The Novel Diagnostic Techniques and Biomarkers of Canine Mammary Tumors
Abstract
Simple Summary
Abstract
1. Introduction
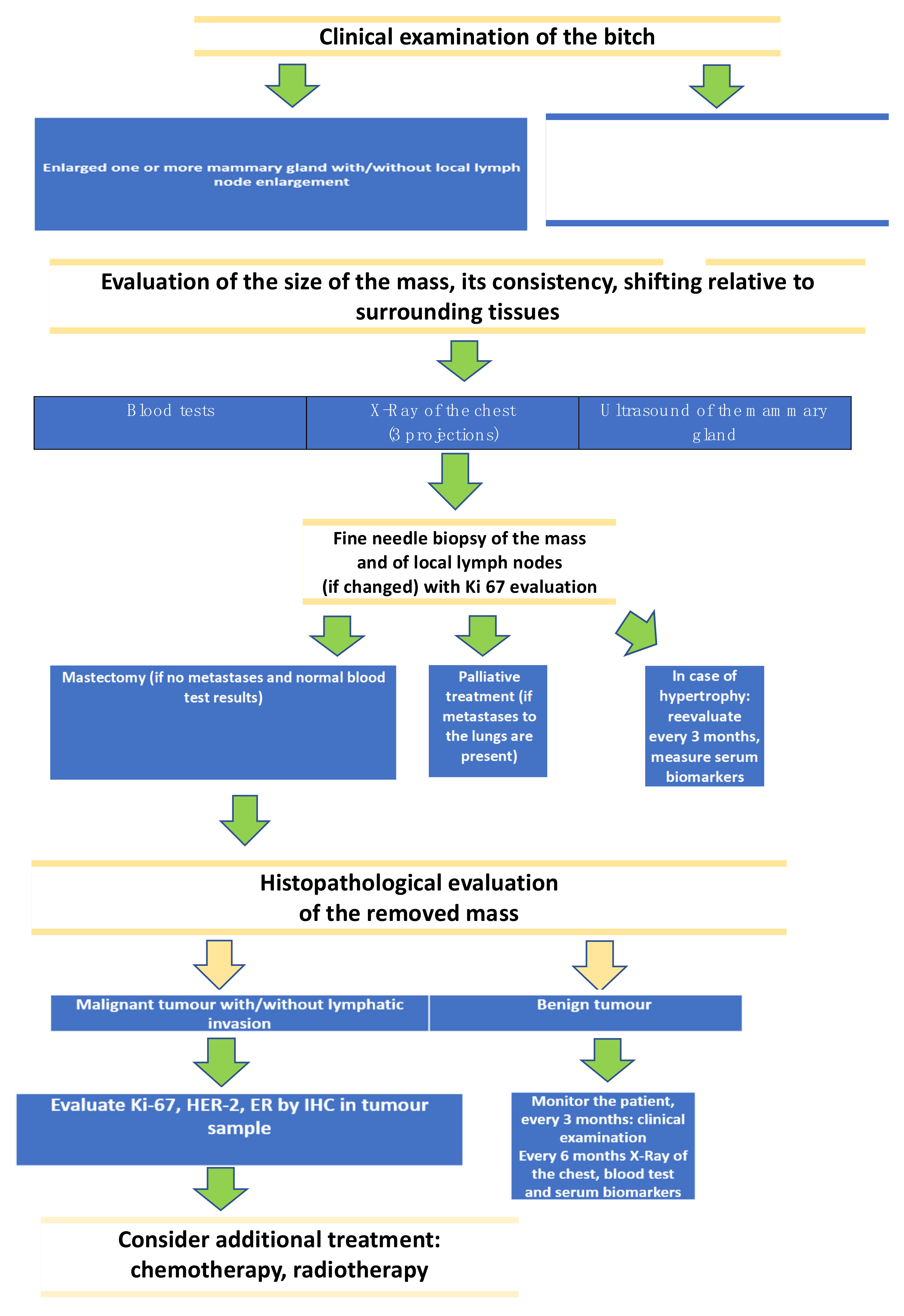
2. Diagnostic Techniques
2.1. Cytology and Histological Grade of Canine mammary Tumors
2.2. Evaluation of Selected Methods of Ultrasound Examination of Mammary Tumors in Bitches
3. Biomarkers of Canine Mammary Tumors
3.1. Various Cell Markers of the Cancer Process
3.1.1. Cell Cycle Markers
3.1.2. Proliferation Markers
3.1.3. Apoptosis Markers
3.2. Metastatic Potential and Prognosis of the Tumor
3.2.1. Cadherins
3.2.2. CEA
3.2.3. CA 15-3
3.3. Hormone Receptors
3.4. “Metabolomic” Markers
3.5. Gene Expression
3.6. miRNA
3.7. Transcriptome Sequencing
3.8. Inflammatory Markers
3.8.1. Inflammatory Cells Infiltration
3.8.2. Other Inflammatory Tissue and Blood CMTs Markers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APPs | acute phase proteins |
| ARFI | Acoustic Radiation Force Impulse |
| Ca15-3 | cancer antigen 15-3 |
| CDK | cyclin -dependent kinases |
| CEA | carcinoembryonic antigen |
| CEUS | contrast-enhanced ultrasound |
| CCL2 | CC chemokine ligand 2 |
| CGS | cytological grading system |
| CMT | canine mammary tumor |
| CMTs | canine mammary tumors |
| COX-2 | cyclo-oxygenase-2 |
| CRP | C- reactive protein |
| DEGs | differentially expressed genes |
| E-cadherin | epithelial cadherin |
| ER | estrogen receptor |
| ER+ | estrogen receptor positive |
| ER- | estrogen receptor negative |
| FNAC | fine needle aspiration cytology |
| HER-2 | human epidermal receptor 2 |
| HSPs | heat shock proteins |
| HBC | human breast cancer |
| HBCs | human breast cancers |
| iNOS | isoform of nitric oxide |
| iTIM | intratumoral tumor-infiltrating macrophages |
| Ki-67 | antigen Ki-67 |
| miRNA | microRNAs |
| MS | mass spectrometry |
| NOS | invasive ductal carcinoma |
| NMR | nuclear magnetic resonance |
| OHE | ovariohysterectomy |
| OTR | oxytocin receptor |
| PD-1 | programmed cell death protein-1 |
| PR | progesterone receptor |
| PR+ | progesterone receptor positive |
| PR- | progesterone receptor negative |
| SR | strain ratio |
| SSMC | staging system of mammary carcinomas |
| sTIMs | stromal tumor-infiltrating macrophages |
| VEGF | vascular endothelial growth factor |
| VEGFR2 | vascular endothelial growth factor receptor 2 |
| TIMs | tumor-infiltrating macrophages |
| TGF-β1 | tumor growth factor-β1 |
| TNBC | triple-negative breast cancer |
| TNF-α | tumor necrosis factor-α |
| TNM | tumor size (T), nodal stage (N), distant metastasis (M) |
| tTIMs | total count tumor-infiltrating macrophages |
| WES | whole-exome sequencing |
| WTS | whole-transcriptome sequencing |
