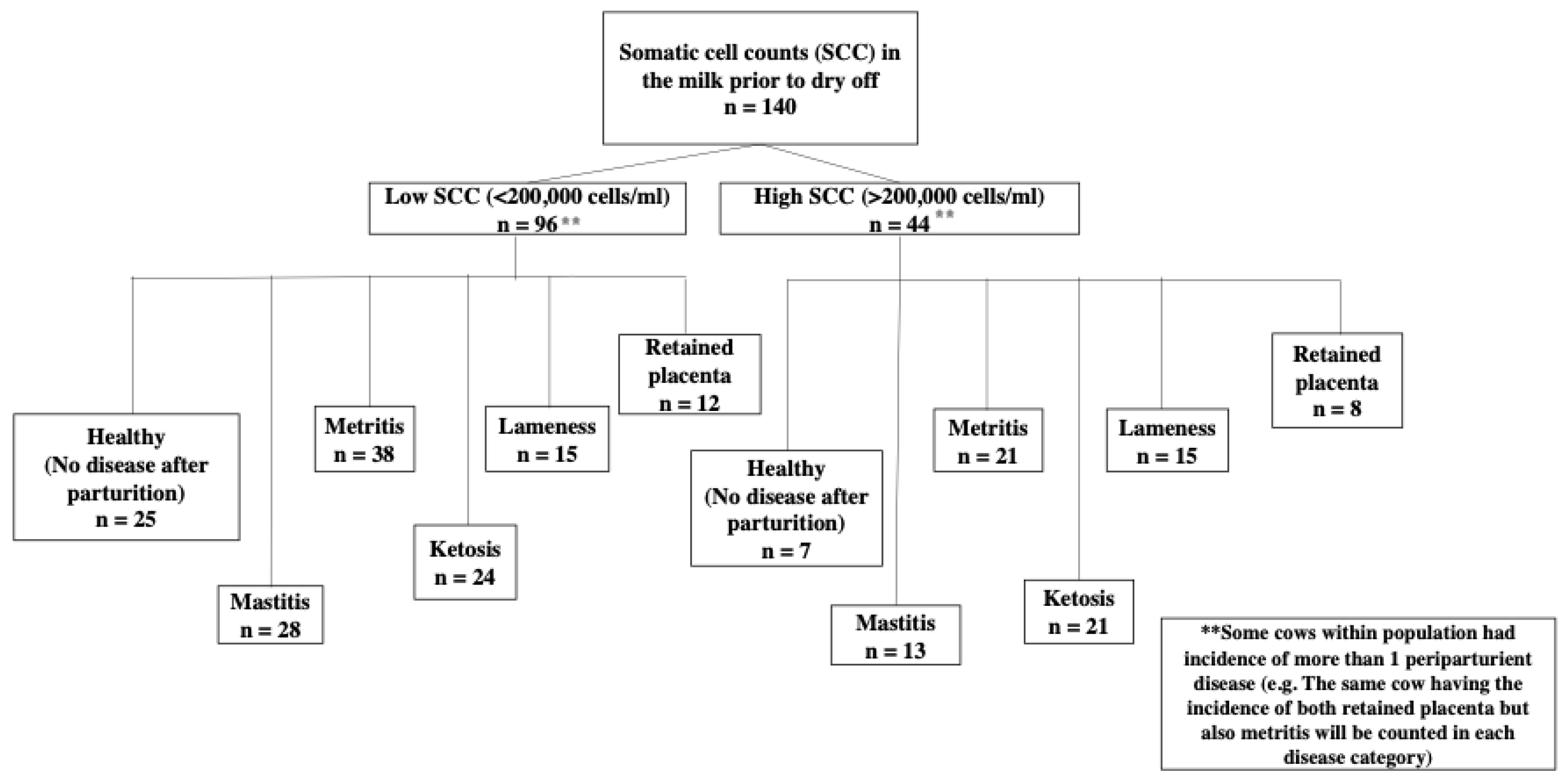
We hypothesized that high SCC in the milk of dairy cow prior to drying off is linked to a higher incidence of postpartum disease, as well as changes in milk composition and milk yield. Cows with high SCC did, in fact, have a higher incidence of periparturient diseases, most notably ketosis. For all diseases studied, milk composition analysis revealed that cows with high SCC had a lower concentration of lactose prior to dry off. Concentrations of protein in the milk were also higher in the HD group prior to dry off, decreasing in the first week after parturition and even more in the second week of lactation. The number of somatic cells in the milk after parturition was higher in the HD group for cows with retained placenta. In mastitis cows, on the other hand, the LD group had higher SCC after parturition than the HD group. For diseases such as mastitis, retained placenta, and ketosis, milk yield was lower in the HD group.
