Relation between the Dam’s Weight on Superficial Temperature of Her Puppies at Different Stages of the Post-Partum
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Facilities
2.2. Study Population
2.3. Clinical History
2.4. Prenatal Procedures
2.5. Puppies
2.6. Infrared Thermography
2.7. Statistical Analysis
2.8. Ethical Statement
3. Results
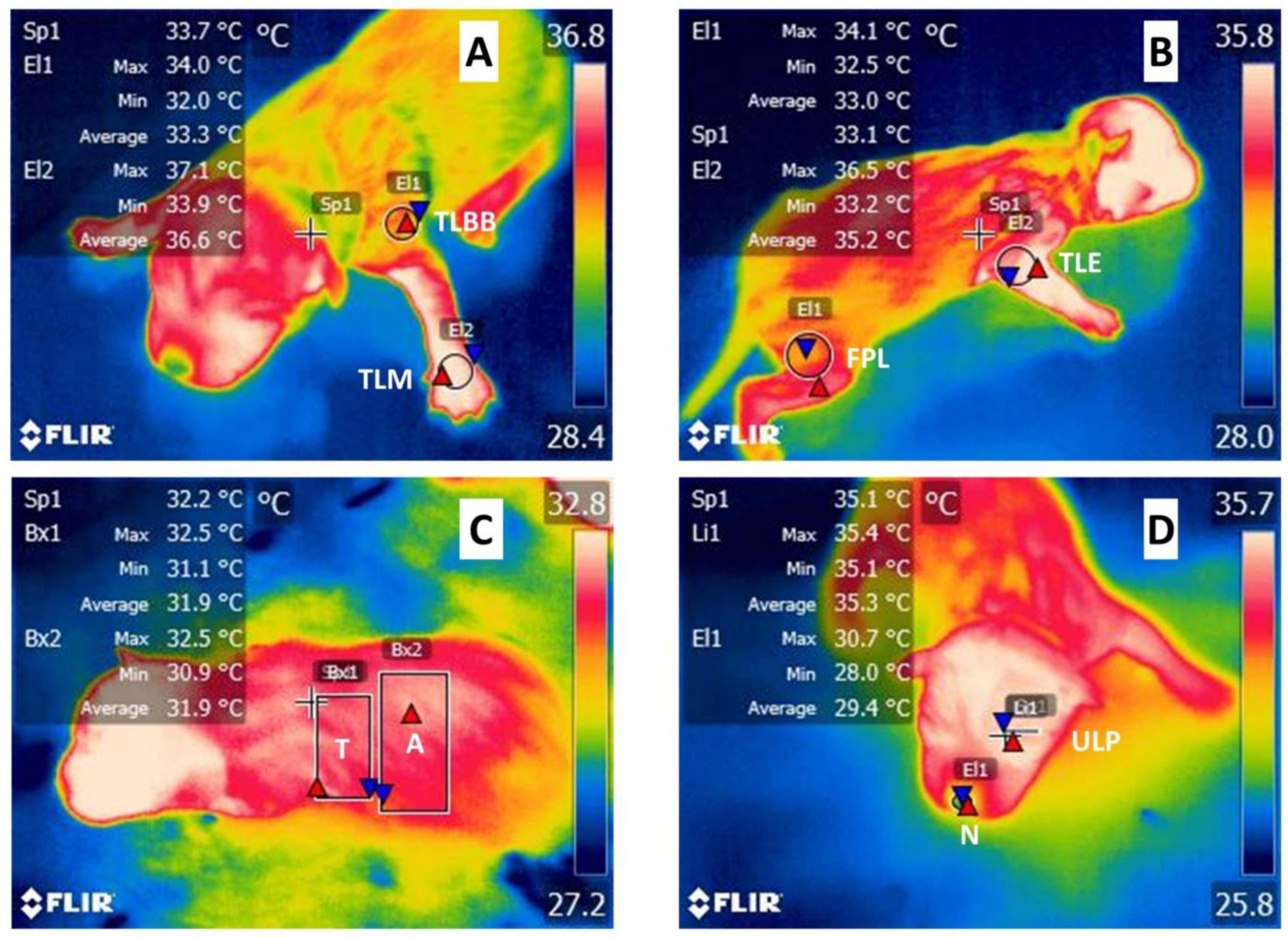
3.1. Infrared Thermography
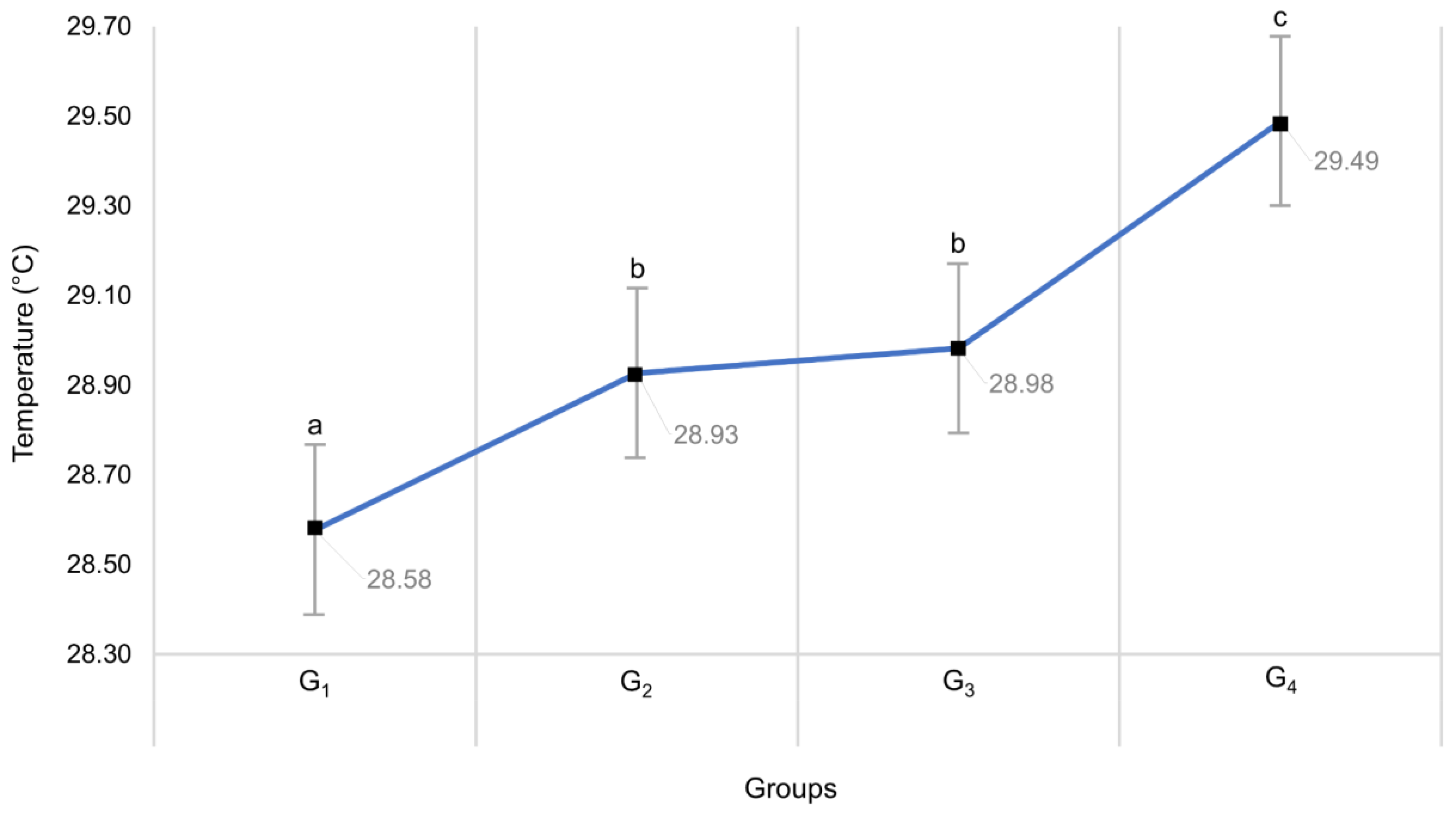
3.2. Effect of Maternal Weight and Litter Size on Neonatal Superficial Temperature Changes
3.3. Effect of Colostrum Consumption on Thermoregulation
3.4. Changes in Temperature according to the Regions Evaluated
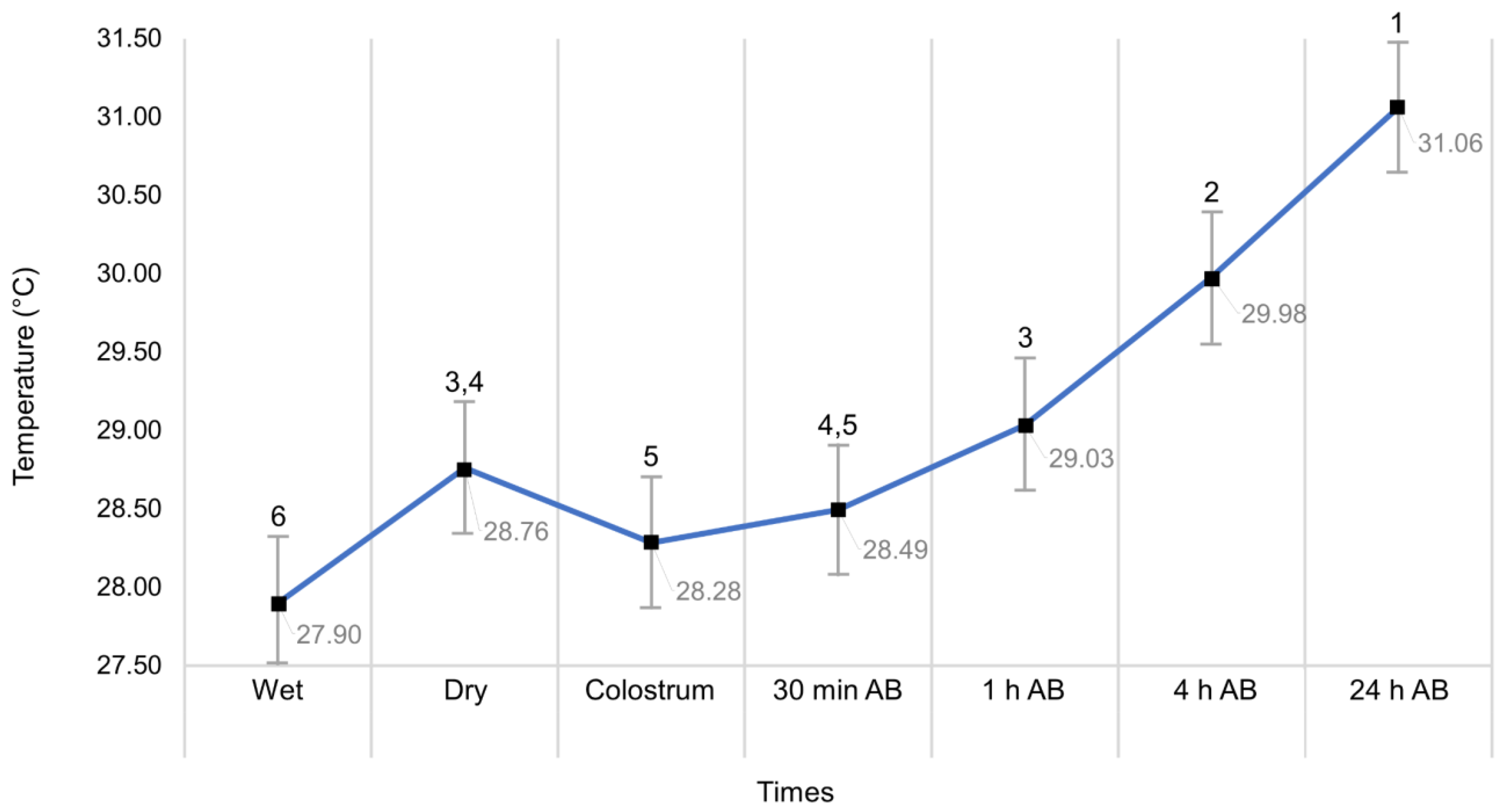
3.5. Thermal Response of the Newborn Due to the Effect of Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mota-Rojas, D.; López, A.; Martínez-Burnes, J.; Muns, R.; Villanueva-García, D.; Mora-Medina, P.; González-Lozano, M.; Olmos-Hernández, A.; Ramírez-Necoechea, R. Is vitality assessment important in neonatal animals? CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2018, 13, 1–13. [Google Scholar] [CrossRef]
- Dwyer, C.M. Maternal behaviour and lamb survival: From neuroendocrinology to practical application. Animal 2014, 8, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Ocepek, M.; Andersen, I.L. What makes a good mother? Maternal behavioural traits important for piglet survival. Appl. Anim. Behav. Sci. 2017, 193, 29–36. [Google Scholar] [CrossRef]
- Lezama-García, K.; Mariti, C.; Mota-Rojas, D.; Martínez-Burnes, J.; Barrios-García, H.; Gazzano, A. Maternal behaviour in domestic dogs. Int. J. Vet. Sci. Med. 2019, 7, 20–30. [Google Scholar] [CrossRef]
- Madani, T.; Allouche, L.; Saffidine, N.; Kaouane, N.; Belkasmi, F.; Semara, L. Maternal and neonatal behaviors of Ouled Djellal sheep breed and their effects on production parameters. Small Rumin. Res. 2013, 114, 46–50. [Google Scholar] [CrossRef]
- Mellor, D. Preparing for Life after Birth: Introducing the Concepts of Intrauterine and Extrauterine Sensory Entrainment in Mammalian Young. Animals 2019, 9, 826. [Google Scholar] [CrossRef]
- Lévy, F.; Keller, M.; Poindron, P. Olfactory regulation of maternal behavior in mammals. Horm. Behav. 2004, 46, 284–302. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Orihuela, A.; Strappini-Asteggiano, A.; Nelly Cajiao-Pachón, M.; Agüera-Buendía, E.; Mora-Medina, P.; Ghezzi, M.; Alonso-Spilsbury, M. Teaching animal welfare in veterinary schools in Latin America. Int. J. Vet. Sci. Med. 2018, 6, 131–140. [Google Scholar] [CrossRef]
- Veronesi, M. Assessment of canine neonatal viability-the Apgar score. Reprod. Domest. Anim. 2016, 51, 46–50. [Google Scholar] [CrossRef]
