; Chieffi Baccari, G.; Santillo, A. Proliferative and Apoptotic Pathways in the Testis of Quail Coturnix coturnix during the Seasonal Reproductive Cycle. Animals 2021, 11, 1729. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.L.; Moore, C.; Waselewsky, D.; Zajac, C.; Russell, L.D. Effects of Cocaine Hydrochloride on Reproductive Function and Sexual Behavior of Male Rats and on the Behavior of Their Offspring. J. Androl. 1989, 10, 17–27. [Google Scholar] [CrossRef]
- Yang, G.S.; Wang, W.; Wang, Y.M.; Chen, Z.D.; Wang, S.; Fang, J.J. Effect of Cocaine on Germ Cell Apoptosis in Rats at Different Ages. Asian J. Androl. 2006, 8, 569–575. [Google Scholar] [CrossRef]
- Li, H.; George, V.K.; Crossland, W.J.; Anderson, G.F.; Dhabuwala, C.B. Characterization of Cocaine Binding Sites in the Rat Testes. J. Urol. 1997, 158, 962–965. [Google Scholar] [CrossRef]
- Vaz, A.; Lefkowitz, S.S.; Castro, A.; Lefkowitz, D.L. The Effects of Cocaine and Its Metabolites on the Production of Reactive Oxygen and Reactive Nitrogen Intermediates. Life Sci. 1994, 55, PL439–PL444. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.D.; Zhou, D.X.; Song, T.B. The Reproductive System Impairment of Adult Male Rats Induced by Cocaine. Fa Yi Xue Za Zhi 2008, 24, 411–413. [Google Scholar]
- Yazigi, R.A.; Odem, R.R.; Polakoski, K.L. Demonstration of Specific Binding of Cocaine to Human Spermatozoa. JAMA 1991, 266, 1956–1959. [Google Scholar] [CrossRef]
- Yelian, F.D.; Sacco, A.G.; Ginsburg, K.A.; Doerr, P.A.; Armant, D.R. The Effects of in Vitro Cocaine Exposure on Human Sperm Motility, Intracellular Calcium, and Oocyte Penetration. Fertil. Steril. 1994, 61, 915–921. [Google Scholar] [CrossRef] [PubMed]
- González, B.; Pantoja, C.R.G.; Sosa, M.H.; Vitullo, A.D.; Bisagno, V.; González, C.R. Cocaine Alters the Mouse Testicular Epigenome with Direct Impact on Histone Acetylation and DNA Methylation Marks. Reprod. Biomed. Online 2018, 37, 269–278. [Google Scholar] [CrossRef]
- Swinford-Jackson, S.E.; Fant, B.; Wimmer, M.E.; Chan, D.; Knouse, M.C.; Sarmiento, M.; Thomas, A.S.; Huffman, P.J.; Mankame, S.; Worobey, S.J.; et al. Cocaine-Induced Changes in Sperm Cdkn1a Methylation Are Associated with Cocaine Resistance in Male Offspring. J. Neurosci. 2022, 42, 2905–2916. [Google Scholar] [CrossRef]
- Yaw, A.M.; Prosser, R.A.; Jones, P.C.; Garcia, B.J.; Jacobson, D.A.; Glass, J.D. Epigenetic Effects of Paternal Cocaine on Reward Stimulus Behavior and Accumbens Gene Expression in Mice. Behav. Brain Res. 2019, 367, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Yazigi, R.A.; Polakoski, K.L. Distribution of Tritiated Cocaine in Selected Genital and Nongenital Organs Following Administration to Male Mice. Arch. Pathol. Lab. Med. 1992, 116, 1036–1039. [Google Scholar]
- George, V.K.; Li, H.; Teloken, C.; Grignon, D.J.; Lawrence, W.D.; Dhabuwala, C.B. Effects of Long-Term Cocaine Exposure on Spermatogenesis and Fertility in Peripubertal Male Rats. J. Urol. 1996, 155, 327–331. [Google Scholar] [CrossRef]
- Mueller, B.A.; Daling, J.R.; Weiss, N.S.; Moore, D.E. Recreational Drug Use and the Risk of Primary Infertility. Epidemiology 1990, 1, 195–200. [Google Scholar] [CrossRef]
- Joesoef, M.R.; Beral, V.; Aral, S.O.; Rolfs, R.T.; Cramer, D.W. Fertility and Use of Cigarettes, Alcohol, Marijuana, and Cocaine. Ann. Epidemiol. 1993, 3, 592–594. [Google Scholar] [CrossRef]
- Mello, N.K.; Mendelson, J.H. Cocaine’s Effects on Neuroendocrine Systems: Clinical and Preclinical Studies. Pharmacol. Biochem. Behav. 1997, 57, 571–599. [Google Scholar] [CrossRef] [PubMed]