4.1. Relation of SCC to Incidence of Periparturient Diseases
To the best of our knowledge, this is the first study to show an association between high SCC before drying off and the incidence of peripartum disease in dairy cows. The most intriguing finding from this study was that ketosis was more common in cows with high SCC before drying off. In fact, a cow with high SCC before drying off was 166% more likely than a cow with low SCC to develop ketosis in the first two weeks after parturition. High SCC in milk indicates subclinical mastitis. A possible explanation for cows with high SCC before drying off being more prone to ketosis or other periparturient diseases could be systemic endotoxemia during dry period. This hypothesis is based on the suggestion by Eckel and Ametaj [
28] that there are three sources of bacterial endotoxins in dairy cows: mammary gland, reproductive tract, and rumen. In fact, the mammary gland is the only source of endotoxin during the dry period. The rumen and uterus are only sources of endotoxins after calving, when the uterus can become infected with pathogenic bacteria causing metritis immediately after calving, whereas grain feeding after parturition is associated with the release of large quantities of endotoxins in the rumen. We hypothesize that endotoxin is translocated from the mammary gland into the systemic circulation of cows with high SCC due to infection but also due to antibiotic treatment at drying off. This suggests that whereas the antibiotic kills the pathogenic bacteria in the mammary gland, the endotoxin released by the dead bacteria escapes into the systemic circulation and triggers a systemic inflammatory response. In fact, our laboratory [
34] reported that cows with postpartum ketosis had higher serum BHBA concentrations 4 weeks before calving. We also showed that blood concentrations of tumor necrosis factor alpha (TNF-a), haptoglobin (Hp), and interleukin-6 (IL-6) were higher in pre-ketotic cows 4 weeks before calving and during disease diagnosis than in healthy controls at the start of ketosis (1–3 weeks after birth). Additionally, [
35] confirmed those findings in ketotic cows, demonstrating increased blood concentrations of LPS prepartum and of serum amyloid A (SAA), Hp, and lipopolysaccharide binding protein (LBP) postpartum versus healthy controls.
Previous research on both natural and experimentally induced mastitis, in which LPS concentrations were observed in the plasma of dairy cows with mastitis, support the possibility of mammary gland endotoxin entering the systemic circulation [
29,
30]. A study from our laboratory [
36] showed that 10 days before calving, blood levels of beta-hydroxybutyric acid (BHBA) increased after parenteral treatment with LPS at increasing doses around parturition. High levels of BHBA are known to be associated with ketosis in dairy cows [
37]. In another study [
34], we showed increases in serum BHBA in pre-ketotic cows 4 weeks before calving, suggesting that endotoxin insults could potentially contribute to increased BHBA. Beta-hydroxybutyric acid has been shown to inhibit NLRP3 macrophage inflammasome activation. When the NLRP3 inflammasome is activated, pro-inflammatory cytokines such as TNF-α, IL-1, IL-6 and acute phase proteins are released [
38]. Consequently, the susceptibility to ketosis of cows with an elevated SCC could be attributed, inter alia, to the translocation of endotoxin into the systemic circulation before drying off and during the dry period, contributing to an increase in ketone bodies during the dry period to control the inflammatory response.
Odds ratio data showed that in cows with elevated SCC before drying off, the likelihoods of metritis, retained placenta, and lameness were increased by 43%, 56% and 31%, respectively. However,
p-values were not statistically significant, implying that other factors may contribute to the incidence of postpartum disease. For example, abortion, twins, dystocia, and short gestation have been associated with retained placenta. Because some of the cows in this study suffered from multiple periparturient diseases, it makes sense that cows with retained placenta are also more prone to metritis. The occurrence of metritis can also be influenced by bacterial infection of the uterus after calving [
23]. Postpartum grain feeding has been strongly associated with an increased incidence of lameness [
39]. Elevated SCC prior to drying off (i.e., subclinical mastitis) may play a role in the etiopathology of metritis, retained placenta, and lameness, possibly through translocation of bacterial endotoxins from mammary glands with elevated SCC; however, more research is needed on this topic.
