Toothed Whales Have Black Neurons in the Blue Spot
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
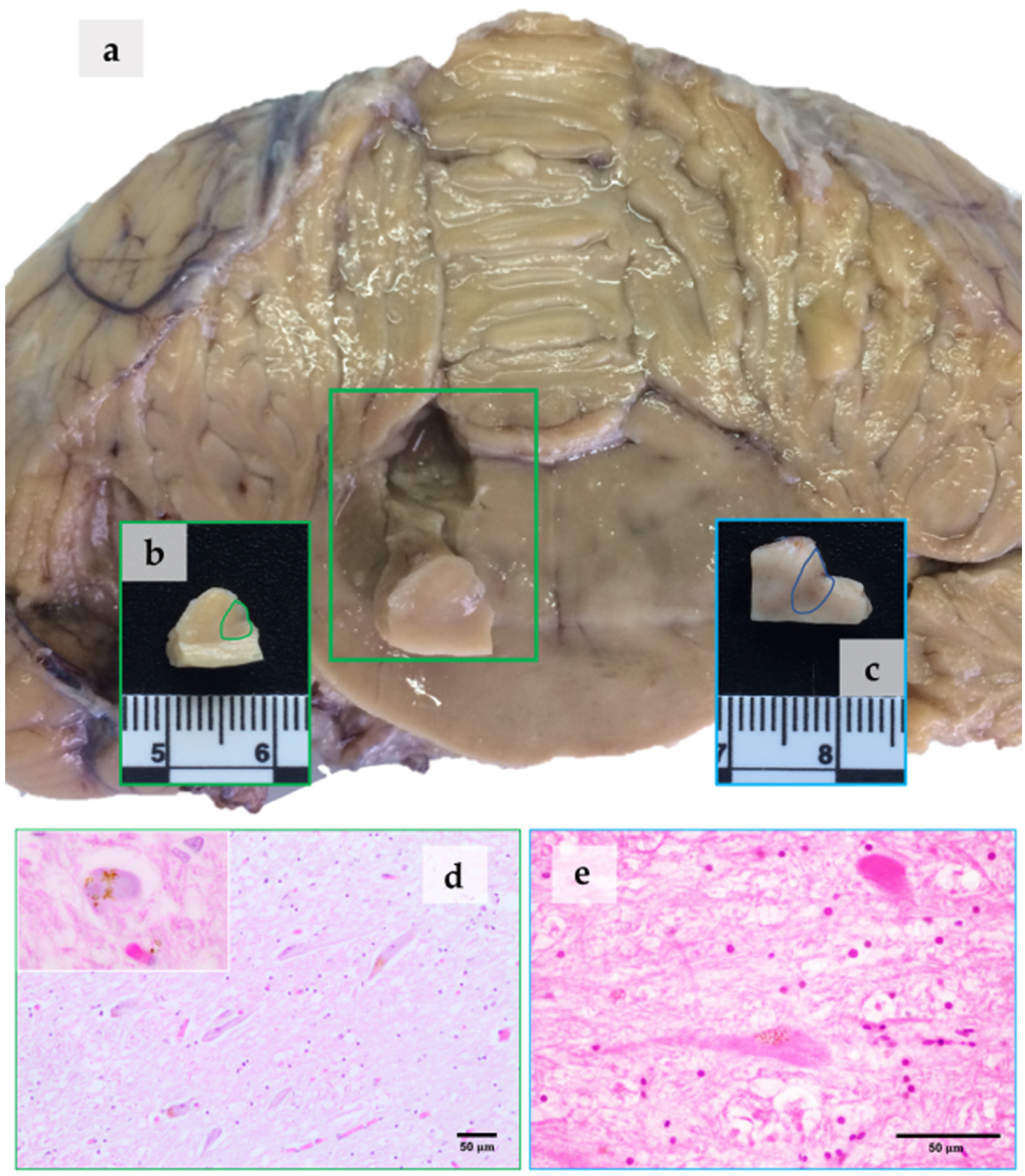
3.1. NM in the LC
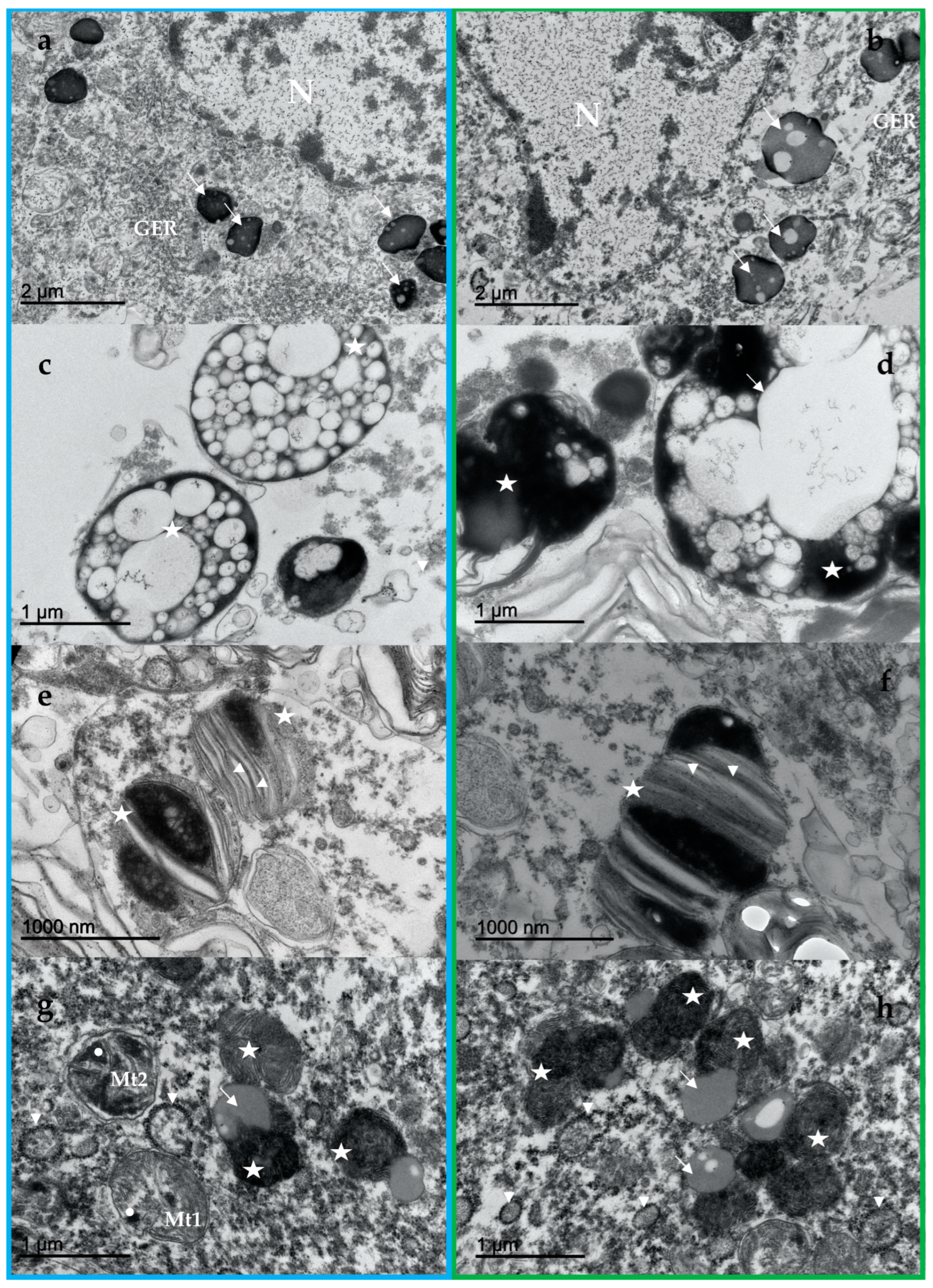
3.2. NM Ultrastructure
3.3. Lipofuscin Ultrastructure