- Münnich, A.; Küchenmeister, U. Causes, diagnosis and therapy of common diseases in neonatal puppies in the first days of life: Cornerstones of practical approach. Reprod. Domest. Anim. 2014, 49, 64–74. [Google Scholar] [CrossRef]
- Uchańska, O.; Ochota, M.; Eberhardt, M.; Niżański, W. Dead or alive? A review of perinatal factors that determine canine neonatal viability. Animals 2022, 12, 1402. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-García, D.; Mota-Rojas, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Mora-Medina, P.; Salmerón, C.; Gómez, J.; Boscato, L.; Gutiérrez-Pérez, O.; Cruz, V.; et al. Hypothermia in newly born piglets: Mechanisms of thermoregulation and pathophysiology of death. J. Anim. Behav. Biometeorol. 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Reyes-Sotelo, B.; Ogi, A.; Mora-Medina, P.; Mariti, C.; Olmos-Hernández, A.; Hernández-Ávalos, I.; Domínguez-Oliva, A.; Rosas, M.E.; Verduzco-Mendoza, A.; Gazzano, A. Early Blood Analysis and Gas Exchange Monitoring in the Canine Neonate: Effect of Dam’s Size and Birth Order. Animals 2022, 12, 1508. [Google Scholar] [CrossRef]
- Nord, A.; Nilsson, J.F.; Sandell, M.I.; Nilsson, J.-Å. Patterns and dynamics of rest-phase hypothermia in wild and captive blue tits during winter. J. Comp. Physiol. B 2009, 179, 737–745. [Google Scholar] [CrossRef]
- Terrien, J.; Perret, M.; Aujard, F. Behavioral thermoregulation in mammals: A review. Front. Biosci. 2011, 16, 1428. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D.; la Fuente, V.C.; et al. Physiological and behavioral mechanisms of thermoregulation in mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef] [PubMed]
- Lezama-García, K.; Mota-Rojas, D.; Pereira, A.M.F.; Martínez-Burnes, J.; Ghezzi, M.; Domínguez, A.; Gómez, J.; de Mira Geraldo, A.; Lendez, P.; Hernández-Ávalos, I.; et al. Transient Receptor Potential (TRP) and thermoregulation in animals: Structural biology and neurophysiological aspects. Animals 2022, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.; Poindron, P. From birth to colostrum: Early steps leading to lamb survival. Reprod. Nutr. Dev. 2006, 46, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, C.I.C.; Rodrigues, J.J.A.; Silva, L.L.C.G.; Lúcio, C.C.F.; Veiga, G.A.L.G. A clinical and hemogasometric survey of neonatal lambs. Small Rumin. Res. 2012, 108, 107–112. [Google Scholar] [CrossRef]
- Traas, A.M. Resuscitation of canine and feline neonates. Theriogenology 2008, 70, 343–348. [Google Scholar] [CrossRef]
- Wilborn, R.R. Small animal neonatal health. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 683–699. [Google Scholar] [CrossRef]