Bacterial endotoxins and inflammatory cytokines, released from the mammary gland of cows with elevated SCC into the systemic circulation, may contribute or increase the likelihood of these three periparturient diseases. Systemic and local administrations of LPS resulted in lesions in the corium and epidermis of the hoof region, implicating a role of endotoxin in the pathogenesis of lameness [
24]. In addition, intermittent and increasing doses of LPS over 3 weeks before calving is associated with an increased risk of retained placenta [
36]. Exposure of neutrophils to LPS can induce LPS tolerance, leading to decreased expression of TLR4 and compromised host immune response [
40]. Tolerance to LPS may explain why cows with high SCC were 56% more likely to retain the placenta.
The reproductive tract, along with the mammary gland and rumen of dairy cows, has been suggested as a source of endotoxin by [
28]. Conversely, mammary gland infections can affect the reproductive tract in dairy cows. For example, induction of mastitis in cows with
Escherichia coli (
E. coli) LPS, has been shown to decrease follicular estrogen, androstenedione, and progesterone by 40%, 13%, and 35%, respectively [
41]. The latter authors also found a 56% reduction in circulating concentrations of estrogen in mastitis induced by
Staphylococcus aureus (
S. aureus). Furthermore, systemic endotoxin can impair the release of hormones from the hypothalamus and pituitary gland. Battagila et al. [
42] demonstrated this in ewes by slowing or blocking luteinizing hormone and follicular-stimulating hormone surges, which interfere with the pre-ovulatory increase in estrogen. Similarly, in dairy cows, this type of delayed response can lead to poor reproductive performance. Therefore, cows with high SCC and increased risk of metritis may also have poor reproductive performance.
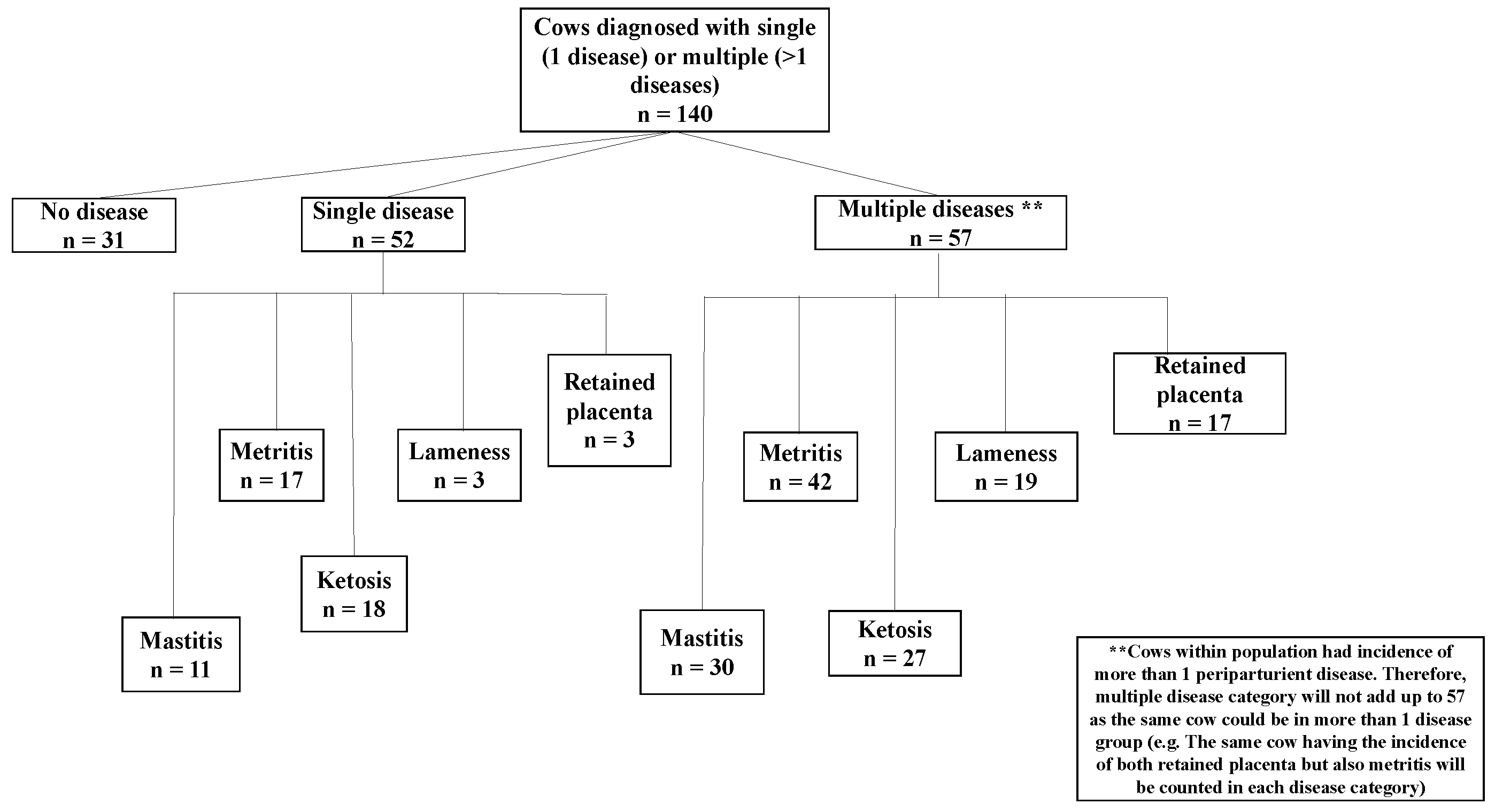
Additionally, previous research by our team found changes in innate immune reactants from 8 and 4 weeks prior to parturition in cows with retained placenta, metritis, lameness, ketosis, and mastitis [
10,
26,
34,
43,
44]. Results from previous studies indicate that dairy cows prior to calving are in a low-grade chronic inflammatory state starting as early as 2 months prior to parturition. Although we did not analyze changes in innate immune reactants prior to cows drying off, it is hypothesized that cows with elevated SCC experience an inflammatory state at the end of the lactation cycle and during the dry period. The odds ratios for the incidence of mastitis for the LD and HD groups were 0.98 and 1.02, respectively, suggesting other factors may predispose cows to post-partum mastitis. These could include the inefficiency of the dry cow therapeutic treatment, insufficient sealant secretion in the teat canal, or sealant before depletion prior to calving [
19].
4.2. Alterations in Milk Composition
Cows who had high SCC before being diagnosed with post-partum disease had significantly higher SCC in their milk postpartum. Significant differences in SCC between groups and diseases were expected. The HD group that was diagnosed with retained placenta after parturition had elevated SCC after calving. In comparison, the SCC after parturition in the LD group remained <200,000 cells/mL. Previously, elevated SCC were observed in cows diagnosed with retained placenta during the diagnosis week, however, the SCC were within the normal range [