3.4. Other Elements of the LC Neurons
4. Discussion
4.1. NM at the Optic Microscope
4.2. NM Ultrastructure and Its Implications
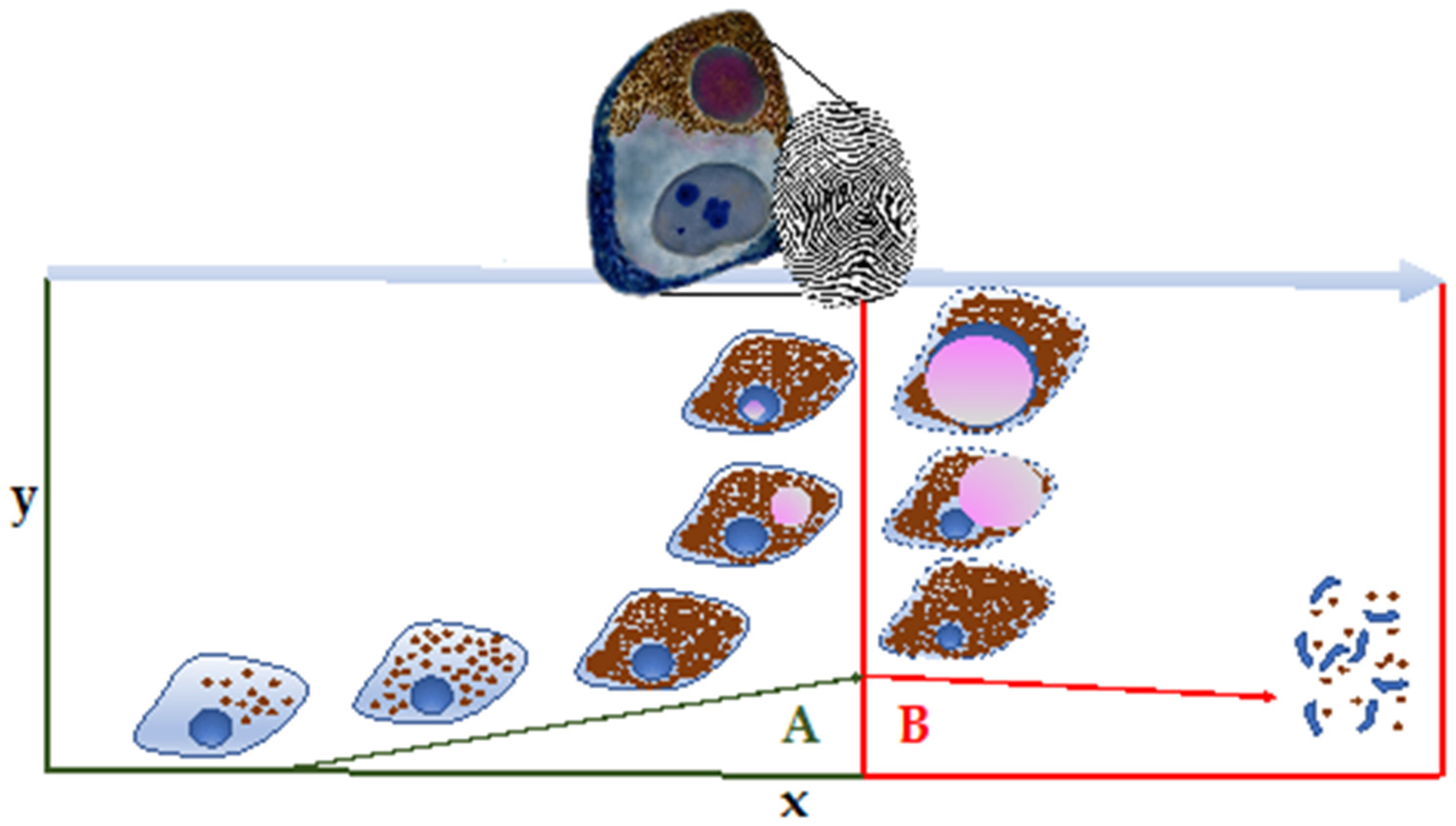
4.3. NM Ultrastructure in Other Animals and Age-Dependent Accumulation
- In the first stage, there is no pigmentation visible by optical microscope; there are a few lysosome-like organelles of medium electron density, surrounded by a single membrane, 0.5 to 1 μm in size, and occasionally lipidic globules and rarely fingerprint-like structures (dogs less than 1 year).