- Indrebø, A.; Trangerud, C.; Moe, L. Canine neonatal mortality in four large breeds. Acta Vet. Scand. 2007, 49, S2. [Google Scholar] [CrossRef]
- Nakamura, K.; Morrison, S.F. Central efferent pathways for cold-defensive and febrile shivering. J. Physiol. 2011, 589, 3641–3658. [Google Scholar] [CrossRef]
- Fisher, A.D.; Morton, R.; Dempsey, J.M.A.; Henshall, J.M.; Hill, J.R. Evaluation of a new approach for the estimation of the time of the LH surge in dairy cows using vaginal temperature and electrodeless conductivity measurements. Theriogenology 2008, 70, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Sevegnani, K.B.; Fernandes, D.P.B.; da Silva, S.H.M.-G. Evaluation of thermorregulatory capacity of dairy buffaloes using infrared thermography. Eng. Agrícola 2016, 36, 1–12. [Google Scholar] [CrossRef]
- Travain, T.; Colombo, E.S.; Heinzl, E.; Bellucci, D.; Prato Previde, E.; Valsecchi, P. Hot dogs: Thermography in the assessment of stress in dogs (Canis familiaris)—A pilot study. J. Vet. Behav. 2015, 10, 17–23. [Google Scholar] [CrossRef]
- McMillan, T.; Spaulding, K. Handling Shelter Dogs. In Animal Behavior for Shelter Veterinarians and Staff; Digangi, B.A., Cussen, V.A., Reid, P.J., Collinds, K.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 177–204. [Google Scholar]
- Casas-Alvarado, A.; Mota-Rojas, D.; Hernández-Ávalos, I.; Mora-Medina, P.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Reyes-Sotelo, B.; Martínez-Burnes, J. Advances in infrared thermography: Surgical aspects, vascular changes, and pain monitoring in veterinary medicine. J. Therm. Biol. 2020, 92, 102664. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, A.; Mota-Rojas, D.; Álvarez-Macias, A.; Mora-Medina, P.; Guerrero-Legarreta, I.; Morales-Canela, A.; Gómez-Prado, J.; José-Pérez, N.; Martínez-Burnes, J. Scientific findings related to changes in vascular microcirculation using infrared thermography in the river buffalo. J. Anim. Behav. Biometeorol. 2020, 8, 288–297. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Titto, C.G.; de Mira Geraldo, A.; Martínez-Burnes, J.; Gómez, J.; Hernández-Ávalos, I.; Casas, A.; Domínguez, A.; José, N.; Bertoni, A.; et al. Efficacy and function of feathers, hair, and glabrous skin in the thermoregulation strategies of domestic animals. Animals 2021, 11, 3472. [Google Scholar] [CrossRef]
- Gama, L.T.; Dickerson, G.E.; Young, L.D.; Leymaster, K.A. Effects of breed, heterosis, age of dam, litter size, and birth weight on lamb mortality1. J. Anim. Sci. 1991, 69, 2727–2743. [Google Scholar] [CrossRef]