10]. The cows in the current study had a significantly higher SCC for the HD group at 1 week after calving, which was the week of diagnosis of retained placenta. At 2 weeks postpartum, the SCC was slightly higher than the subclinical mastitis cut off value. The possible reason for the high SCC in all three time periods measured in the retained placenta group could be attributed to the number of diseases the cows were experiencing when diagnosed with retained placenta. The effect of the number of diseases (multiple diseases) was taken into account when modeling SCC for retained placenta, but this effect was not significant, suggesting that other factors may contribute to the increase in SCC after parturition in cows with retained placenta.
The number of somatic cells in the mastitis group increased after parturition in the LD and HD groups. The LD group may have had a new IMI during the dry off period, leading to increased SCC in milk after parturition. According to [
19], 95% of all new IMI occur between 2 and 3 weeks before calving. The incidence of IMI has been reported to be highest at the start of the dry off period and towards the end of the pregnancy [
45]. Furthermore, the type of bacterial strain could be a factor in low SCC cows becoming sick or high SCC cows still having a high incidence of mastitis after drying off. For example, bacterial infection by
S. aureus has been found to be more frequent at the start of lactation [
46]. Moreover, the host immune response to
S. aureus infections has been reported to be weaker (Bannerman et al., 2004). The slow response could be attributed to
S. aureus biofilm formation, which protects the pathogen from neutrophil phagocytosis [
47]. It would be interesting to perform an analysis of the bacterial strains of the milk microbiota in cows with low and high SCC in order to determine the type of pathogens that can cause mastitis.
Cows in the HD group prepartum who were diagnosed with lameness, ketosis, or metritis after parturition had normal SCC in the milk postpartum. It has previously been shown that cows diagnosed with metritis, ketosis, and lameness have a higher SCC during disease diagnosis than healthy cows; however, the number of SCC was within the normal range, below 200,000 cells/mL, which is consistent with the current study [
34,
43,
44]. Several authors have suggested that the decrease in milk SCC for the HD group may be due to the sensation of pain associated with hoof inflammation, and that cows may be standing more than lying down, as the lying position is extremely painful for lame cows [
48]. However, this remains controversial. Archer et al. [
25] attempted to find an association between milk SCC and lameness. Those authors showed that lame cows had lower SCC than non-lame cows and concluded that lame cows spend more time standing than lying down, reducing mammary gland exposure to bedding bacteria [
25].