- In the second stage, highly electron-dense organelles, 1.5 to 3 μm in size, are surrounded by an irregular membrane. The lipidic component is more abundant, as well as the electron-dense granular matrix. Optically, only a few small NM granules are visible (dogs up to 5 years)
- In the third stage, NM shows great polymorphism, and contains a larger quantity of highly electron-dense organelles, 2 to 4 μm in size, and lipid droplets. At this stage, groups of organelles merge and form larger and more numerous electron-dense masses. Optically, NM granules are quite visible (dogs aged 6 years onwards).
4.4. NM and Neuronal Vulnerability
4.5. NM-Related Intraneuronal Inclusions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zecca, L.; Bellei, C.; Costi, P.; Albertini, A.; Monzani, E.; Casella, L.; Gallorini, M.; Bergamaschi, L.; Moscatelli, A.; Turro, N.J.; et al. New Melanic Pigments in the Human Brain That Accumulate in Aging and Block Environmental Toxic Metals. Proc. Natl. Acad. Sci. USA 2008, 105, 17567–17572. [Google Scholar] [CrossRef] [PubMed]
- ICVGAN. International Committee on Veterinary Gross Anatomical Nomenclature—Nomina Anatomica Veterinaria; World Association of Veterinary Anatomists (WAVA) Editorial Committee: Hannover, Germany; Ghent, Belgium; Columbia, MO, USA; Rio de Janeiro, Brazil, 2017; p. 160. [Google Scholar]
- Kemali, M.; Gioffré, D. Anatomical localisation of neuromelanin in the brains of the frog and tadpole. Ultrastructural comparison of neuromelanin with other melanins. J. Anat. 1985, 142, 73–83. [Google Scholar] [PubMed]
- Sulzer, D.; Mosharov, E.; Talloczy, Z.; Zucca, F.A.; Simon, J.D.; Zecca, L. Neuronal pigmented autophagic vacuoles: Lipofuscin, neuromelanin, and ceroid as macroautophagic responses during aging and disease. J. Neurochem. 2008, 106, 24–36. [Google Scholar] [CrossRef]
- Beason-held, L.L.; Horwitz, B. Aging Brain. In Encyclopedia of the Human Brain; Ramachandran, V.S., Ed.; Academic Press: New York, NY, USA, 2002; pp. 43–57. [Google Scholar] [CrossRef]
- Cotzias, G.C.; Papavasiliou, P.S.; Vanwoert, M.H.; Sakamoto, A. Melanogenesis and Extrapyramidal Diseases. Fed. Proc. 1964, 23, 713–718. [Google Scholar]
- Liu, L.; Luo, S.; Zeng, L.; Wang, W.; Yuan, L.; Jian, X. Degenerative alterations in noradrenergic neurons of the locus coeruleus in Alzheimer’s disease. Neural Regen. Res. 2013, 8, 2249–2255. [Google Scholar] [CrossRef] [PubMed]
- Zucca, F.A.; Basso, E.; Cupaioli, F.A.; Ferrari, E.; Sulzer, D.; Casella, L.; Zecca, L. Neuromelanin of the Human Substantia Nigra: An Update. Neurotox. Res. 2014, 25, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Simic, G.; Babic Leko, M.; Wray, S.; Harrington, C.R.; Delalle, I.; Jovanov-Milosevic, N.; Bazadona, D.; Buee, L.; de Silva, R.; Di Giovanni, G.; et al. Monoaminergic neuropathology in Alzheimer’s disease. Prog. Neurobiol. 2017, 151, 101–138. [Google Scholar] [CrossRef] [PubMed]
- Merighi, A.; Peirone, S.M. Histochemical and ultrastructural features of neuronal pigment in some encephalic nuclei of ruminants. Exp. Biol. 1985, 44, 109–121. [Google Scholar]
- Cozzi, B.; Pellegrini, M.; Droghi, A. Neuromelanin in the substantia nigra of adult horses. Anat. Anz. 1988, 166, 53–61. [Google Scholar]
- Sukhorukova, E.G.; Alekseeva, O.S.; Korzhevsky, D.E. Catecholaminergic neurons of mammalian brain and neuromelanin. J. Evol. Biochem. Physiol. 2014, 50, 383–391. [Google Scholar] [CrossRef]