- Dwyer, C.M.; Morgan, C.A. Maintenance of body temperature in the neonatal lamb: Effects of breed, birth weight, and litter size1. J. Anim. Sci. 2006, 84, 1093–1101. [Google Scholar] [CrossRef]
- Tuchscherer, M.; Puppe, B.; Tuchscherer, A.; Tiemann, U. Early identification of neonates at risk: Traits of newborn piglets with respect to survival. Theriogenology 2000, 54, 371–388. [Google Scholar] [CrossRef]
- Kirkden, R.D.; Broom, D.M.; Andersen, I.L. Invited review: Piglet mortality: Management solutions. J. Anim. Sci. 2013, 91, 3361–3389. [Google Scholar] [CrossRef] [PubMed]
- Darwish, R.A.; Abou-Ismail, U.A.; El-Kholya, S.Z. Differences in post-parturient behaviour, lamb performance and survival rate between purebred Egyptian Rahmani and its crossbred Finnish ewes. Small Rumin. Res. 2010, 89, 57–61. [Google Scholar] [CrossRef]
- Groppetti, D.; Ravasio, G.; Bronzo, V.; Pecile, A. The role of birth weight on litter size and mortality within 24 h of life in purebred dogs: What aspects are involved? Anim. Reprod. Sci. 2015, 163, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Mila, H.; Grellet, A.; Feugier, A.; Chastant-Maillard, S. Differential impact of birth weight and early growth on neonatal mortality in puppies1,2. J. Anim. Sci. 2015, 93, 4436–4442. [Google Scholar] [CrossRef]
- Veronesi, M.C.; Panzani, S.; Faustini, M.; Rota, A. An Apgar scoring system for routine assessment of newborn puppy viability and short-term survival prognosis. Theriogenology 2009, 72, 401–407. [Google Scholar] [CrossRef]
- Reyes-Sotelo, B.; Mota-Rojas, D.; Mora-Medina, P.; Ogi, A.; Mariti, C.; Olmos-Hernández, A.; Martínez-Burnes, J.; Hernández-Ávalos, I.; Sánchez-Millán, J.; Gazzano, A. Blood biomarker profile alterations in newborn canines: Effect of the mother’s weight. Animals 2021, 11, 2307. [Google Scholar] [CrossRef]
- Casas-Alvarado, A.; Martínez-Burnes, J.; Mora-Medina, P.; Hernández-Avalos, I.; Domínguez-Oliva, A.; Lezama-García, K.; Gómez-Prado, J.; Mota-Rojas, D. Thermal and circulatory changes in diverse body regions in dogs and cats evaluated by infrared thermography. Animals 2022, 12, 789. [Google Scholar] [CrossRef]
- World Small Animal Veterinary Association (WSAVA). Global Nutritional Assessment Guidelines. Available online: http://wsava.org/wp-content/uploads/2020/01/Global-Nutritional-Assesment-Guidelines-Spanish.pdf (accessed on 24 September 2019).
- Tønnessen, R.; Borge, K.S.; Nødtvedt, A.; Indrebø, A. Canine perinatal mortality: A cohort study of 224 breeds. Theriogenology 2012, 77, 1788–1801. [Google Scholar] [CrossRef]
- Federation Cynologigue Internationale (FCI). Available online: http://www.fci.be (accessed on 24 September 2019).