In all diseases, lowered lactose concentrations in the milk were observed in the HD group. The mammary gland consists of a network of alveoli that are lined with mammary epithelial cells (MECs) that secrete milk and are connected by tight junctions to prevent milk from entering into the systemic circulation [
49]. During infections, an influx of leukocytes into the mammary gland to remove pathogens causes an increase in the gap between the epithelial cells [
15,
50]. The wider gap allows lactose to escape into the systemic circulation, which is supported by several authors who have observed increases in lactose concentrations in the blood and urine of cows with mastitis [
15,
50]. Other factors that may contribute to the degradation of lactose in milk include the ability of some bacterial serotypes to use lactose for their needs [
15], as well as physical damage to the MECs, resulting in reduced lactose synthesis. Furthermore, lactose acts as the primary osmotic regulator for milk synthesis [
8], so if lactose concentrations in milk decrease, milk production will also decrease in cows with a high SCC. In addition, pro-inflammatory cytokines, as well as pathogens and their associated endotoxins, are believed to play a role in lactose synthesis. TNF-a, a potent pro-inflammatory cytokine, has been shown to influence the lactose secretion pathway by downregulating lactose synthesis-related genes such as a-lactalbumin gene and the degradation glucose transporter-1 (GLUT1) from the basolateral membrane [
51]. As a result, the decrease in lactose in cows with high SCC can be attributed to an increase in inflammatory mediators during infection, as well as the suppression of genes involved in lactose synthesis.
Moreover, we identified changes in milk protein concentrations for lameness over all time periods, ketosis in the first and second weeks after calving, and retained placenta that had a tendency prior to dry off period. Interestingly, for all diseases studied, concentrations of protein in the milk, in the HD group were higher before dry-off and lowered after calving. During a mammary gland infection, the influx of blood-borne proteins, such as serum albumin and immunoglobulins, increases during a mammary gland infection, due to the immune response [
7,
8,
15]. A study using proteomics in bovine milk from mastitis cows, identified proteins involved in the immune response, including lactoferrin, transferrin, fibrinogen, apolipoprotein A1, glycosylation-dependent cell adhesion molecule-1, peptidoglycan recognition proteins, as well as cathelicidin-1 [
52]. Furthermore, lactoferrin (an iron-binding protein) has been reported to increase nearly 100-fold during the involution phase of the dry period to prevent iron utilization by iron-consuming bacteria. Lameness has previously been shown to be associated with a decrease in both milk protein and milk fat.
Additionally, key milk proteins have been shown to decrease during infection, including casein and whey proteins such as a-lactalbumin and b-lactoglobulin. The decrease in milk proteins can be caused by bacterial proteinases, leakage of proteins from the mammary gland, and a decrease in synthesis due to damage to the MECs [
8]. Furthermore, secretion of TNF-a in rats has been shown to suppress both transcriptional and posttranscriptional gene expression for b-casein [
53]. Under normal conditions, TNF-a is important for the proliferation and differentiation of MECs in the mammary glands of rats [
54]. In dairy cows, the increase in TNF-a along with other pro-inflammatory cytokines is important for the host’s immune response to infection and can therefore inhibit the expression of milk proteins.
Strong associations between milk protein concentration and energy balance have been shown, with low milk protein content reflecting negative energy balance (NEB) and poor reproductive performance [
55]. A negative energy balance is strongly associated with ketosis, especially during early lactation when the energy demand for milk production is high [
56]. We observed a reduction in milk protein after calving in cows diagnosed with ketosis, with the HD group showing the lowest concentration. The decrease in milk protein suggests that cows suffer NEB and have insufficient feed intake. Additionally, neutrophil granules contain both enzymes and antibacterial peptides which are important for killing bacteria during infection but can also change milk protein synthesis during infection [
57].
Additionally, endotoxins or pro-inflammatory cytokines translocated from the mammary gland of cows with high SCC cows during the dry period may play a role in NEB and indirectly contribute to low milk protein, leading to the development of ketosis. Systemic circulation of pro-inflammatory cytokines triggers the expression of acute phase proteins from the liver [
58]. Reports from LPS-induced mastitis models have shown induction of transcriptome response by the liver and increased expression of acute phase protein-related genes [
59]. Zhang et al., (2016) observed an upregulation of TNF-a and serum amyloid A in ketotic cows during the week of disease diagnosis and 8 and 4 weeks before calving. The current study has confirmed that the onset of ketosis is significantly associated with high SCC before drying off, and decreased milk protein concentration in the HD group, further supporting this hypothesis.