- Lindquist, N.G.; Larsson, B.S.; Lydén-Sokolowski, A. Autoradiography of [14C]paraquat or [14C]diquat in frogs and mice: Accumulation in neuromelanin. Neurosci. Lett. 1988, 93, 1. [Google Scholar] [CrossRef]
- Double, K.L.; Dedov, V.N.; Fedorow, H.; Kettle, E.; Halliday, G.M.; Garner, B.; Brunk, U.T. The comparative biology of neuromelanin and lipofuscin in the human brain. Cell Mol. Life Sci. 2008, 65, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Sarasa, M.; Pesini, P. Natural non-trasgenic animal models for research in Alzheimer’s disease. Curr. Alzheimer Res. 2009, 6, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Gunn-Moore, D.; Kaidanovich-Beilin, O.; Gallego Iradi, M.C.; Gunn-Moore, F.; Lovestone, S. Alzheimer’s disease in humans and other animals: A consequence of postreproductive life span and longevity rather than aging. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2017, 14, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Di Guardo, G. Alzheimer’s disease, cellular prion protein, and dolphins. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2018, 14, 259–260. [Google Scholar] [CrossRef]
- Sacchini, S.; Arbelo, M.; Bombardi, C.; Fernández, A.; Cozzi, B.; Bernaldo de Quirós, Y.; Herráez, P. Locus coeruleus complex of the family Delphinidae. Sci. Rep. 2018, 8, 5486. [Google Scholar] [CrossRef]
- Davis, D.A.; Mondo, K.; Stern, E.; Annor, A.K.; Murch, S.J.; Coyne, T.M.; Brand, L.E.; Niemeyer, M.E.; Sharp, S.; Bradley, W.G.; et al. Cyanobacterial neurotoxin BMAA and brain pathology in stranded dolphins. PLoS ONE 2019, 14, e0213346. [Google Scholar] [CrossRef]
- Stylianaki, I.; Komnenou, A.T.; Posantzis, D.; Nikolaou, K.; Papaioannou, N. Alzheimer’s disease-like pathological lesions in an aged bottlenose dolphin (Tursiops truncatus). Vet. Rec. Case Rep. 2019, 7, e000700. [Google Scholar] [CrossRef]
- Di Guardo, G. Do dolphins get Alzheimer’s disease? Vet. Rec. 2019, 185, 762. [Google Scholar] [CrossRef]
- Di Guardo, G. Cetaceans, models for human disease? Res. Vet. Sci. 2018, 119, 43–44. [Google Scholar] [CrossRef]
- Sacchini, S.; Díaz-Delgado, J.; Espinosa de Los Monteros, A.; Paz, Y.; Bernaldo de Quirós, Y.; Sierra, E.; Arbelo, M.; Herráez, P.; Fernández, A. Amyloid-beta peptide and phosphorylated tau in the frontopolar cerebral cortex and in the cerebellum of toothed whales: Aging versus hypoxia. Biol. Open 2020, 9, bio054734. [Google Scholar] [CrossRef] [PubMed]
- Manger, P.R.; Fuxe, K.; Ridgway, S.H.; Siegel, J.M. The distribution and morphological characteristics of catecholaminergic cells in the diencephalon and midbrain of the bottlenose dolphin (Tursiops truncatus). Brain Behav. Evol. 2004, 64, 42–60. [Google Scholar] [CrossRef] [PubMed]
- Manger, P.R.; Ridgway, S.H.; Siegel, J.M. The locus coeruleus complex of the bottlenose dolphin (Tursiops truncatus) as revealed by tyrosine hydroxylase immunohistochemistry. J. Sleep Res. 2003, 12, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Dell, L.A.; Patzke, N.; Spocter, M.A.; Siegel, J.M.; Manger, P.R. Organization of the sleep-related neural systems in the brain of the harbour porpoise (Phocoena phocoena). J. Comp. Neurol. 2016, 524, 1999–2017. [Google Scholar] [CrossRef] [PubMed]
- Dell, L.A.; Karlsson, K.A.; Patzke, N.; Spocter, M.A.; Siegel, J.M.; Manger, P.R. Organization of the sleep-related neural systems in the brain of the minke whale (Balaenoptera acutorostrata). J. Comp. Neurol. 2016, 524, 2018–2035. [Google Scholar] [CrossRef]