- Potter, D.A.; Moreno, A.; Luther, M.F. Effects of Follicularphase Cocaine Administration on Menstrual and Ovarian Cyclicity in Rhesus Monkeys. Am. J. Obstet. Gynecol. 1998, 78, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Thyer, A.C.; King, T.S.; Moreno, A.C.; Eddy, C.A.; Siler-Khodr, T.M.; Schenken, R.S. Cocaine Impairs Ovarian Response to Exogenous Gonadotropins in Nonhuman Primates. J. Soc. Gynecol. Investig. 2001, 8, 358–362. [Google Scholar] [CrossRef]
- Atlas, S.J.; Wallach, E.E. Effects of Intravenous Cocaine on Reproductive Function in the Mated Rabbit. Am. J. Obstet. Gynecol. 1991, 165, 1785–1790. [Google Scholar] [CrossRef]
- Chen, C.J.; Vandenbergh, J.G. Effect of Chronic Cocaine on Reproduction in Female House Mice. Pharmacol. Biochem. Behav. 1994, 48, 909–913. [Google Scholar] [CrossRef]
- Raap, D.K.; Morin, B.; Medici, C.N.; Smith, R.F. Adolescent Cocaine and Injection Stress Effects on the Estrous Cycle. Physiol. Behav. 2000, 70, 417–424. [Google Scholar] [CrossRef]
- Kaufmann, R.A.; Savoy-Moore, R.T.; Sacco, A.G.; Subramanian, M.G. The Effect of Cocaine on Oocyte Development and the Follicular Microenvironment in the Rabbit. Fertil. Steril. 1990, 54, 921–926. [Google Scholar] [CrossRef]
- Combelles, C.M.; Carabatsos, M.J.; London, S.N.; Mailhes, J.B.; Albertini, D.F. Centrosome-specific perturbations during in vitro maturation of mouse oocytes exposed to cocaine. Exp. Cell Res. 2000, 260, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.; Goldstein, E.G.; Dannenhoffer, R. Induction by cocaine of defective spindle formation in cultured mouse oocytes. Toxicol. In Vitro 1996, 10, 377–382. [Google Scholar] [CrossRef]
- Maranho, L.A.; Fontes, M.K.; Kamimura, A.S.S.; Nobre, C.R.; Moreno, B.B.; Pusceddu, F.H.; Cortez, F.S.; Lebre, D.T.; Marques, J.R.; Abessa, D.M.S.; et al. Exposure to Crack Cocaine Causes Adverse Effects on Marine Mussels Perna perna. Mar. Pollut. Bull. 2017, 123, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Souza, L.; Pusceddu, F.H.; Cortez, F.S.; De Orte, M.R.; Seabra, A.A.; Cesar, A.; Ribeiro, D.A.; Del Valls Casillas, T.A.; Pereira, C.D.S. Harmful Effects of Cocaine Byproduct in the Reproduction of Sea Urchin in Different Ocean Acidification Scenarios. Chemosphere 2019, 236, 124284. [Google Scholar] [CrossRef] [PubMed]
- Willard, S.S.; Koss, C.M.; Cronmiller, C. Chronic Cocaine Exposure in Drosophila: Life, Cell Death and Oogenesis. Dev. Biol. 2006, 296, 150–163. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosati, L.; Chianese, T.; Mileo, A.; De Falco, M.; Capaldo, A. Cocaine Effects on Reproductive Behavior and Fertility: An Overview. Vet. Sci. 2023, 10, 484. https://doi.org/10.3390/vetsci10080484
Rosati L, Chianese T, Mileo A, De Falco M, Capaldo A. Cocaine Effects on Reproductive Behavior and Fertility: An Overview. Veterinary Sciences. 2023; 10(8):484. https://doi.org/10.3390/vetsci10080484
Chicago/Turabian StyleRosati, Luigi, Teresa Chianese, Aldo Mileo, Maria De Falco, and Anna Capaldo. 2023. "Cocaine Effects on Reproductive Behavior and Fertility: An Overview" Veterinary Sciences 10, no. 8: 484. https://doi.org/10.3390/vetsci10080484
APA StyleRosati, L., Chianese, T., Mileo, A., De Falco, M., & Capaldo, A. (2023). Cocaine Effects on Reproductive Behavior and Fertility: An Overview. Veterinary Sciences, 10(8), 484. https://doi.org/10.3390/vetsci10080484