- Eneroth, A.; Linde-Forsberg, C.; Uhlhorn, M.; Hall, M. Radiographic pelvimetry for assessment of dystocia in bitches: A clinical study in two terrier breeds. J. Small Anim. Pract. 1999, 40, 257–264. [Google Scholar] [CrossRef]
- Kammersgaard, T.S.; Malmkvist, J.; Pedersen, L.J. Infrared thermography—A noninvasive tool to evaluate thermal status of neonatal pigs based on surface temperature. Animal 2013, 7, 2026–2034. [Google Scholar] [CrossRef]
- Sherwin, C.M.; Christiansen, S.B.; Duncan, I.J.; Erhard, H.W.; Lay, D.C.; Mench, J.A.; O’Connor, C.E.; Petherick, J.C. Guidelines for the ethical use of animals in applied ethology studies. Appl. Anim. Behav. Sci. 2003, 81, 291–305. [Google Scholar] [CrossRef]
- Piccione, G.; Fazio, F.; Giudice, E.; Refinetti, R. Body size and the daily rhythm of body temperature in dogs. J. Therm. Biol. 2009, 34, 171–175. [Google Scholar] [CrossRef]
- Rigotti, C.F.; Jolliffe, C.T.; Leece, E.A. Effect of prewarming on the body temperature of small dogs undergoing inhalation anesthesia. J. Am. Vet. Med. Assoc. 2015, 247, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.J.; Brundage, C.M. Quantifying body surface temperature differences in canine coat types using infrared thermography. J. Therm. Biol. 2019, 82, 18–22. [Google Scholar] [CrossRef]
- Jordan, M.; Bauer, A.E.; Stella, J.L.; Croney, C. Temperature Requirements for Dogs. Available online: https://www.extension.purdue.edu/extmedia/va/va-16-w.pdf (accessed on 1 May 2022).
- Schrank, M.; Mollo, A.; Contiero, B.; Romagnoli, S. Bodyweight at Birth and growth rate during the neonatal period in three canine breeds. Animals 2019, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, M.C.; Faustini, M.; Probo, M.; Rota, A.; Fusi, J. Refining the APGAR score cutoff values and viability classes according to breed body size in newborn dogs. Animals 2022, 12, 1664. [Google Scholar] [CrossRef]
- Harri, M.; Mononen, J.; Haapanen, K.; Korhonen, H. Postnatal changes in hypothermic response in farmborn blue foxes and raccoon dogs. J. Therm. Biol. 1991, 16, 71–76. [Google Scholar] [CrossRef]
- Trangerud, C.; Grøndalen, J.; Indrebø, A.; Tverdal, A.; Ropstad, E.; Moe, L. A longitudinal study on growth and growth variables in dogs of four large breeds raised in domestic environments1. J. Anim. Sci. 2007, 85, 76–83. [Google Scholar] [CrossRef]
- Tattersall, G.J. Infrared thermography: A non-invasive window into termal physiology. Comp. Biol. Physiol. 2016, 202, 78–98. [Google Scholar] [CrossRef] [PubMed]
- Travain, T.; Colombo, E.S.; Grandi, L.C.; Heinzl, E.; Pelosi, A.; Prato Previde, E.; Valsecchi, P. How good is this food? A study on dogs’ emotional responses to a potentially pleasant event using infrared thermography. Physiol. Behav. 2016, 159, 80–87. [Google Scholar] [CrossRef]
- Nitrini, A.G.C.; Cogliati, B.; Matera, J.M. Thermographic assessment of skin and soft tissue tumors in cats. J. Feline Med. Surg. 2021, 23, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Waddell, R.E.; Marino, D.J.; Loughin, C.A.; Tumulty, J.W.; Dewey, C.W.; Sackman, J. Medical infrared thermal imaging of cats with hyperthyroidism. Am. J. Vet. Res. 2015, 76, 53–59. [Google Scholar] [CrossRef]
- Asakura, H. Fetal and neonatal thermoregulation. J. Nippon Med. Sch. 2004, 71, 360–370. [Google Scholar] [CrossRef]
- Pineda, M.; Dooley, M. McDonald’s Veterinary Endocrinology and Reproduction; Wiley-Blackwell: New York, NY, USA, 2008; p. 597. [Google Scholar]
- Lawler, D. Neonatal and pediatric care of the puppy and kitten. Theriogenology 2008, 70, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Le Dividich, J.; Noblet, J. Colostrum intake and thermoregulation in the neonatal pig in relation to environmental temperature. Neonatology 1981, 40, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Mugnier, A.; Chastant, S.; Saegerman, C.; Gaillard, V.; Grellet, A.; Mila, H. Management of low birth weight in canine and feline species: Breeder profiling. Animals 2021, 11, 2953. [Google Scholar] [CrossRef]