In the present study, there was a difference in the FPR in the HD group diagnosed with ketosis 2 weeks after calving. The FPR has been proposed as an indicator for the diagnosis of cows in ketosis [
60]. Several authors have proposed different threshold values to diagnose ketosis using FPR values. Heuer et al. [
60] found that cows with an FPR of >1.5 had an increased risk of clinical ketosis. The same authors also reported that at that cut-off there was a higher incidence of other post-partum diseases, including displaced abomasum, ovarian cysts, lameness, and mastitis [
60]. Other researchers have reported an increased incidence of retained placenta, displaced abomasum, metritis, endometritis, and risk of culling at an FPR > 2.0 at 7 DIM [
61]. Cows in the HD group diagnosed with ketosis in our study had higher FPR before drying off (1.33 ± 0.19), 1 week (1.57 ± 0.20) and at 2 weeks (1.71 ± 0.19) after parturition compared to cows from the HG and LD groups, further showing that cows with high SCC before drying off are more prone to onset of ketosis.
Changes in milk fat content showed no differences between the groups for four diseases, whereas cows with metritis tended to have higher milk fat content before drying off. Milk fat concentrations were higher in the HD group of pre-metritic cows. Previously, milk fat concentrations were lowest in cows during the week of metritis diagnosis [
44]. On the other hand, [
50] reported an increase in milk fat and a decrease in milk lactose during mammary gland infections. Enlarged gaps between the MEC tight junctions lead to leakage of milk components from the mammary gland; however, because milk fat globules are too large to move through the tight junctions, they remain in the mammary gland [
14]. The increase in milk fat concentration of HD group cows before drying off can be attributed to the decrease in other milk components during infection and is less likely to be associated with the occurrence of metritis.
There was no difference in MUN and TS concentrations between the three disease incidence groups (HG, HD, and LD) and will not be discussed any further here.
4.3. Alterations in Milk Production
Milk production was shown to be lower in all diseases studied during the first 60 DIM, with retained placenta, mastitis, and ketosis being the most important in the loss of production in cows with a high SCC at drying off. We have previously found that cows with metritis, mastitis, retained placenta, lameness, and ketosis have lower daily milk production [
10,
26,
34,
43,
44]. The emergence of new IMI can have a significant impact on both milk synthesis and secretion, leading to a decrease in milk yield [
62]. Decreased milk production associated with elevated SCC may be due to bacterial infection, causing influx of leukocytes in the mammary gland and the secretion of inflammatory mediators, leading to rupture of tight junctions [
15,
50].
Furthermore, decreased lactose synthesis may contribute to the loss of milk production [
8], which was identified in the HD group of this study. Further to that, prolactin released from anterior pituitary gland regulates both cellular and humoral immune responses as well as milk yield. There is an increase in prolactin secretion towards the end of pregnancy, which stimulates the proliferation of the alveoli in the mammary gland, resulting in increased milk production after calving [
16]. External LPS has been shown to activate the hypothalamic-pituitary–adrenocortical axis and cause the release of pro-inflammatory cytokines [
63]. Additionally, pro-inflammatory cytokines have been shown to inhibit prolactin secretion in rodents [
64]. Therefore, it is hypothesized that high SCC in cows with lower postpartum milk production may be related to suppression of prolactin secretion by the pituitary.
The dry period is critical for the regeneration of milk-secreting cells in the mammary gland [
16]. Our results on reduced milk production in cows with high SCC suggest that inflammation of the mammary gland before drying off influences milk production and milk composition in the following lactation. In addition, cows with a high SCC had lower daily milk production than cows with low SCC. This is particularly important for producers, as cows with a high SCC before dry period can lead to large economic losses during the next lactation. The additional management and labor costs, the higher culling rate, and milk waste are all negative effects of the high SCC on the farm profitability [
65]. As a result, better management of late lactating dairy cows may be needed before the dry off period to increase future production and reduce disease incidence. This includes screening the cows for SCC prior to drying them off. Finally, further research on the etiological factors of cows with high SCC and their association with the incidence of periparturient diseases is needed to gain a better understanding of the pathological mechanisms involved in the disease process, the health of dairy cows during the periparturient period, and reduce to a minimum the loss of production.