- Geraci, J.; Lounsbury, V. Marine Mammals Ashore: A Field Guide for Strandings, 2nd ed.; National Aquarium in Baltimore: Baltimore, MD, USA, 2005. [Google Scholar]
- Díaz-Delgado, J.; Fernández, A.; Sierra, E.; Sacchini, S.; Andrada, M.; Vela, A.I.; Quesada-Canales, Ó.; Paz, Y.; Zucca, D.; Groch, K.; et al. Pathologic findings and causes of death of stranded cetaceans in the Canary Islands (2006–2012). PLoS ONE 2018, 13, e0204444. [Google Scholar] [CrossRef]
- Sacchini, S.; Herráez, P.; Arbelo, M.; Espinosa de los Monteros, A.; Sierra, E.; Rivero, M.; Bombardi, C.; Fernández, A. Methodology and Neuromarkers for Cetaceans’ Brains. Vet. Sci. 2022, 9, 38. [Google Scholar] [CrossRef]
- Sabatini, D.D.; Miller, F.; Barrnett, R.J. Aldehyde fixation for morphological and enzyme histochemical studies with the electron microscope. J. Histochem. Cytochem. Off. J. Histochem. Soc. 1964, 12, 57–71. [Google Scholar] [CrossRef]
- Moses, H.L.; Ganote, C.E.; Beaver, D.L.; Schuffman, S.S. Light and electron microscopic studies of pigment in human and rhesus monkey substantia nigra and locus coeruleus. Anat. Rec. 1966, 155, 167–183. [Google Scholar] [CrossRef]
- Duffy, P.E.; Tennyson, V.M. Phase and Electron Microscopic Observations of Lewy Bodies and Melanin Granules in the Substantia Nigra and Locus Caeruleus in Parkinson’s Disease. J. Neuropathol. Exp. Neurol. 1965, 24, 398–414. [Google Scholar] [CrossRef]
- Zucca, F.A.; Vanna, R.; Cupaioli, F.A.; Bellei, C.; De Palma, A.; Di Silvestre, D.; Mauri, P.; Grassi, S.; Prinetti, A.; Casella, L.; et al. Neuromelanin organelles are specialized autolysosomes that accumulate undegraded proteins and lipids in aging human brain and are likely involved in Parkinson’s disease. npj Parkinson’s Dis. 2018, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Pannese, E.V. The Structure of Neurons. In Neurocytology: Fine Structure of Neurons, Nerve Processes, and Neuroglial Cells; Springer: Cham, Switzerland, 2015; pp. 35–98. [Google Scholar] [CrossRef]
- Brandt, T.; Mourier, A.; Tain, L.S.; Partridge, L.; Larsson, N.-G.; Kühlbrandt, W. Changes of mitochondrial ultrastructure and function during ageing in mice and Drosophila. eLife 2017, 6, e24662. [Google Scholar] [CrossRef] [PubMed]
- DeMattei, M.; Levi, A.C.; Fariello, R.G. Neuromelanic pigment in substantia nigra neurons of rats and dogs. Neurosci. Lett. 1986, 72, 37–42. [Google Scholar] [CrossRef]
- Brayda-Bruno, M.; Levi, A.C. Ultrastructure of neuromelanin granules in dog substantia nigra at different ages. Boll. Soc. Ital. Biol. Sper. 1979, 55, 1902–1908. [Google Scholar] [PubMed]
- Vila, M. Neuromelanin, aging, and neuronal vulnerability in Parkinson’s disease. Mov. Disord. 2019, 34, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Carballo-Carbajal, I.; Laguna, A.; Romero-Giménez, J.; Cuadros, T.; Bové, J.; Martinez-Vicente, M.; Parent, A.; Gonzalez-Sepulveda, M.; Peñuelas, N.; Torra, A.; et al. Brain tyrosinase overexpression implicates age-dependent neuromelanin production in Parkinson’s disease pathogenesis. Nat. Commun. 2019, 10, 973. [Google Scholar] [CrossRef]
- Fu, H.; Hardy, J.; Duff, K.E. Selective vulnerability in neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1350–1358. [Google Scholar] [CrossRef]