- Mila, H.; Feugier, A.; Grellet, A.; Anne, J.; Gonnier, M.; Martin, M.; Rossig, L.; Chastant-Maillard, S. Immunoglobulin G concentration in canine colostrum: Evaluation and variability. J. Reprod. Immunol. 2015, 112, 24–28. [Google Scholar] [CrossRef]
- Chastant-Maillard, S.; Aggouni, C.; Albaret, A.; Fournier, A.; Mila, H. Canine and feline colostrum. Reprod. Domest. Anim. 2017, 52, 148–152. [Google Scholar] [CrossRef]
- Bühler, C.; Hammon, H.; Rossi, G.L.; Blum, J.W. Small intestinal morphology in eight-day-old calves fed colostrum for different durations or only milk replacer and treated with long-R3-insulin-like growth factor I and growth hormone. J. Anim. Sci. 1998, 76, 758. [Google Scholar] [CrossRef] [PubMed]
- Burrin, D.G.; Shulman, R.J.; Reeds, P.J.; Davis, T.A.; Gravitt, K.R. Porcine Colostrum and milk stimulate visceral organ and skeletal muscle protein synthesis in neonatal piglets. J. Nutr. 1992, 122, 1205–1213. [Google Scholar] [CrossRef]
- Schwarz, S.M.; Heird, W.C. Effects of feeding on the small intestinal mucosa of beagle pups during the first 5 d of life. Am. J. Clin. Nutr. 1994, 60, 879–886. [Google Scholar] [CrossRef]
- Bartolomé, E.; Sánchez, M.J.; Molina, A.; Schaefer, A.L.; Cervantes, I.; Valera, M. Using eye temperature and heart rate for stress assessment in young horses competing in jumping competitions and its possible influence on sport performance. Animal 2013, 7, 2044–2053. [Google Scholar] [CrossRef]
- Church, J.S.; Hegadoren, P.R.; Paetkau, M.J.; Miller, C.C.; Regev-Shoshani, G.; Schaefer, A.L.; Schwartzkopf-Genswein, K.S. Influence of environmental factors on infrared eye temperature measurements in cattle. Res. Vet. Sci. 2014, 96, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.; Webster, J.R.; Verkerk, G.A.; Schaefer, A.L.; Colyn, J.J.; Stafford, K.J. Non-invasive measurement of stress in dairy cows using infrared thermography. Physiol. Behav. 2007, 92, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, M.A.; Worth, G.M.; Dowling, S.K.; Lowe, G.L.; Cave, V.M.; Stewart, M. Evaluation of infrared thermography as a non-invasive method of measuring the autonomic nervous response in sheep. PLoS ONE 2020, 15, e0233558. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Cho, G.-J. Validation of eye temperature assessed using infrared thermography as an indicator of welfare in horses. Appl. Sci. 2021, 11, 7186. [Google Scholar] [CrossRef]
- Lezama-García, K.; Mota-Rojas, D.; Martínez-Burnes, J.; Villanueva-García, D.; Domínguez-Oliva, A.; Gómez-Prado, J.; Mora-Medina, P.; Casas-Alvarado, A.; Olmos-Hernández, A.; Soto, P.; et al. Strategies for hypothermia compensation in altricial and precocial newborn mammals and their monitoring by infrared thermography. Vet. Sci. 2022, 9, 246. [Google Scholar] [CrossRef]
- Hillman, N.H.; Kallapur, S.G.; Jobe, A.H. Physiology of transition from intrauterine to extrauterine life. Clin. Perinatol. 2012, 39, 769–783. [Google Scholar] [CrossRef]
- Mallet, M.L. Pathophysiology of accidental hypothermia. QJM 2002, 95, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Oncken, A.; Kirby, R.; Rudloff, E. Hypotherma in critically ill dogs and cats. Compend. Contin. Educ. Pract. Vet. 2001, 23, 506–520. [Google Scholar]
- Dwyer, C.M.; Conington, J.; Corbiere, F.; Holmøy, I.H.; Muri, K.; Nowak, R.; Rooke, J.; Vipond, J.; Gautier, J.M. Invited review: Improving neonatal survival in small ruminants: Science into practice. Animal 2016, 10, 449–459. [Google Scholar] [CrossRef]
- Kozat, S. Hypothermia in newborn calves. J. Istanb. Vet. Sci. 2018, 2, 30–37. [Google Scholar] [CrossRef]
- Nelson, S.M.; Matthews, P.; Poston, L. Maternal metabolism and obesity: Modifiable determinants of pregnancy outcome. Hum. Reprod. Update 2010, 16, 255–275. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, L.J.; Hawkins, S.S.; Cole, T.J.; Dezateux, C.; Millennium Cohort Study Child Health Group. Risk factors for rapid weight gain in preschool children: Findings from UK-wide prospective study. Int. J. Obes. 2010, 34, 624–632. [Google Scholar] [CrossRef] [PubMed]

| Time | G1 n = 47 | G2 n = 68 | G3 n = 79 | G4 n = 96 |
|---|---|---|---|---|
| Wet | 27.43 ± 0.065 c,5 | 27.76 ± 0.051 b,6 | 27.80 ± 0.046 b,6 | 28.29 ± 0.042 a,6 |