References
- National Cancer Institute. Available online: http://www.cancer.gov (accessed on 10 October 2017).
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017, 50, 33. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, M.; Peña, L.; Rasotto, R.; Zappulli, V. Classification and Grading of Canine Mammary Tumors. Veter. Pathol. 2011, 48, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, K.; Rajan, A.; Warrier, A.; Nadhan, R.; Patra, D.; Srinivas, P. Cytological Grading of Breast Tumors—The Human and Canine Perspective. Front. Veter. Sci. 2019, 6, 283. [Google Scholar] [CrossRef] [PubMed]
- Abdelmegeed, S.M.; Mohammed, S. Canine mammary tumors as a model for human disease. Oncol. Lett. 2018, 15, 8195–8205. [Google Scholar] [CrossRef] [PubMed]
- Queiroga, F.L.; Raposo, T.; Carvalho, M.I.; Prada, J.; Pires, I. Canine mammary tumours as a model to study human breast can-cer: Most recent findings. In Vivo 2011, 25, 455–565. [Google Scholar] [PubMed]
- Liu, D.; Xiong, H.; Ellis, A.E.; Northrup, N.C.; Rodriguez, C.O.; O’Regan, R.M.; Dalton, S.; Zhao, S. Molecular Homology and Difference between Spontaneous Canine Mammary Cancer and Human Breast Cancer. Cancer Res. 2014, 74, 5045–5056. [Google Scholar] [CrossRef] [PubMed]
- Sorenmo, K. Canine mammary gland tumors. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 573–596. [Google Scholar] [CrossRef]
- Salas, Y.; Márquez, A.; Diaz, D.; Romero, L. Epidemiological Study of Mammary Tumors in Female Dogs Diagnosed during the Period 2002–2012: A Growing Animal Health Problem. PLoS ONE 2015, 10, e0127381. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.B.M.; Kumar, P.; Kumar, R.; Singh Pawaiya, R.; Ravindran, R. Histopathological classification and incidence of canine mammary tumours. Indian J. Vet. Pathol. 2009, 33, 152–155. [Google Scholar]
- Benavente, M.A.; Bianchi, C.P.; Aba, M.A. Canine Mammary Tumors: Risk Factors, Prognosis and Treatments. J. Veter. Adv. 2016, 6, 1291–1300. [Google Scholar] [CrossRef]
- Vascellari, M.; Capello, K.; Carminato, A.; Zanardello, C.; Baioni, E.; Mutinelli, F. Incidence of mammary tumors in the canine population living in the Veneto region (Northeastern Italy): Risk factors and similarities to human breast cancer. Prev. Veter. Med. 2016, 126, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Sinn, H.P.; Kreipe, H. A Brief Overview of the WHO Classification of Breast Tumors, 4th Edition, Focusing on Issues and Updates from the 3rd Edition. Breast Care 2013, 8, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Beauvais, W.; Cardwell, J.M.; Brodbelt, D. The effect of neutering on the risk of mammary tumours in dogs—A systematic review. J. Small Anim. Pr. 2012, 53, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Sorenmo, K.; Shofer, F.S.; Goldschmidt, M.H. Effect of spaying and timing of spaying on survival of dogs with mammary carcinoma. J. Vet. Intern. Med. 2000, 14, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Tsai, M.