- Halliday, G.M.; Leverenz, J.B.; Schneider, J.S.; Adler, C.H. The neurobiological basis of cognitive impairment in Parkinson’s disease. Mov. Disord. 2014, 29, 634–650. [Google Scholar] [CrossRef]
- Zecca, L.; Zucca, F.A.; Wilms, H.; Sulzer, D. Neuromelanin of the substantia nigra: A neuronal black hole with protective and toxic characteristics. Trends Neurosci. 2003, 26, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Clewett, D.V.; Lee, T.-H.; Greening, S.; Ponzio, A.; Margalit, E.; Mather, M. Neuromelanin marks the spot: Identifying a locus coeruleus biomarker of cognitive reserve in healthy aging. Neurobiol. Aging 2016, 37, 117–126. [Google Scholar] [CrossRef]
- Baker, C.S.; Dalebout, M.L. Forensic Genetics A2. In Encyclopedia of Marine Mammals, 2nd ed.; Perrin, W.F., Würsig, B., Thewissen, J.G.M., Eds.; Academic Press: London, UK, 2009; pp. 453–459. [Google Scholar] [CrossRef]
- Halliday, G.M.; Ophof, A.; Broe, M.; Jensen, P.H.; Kettle, E.; Fedorow, H.; Cartwright, M.I.; Griffiths, F.M.; Shepherd, C.E.; Double, K.L. Alpha-synuclein redistributes to neuromelanin lipid in the substantia nigra early in Parkinson’s disease. Brain 2005, 128, 2654–2664. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Spillantini, M.G.; Del Tredici, K.; Braak, H. 100 years of Lewy pathology. Nat. Rev. Neurol. 2013, 9, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, C.; Zucca, F.A.; Mauri, P.; Steinbeck, J.A.; Studer, L.; Scherzer, C.R.; Kanter, E.; Budhu, S.; Mandelbaum, J.; Vonsattel, J.P.; et al. MHC-I expression renders catecholaminergic neurons susceptible to T-cell-mediated degeneration. Nat. Commun. 2014, 5, 3633. [Google Scholar] [CrossRef] [PubMed]
- Double, K.L.; Maruyama, W.; Naoi, M.; Gerlach, M.; Riedere, P. Biological Role of Neuromelanin in the Human Brain and Its Importance in Parkinson’s Disease. In Melanins and Melanosomes; Borovanský, J., Riley, P.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 225–246. [Google Scholar] [CrossRef]
| Species/Diving Habit | Sex | AC | S | CC | CD |
|---|---|---|---|---|---|
| ASD: shallow diver | male | adult | alive | fresh | Interaction with fishing activities. |
| BBW: deep diver | female | adult | dead | fresh | Pathology associated with significant loss of nutritional status. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacchini, S.; Fernández, A.; Mompeó, B.; Ramírez, R.; Arbelo, M.; Holgersen, U.; Quesada-Canales, O.; Castro-Alonso, A.; Andrada, M. Toothed Whales Have Black Neurons in the Blue Spot. Vet. Sci. 2022, 9, 525. https://doi.org/10.3390/vetsci9100525
Sacchini S, Fernández A, Mompeó B, Ramírez R, Arbelo M, Holgersen U, Quesada-Canales O, Castro-Alonso A, Andrada M. Toothed Whales Have Black Neurons in the Blue Spot. Veterinary Sciences. 2022; 9(10):525. https://doi.org/10.3390/vetsci9100525
Chicago/Turabian StyleSacchini, Simona, Antonio Fernández, Blanca Mompeó, Raquel Ramírez, Manuel Arbelo, Unn Holgersen, Oscar Quesada-Canales, Ayoze Castro-Alonso, and Marisa Andrada. 2022. "Toothed Whales Have Black Neurons in the Blue Spot" Veterinary Sciences 9, no. 10: 525. https://doi.org/10.3390/vetsci9100525
APA StyleSacchini, S., Fernández, A., Mompeó, B., Ramírez, R., Arbelo, M., Holgersen, U., Quesada-Canales, O., Castro-Alonso, A., & Andrada, M. (2022). Toothed Whales Have Black Neurons in the Blue Spot. Veterinary Sciences, 9(10), 525. https://doi.org/10.3390/vetsci9100525