| Dry | 28.27 ± 0.120 b,3,4 | 28.61 ± 0.114 b,3,4 | 28.65 ± 0.114 b,3,4 | 29.19 ± 0.096 a,3,4 |
| Colostrum | 27.75 ± 0.057 c,4,5 | 28.11 ± 0.047 b,5 | 28.20 ± 0.049 b,5 | 28.72 ± 0.045 a,5 |
| 30 min AB | 28.03 ± 0.104 c,4 | 28.33 ± 0.093 b,c,4,5 | 28.40 ± 0.075 b,4,5 | 28.91 ± 0.073 a,4,5 |
| 1 h AB | 28.53 ± 0.094 c,3 | 28.86 ± 0.078 b,3 | 28.95 ± 0.075 b,3 | 29.47 ± 0.063 a,3 |
| 4 h AB | 29.43 ± 0.110 c,2 | 29.85 ± 0.096 b,2 | 29.91 ± 0.082 b,2 | 30.38 ± 0.076 a,2 |
| 24 h AB | 30.59 ± 0.103 c,1 | 30.95 ± 0.083 b,1 | 30.95 ± 0.070 b,1 | 31.45 ± 0.068 a,1 |
| Time | G1 n = 47 | G2 n = 68 | G3 n = 79 | G4 n = 96 |
|---|---|---|---|---|
| Wet | 27.11 ± 0.057 c,5 | 27.30 ± 0.051 b,c,7 | 27.42 ± 0.047 b,7 | 28.01 ± 0.039 a,4 |
| Dry | 30.78 ± 0.074 c,4 | 30.96 ± 0.058 b,c,4 | 31.11 ± 0.058 b,4 | 31.40 ± 0.060 a,3 |
| Colostrum | 30.29 ± 0.067 c,3 | 30.52 ± 0.048 b,c,5 | 30.64 ± 0.049 b,5 | 30.87 ± 0.078 a,2 |
| 30 min AB | 31.04 ± 0.059 c,4 | 31.23 ± 0.046 b,c,3 | 31.34 ± 0.044 a,b,3 | 31.57 ± 0.086 a,3 |
| 1 h AB | 30.04 ± 0.071 c,3 | 30.22 ± 0.061 b,c,6 | 30.36 ± 0.060 b,6 | 30.84 ± 0.055 a,2 |
| 4 h AB | 32.13 ± 0.068 b,2 | 32.33 ± 0.057 a,b,2 | 32.47 ± 0.059 a,2 | 32.56 ± 0.078 a,1 |
| 24 h AB | 32.81 ± 0.073 a,b,1 | 33.05 ± 0.066 b,1 | 33.12 ± 0.059 b,1 | 32.62 ± 0.094 a,1 |
| Time | G1 n = 47 | G2 n = 68 | G3 n = 79 | G4 n = 96 |
|---|---|---|---|---|
| Wet | 26.98 ± 0.073 c,5 | 27.14 ± 0.061 b,c,5 | 27.30 ± 0.054 b,5 | 27.75 ± 0.056 a,6 |
| Dry | 29.84 ± 0.073 c,2,3 | 30.02 ± 0.062 b,c,2,3 | 30.19 ± 0.062 b,2 | 30.46 ± 0.063 a,4 |
| Colostrum | 28.96 ± 0.043 c,4 | 29.16 ± 0.033 b,4 | 29.25 ± 0.036 b,4 | 29.71 ± 0.031 a,7 |
| 30 min AB | 29.43 ± 0.10 b,3,4 | 29.63 ± 0.078 b,3 | 29.73 ± 0.081 b,3 | 30.14 ± 0.072 a,5 |
| 1 h AB | 30.24 ± 0.063 c,1,2 | 30.38 ± 0.058 b,c,2 | 30.49 ± 0.053 b,2 | 30.95 ± 0.047 a,3 |
| 4 h AB | 30.08 ± 0.33 b,2 | 30.37 ± 0.24 b,2 | 30.46 ± 0.20 b,2 | 31.19 ± 0.052 a,2 |
| 24 h AB | 30.83 ± 0.10 b,1 | 30.89 ± 0.091 b,1 | 31.04 ± 0.083 b,1 | 31.54 ± 0.073 a,1 |
| Time | G1 n = 47 | G2 n = 68 | G3 n = 79 | G4 n = 96 |
|---|---|---|---|---|
| Wet | 28.79 ± 0.18 a,6 | 28.80 ± 0.19 a,6 | 29.06 ± 0.13 a,5 | 29.20 ± 0.13 a,5 |
| Dry | 31.44 ± 0.049 c,5 | 31.64 ± 0.042 b,5 | 31.69 ± 0.040 b,4 | 31.93 ± 0.035 a,4 |
| Colostrum | 31.66 ± 0.074 b,4,5 | 31.87 ± 0.056 b,4,5 | 31.86 ± 0.052 b,4 | 32.16 ± 0.047 a,4 |
| 30 min AB | 31.99 ± 0.052 c,3,4 | 32.17 ± 0.039 b,3,4 | 32.26 ± 0.037 b,3 | 32.51 ± 0.033 a,2 |
| 1 h AB | 32.12 ± 0.063 c,2,3 | 32.35 ± 0.052 b,2,3 | 32.38 ± 0.045 b,2,3 | 32.62 ± 0.041 a,2 |
| 4 h AB | 32.40 ± 0.066 c,2 | 32.68 ± 0.054 b,2 | 32.63 ± 0.059 b,c,2 | 32.92 ± 0.050 a,2 |
| 24 h AB | 33.22 ± 0.054 c,1 | 33.38 ± 0.052 b,c,1 | 33.49 ± 0.045 b,1 | 33.72 ± 0.041 a,1 |
| Time | G1 n = 47 | G2 n = 68 | G3 n = 79 | G4 n = 96 |
|---|---|---|---|---|
| Wet | 29.47 ± 0.12 b,6 | 29.77 ± 0.10 a,b,5 | 29.76 ± 0.098 a,b,5 | 29.95 ± 0.085 a,5 |
| Dry | 32.23 ± 0.046 c,5 | 32.49 ± 0.037 b,4 | 32.55 ± 0.037 b,4 | 32.75 ± 0.032 a,4 |
| Colostrum | 32.46 ± 0.075 b,4,5 | 32.66 ± 0.062 b,4 | 32.59 ± 0.060 b,4 | 32.90 ± 0.054 a,4 |
| 30 min AB | 32.75 ± 0.073 b,3,4 | 32.99 ± 0.061 b,3 | 32.96 ± 0.060 b,3 | 33.22 ± 0.055 a,3 |
| 1 h AB | 32.84 ± 0.086 b,3 | 33.09 ± 0.072 a,b,3 | 33.08 ± 0.063 a,b,3 | 33.30 ± 0.061 a,3 |
| 4 h AB | 33.20 ± 0.083 b,2 | 33.46 ± 0.068 a,b,2 | 33.38 ± 0.070 b,2 | 33.63 ± 0.063 a,2 |
| 24 h AB | 33.74 ± 0.061 c,1 | 33.92 ± 0.060 b,c,1 | 34.02 ± 0.053 b,1 | 34.23 ± 0.047 a,1 |
| Time | G1 n = 47 | G2 n = 68 | G3 n = 79 | G4 n = 96 |
|---|---|---|---|---|
| Wet | 27.54 ± 0.071 c,6 | 27.90 ± 0.057 b,6 | 27.97 ± 0.051 b,5 | 28.29 ± 0.046 a,5 |
| Dry | 28.49 ± 0.12 b,3,4 | 28.83 ± 0.11 b,3,4 | 28.84 ± 0.11 b,3 | 29.24 ± 0.095 a,3 |
| Colostrum | 27.86 ± 0.065 c,5,6 | 28.25 ± 0.057 b,5,6 | 28.39 ± 0.062 b,4 | 28.72 ± 0.055 a,4 |
| 30 min AB | 28.15 ± 0.12 b,4,5 | 28.54 ± 0.10 b,4,5 | 28.51 ± 0.087 b,4 | 28.89 ± 0.085 a,4 |
| 1 h AB | 28.61 ± 0.097 c,3 | 28.97 ± 0.084 b,3 | 29.03 ± 0.077 b,3 | 29.40 ± 0.066 a,3 |
| 4 h AB | 29.62 ± 0.082 c,2 | 30.12 ± 0.071 b,2 | 30.08 ± 0.064 b,2 | 30.43 ± 0.061 a,2 |
| 24 h AB | 30.70 ± 0.11 c,1 | 31.06 ± 0.088 b,c,1 | 31.15 ± 0.087 b,1 | 31.46 ± 0.075 a,1 |
| Time | G1 n = 47 | G2 n = 68 | G3 n = 79 | G4 n = 96 |
|---|---|---|---|---|
| Wet | 28.09 ± 0.079 c,3 | 28.53 ± 0.069 b,3 | 28.64 ± 0.061 b,3 | 29.05 ± 0.055 a,3 |
| Dry | 29.56 ± 0.067 c,1,2 | 30.02 ± 0.059 b,1,2 | 30.07 ± 0.053 b,1,2 | 30.52 ± 0.046 a,1,2 |
| Colostrum | 29.32 ± 0.090 c,1,2 | 29.79 ± 0.081 b,2 | 29.89 ± 0.072 b,2 | 30.28 ± 0.065 a,2 |
| 30 min AB | 29.20 ± 0.098 c,2 | 29.65 ± 0.088 b,2 | 29.75 ± 0.080 b,2 | 30.17 ± 0.070 a,2 |
| 1 h AB | 29.31 ± 0.18 b,1,2 | 29.66 ± 0.15 b,2 | 29.81 ± 0.14 a,b,2 | 30.21 ± 0.12 a,2 |
| 4 h AB | 29.20 ± 0.19 c,2 | 29.64 ± 0.15 b,c,2 | 29.89 ± 0.14 a,b,2 | 30.35 ± 0.12 a,2 |
| 24 h AB | 29.75 ± 0.14 c,1 | 30.26 ± 0.11 b,1 | 30.35 ± 0.097 a,b,1 | 30.68 ± 0.093 a,1 |
| Time | G1 n = 47 | G2 n = 68 | G3 n = 79 | G4 n = 96 |
|---|---|---|---|---|
| Wet | 30.69 ± 0.079 b,5 | 30.78 ± 0.066 b,5 | 30.83 ± 0.060 b,5 | 31.09 ± 0.058 a,5 |
| Dry | 31.56 ± 0.097 b,4 | 31.67 ± 0.084 a,b,4 | 31.68 ± 0.081 a,b,4 | 31.95 ± 0.075 a,4 |
| Colostrum | 32.00 ± 0.089 b,3 | 32.01 ± 0.077 b,3 | 32.07 ± 0.068 b,3 | 32.39 ± 0.062 a,3 |
| 30 min AB | 32.88 ± 0.063 b,2 | 32.92 ± 0.054 b,2 | 32.99 ± 0.049 b,2 | 33.30 ± 0.045 a,2 |
| 1 h AB | 32.85 ± 0.048 c,2 | 32.93 ± 0.040 b,c,2 | 33.03 ± 0.036 b,2 | 33.29 ± 0.032 a,2 |
| 4 h AB | 33.02 ± 0.095 b,2 | 33.01 ± 0.079 b,2 | 33.16 ± 0.073 b,2 | 33.48 ± 0.069 a,2 |
| 24 h AB | 33.62 ± 0.066 b,1 | 33.65 ± 0.050 b,1 | 33.79 ± 0.048 b,1 | 34.07 ± 0.044 a,1 |
| Variables | Correlation Coefficient (r) | p-Value |
|---|---|---|
| Wet | 0.540 | <0.0001 |
| Dry | 0.301 | <0.0001 |
| Colostrum | 0.601 | <0.0001 |
| 30 min AB | 0.399 | <0.0001 |
| 1 h AB | 0.435 | <0.0001 |
| 4 h AB | 0.369 | <0.0001 |
| 24 h AB | 0.365 | <0.0001 |
| Variables | Correlation Coefficient (r) | p-Value |
|---|---|---|
| Wet | 0.610 | <0.0001 |
| Dry | 0.380 | <0.0001 |
| Colostrum | 0.326 | <0.0001 |
| 30 min AB | 0.286 | <0.0001 |
| 1 h AB | 0.484 | <0.0001 |
| 4 h AB | 0.222 | <0.001 |
| 24 h AB | −0.177 | 0.0026 |
| Variables | Correlation Coefficient (r) | p-Value |
|---|---|---|
| Wet | 0.475 | <0.0001 |
| Dry | 0.358 | <0.0001 |
| Colostrum | 0.634 | <0.0001 |
| 30 min AB | 0.334 | <0.0001 |
| 1 h AB | 0.485 | <0.0001 |
| 4 h AB | 0.241 | <0.0001 |
| 24 h AB | 0.342 | <0.0001 |
| Variables | Correlation Coefficient (r) | p-Value |
|---|---|---|
| Wet | 0.127 | 0.031 |
| Dry | 0.429 | <0.0001 |
| Colostrum | 0.316 | <0.0001 |
| 30 min AB | 0.467 | <0.0001 |
| 1 h AB | 0.342 | <0.0001 |
| 4 h AB | 0.286 | <0.0001 |
| 24 h AB | 0.391 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lezama-García, K.; Martínez-Burnes, J.; Pérez-Jiménez, J.C.; Domínguez-Oliva, A.; Mora-Medina, P.; Olmos-Hernández, A.; Hernández-Ávalos, I.; Mota-Rojas, D. Relation between the Dam’s Weight on Superficial Temperature of Her Puppies at Different Stages of the Post-Partum. Vet. Sci. 2022, 9, 673. https://doi.org/10.3390/vetsci9120673
Lezama-García K, Martínez-Burnes J, Pérez-Jiménez JC, Domínguez-Oliva A, Mora-Medina P, Olmos-Hernández A, Hernández-Ávalos I, Mota-Rojas D. Relation between the Dam’s Weight on Superficial Temperature of Her Puppies at Different Stages of the Post-Partum. Veterinary Sciences. 2022; 9(12):673. https://doi.org/10.3390/vetsci9120673
Chicago/Turabian StyleLezama-García, Karina, Julio Martínez-Burnes, Juan Carlos Pérez-Jiménez, Adriana Domínguez-Oliva, Patricia Mora-Medina, Adriana Olmos-Hernández, Ismael Hernández-Ávalos, and Daniel Mota-Rojas. 2022. "Relation between the Dam’s Weight on Superficial Temperature of Her Puppies at Different Stages of the Post-Partum" Veterinary Sciences 9, no. 12: 673. https://doi.org/10.3390/vetsci9120673
APA StyleLezama-García, K., Martínez-Burnes, J., Pérez-Jiménez, J. C., Domínguez-Oliva, A., Mora-Medina, P., Olmos-Hernández, A., Hernández-Ávalos, I., & Mota-Rojas, D. (2022). Relation between the Dam’s Weight on Superficial Temperature of Her Puppies at Different Stages of the Post-Partum. Veterinary Sciences, 9(12), 673. https://doi.org/10.3390/vetsci9120673