-H.; Liao, J.-W.; Chan, J.P.-W.; Wong, M.-L.; Chang, S.-C. Evaluation of hormone receptor expression for use in predicting survival of female dogs with malignant mammary gland tumors. J. Am. Veter. Med. Assoc. 2009, 235, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, V.; Nødtvedt, A.; Breen, A.; Langeland, M.; Teige, J.; Goldschmidt, M.; Jonasdottir, T.; Grotmol, T.; Sørenmo, K. Effect of Ovariohysterectomy at the Time of Tumor Removal in Dogs with Benign Mammary Tumors and Hyperplastic Lesions: A Randomized Controlled Clinical Trial. J. Veter. Intern. Med. 2013, 27, 935–942. [Google Scholar] [CrossRef] [PubMed]
- A Santos, A.; Lopes, C.C.; Ribeiro, J.R.; Martins, L.R.; Santos, J.C.; Amorim, I.F.; Gärtner, F.; Matos, A.J. Identification of prognostic factors in canine mammary malignant tumours: A multivariable survival study. BMC Veter. Res. 2013, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Alenza, M.D.P.; Pena, L.; del Castillo, N.; Nieto, A.I. Factors influencing the incidence and prognosis of canine mammary tumours. J. Small Anim. Pract. 2000, 41, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Sarli, G.; Preziosi, R.; Benazzi, C.; Castellani, G.; Marcato, P.S. Prognostic value of histologic stage and proliferative activity in canine malignant mammary tumors. J. Veter. Diagn. Investig. 2002, 14, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Tavasoly, A.; Golshahi, H.; Rezaie, A.; Farhadi, M. Classification and grading of canine malignant mammary tumors. Veter. Res. Forum Int. Q. J. 2013, 4, 25–30. [Google Scholar]
- Mobasheri, A.; Cassidy, J.P. Biomarkers in veterinary medicine: Towards targeted, individualised therapies for companion animals. Veter. J. 2010, 185, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.J. Biomarkers in veterinary cancer screening: Applications, limitations and expectations. Veter. J. 2010, 185, 10–14. [Google Scholar] [CrossRef]
- Dolka, I.; Czopowicz, M.; Gruk-Jurka, A.; Wojtkowska, A.; Sapierzynski, R.; Jurka, P. Diagnostic efficacy of smear cytology and Robinson’s cytological grading of canine mammary tumors with respect to histopathology, cytomorphometry, metastases and overall survival. PLoS ONE 2018, 13, e0191595. [Google Scholar] [CrossRef]
- Yildirim, F.; Gurel, A. Comparison between cytological and histopathological evaluations of canine mammary tumours. Rev. Med. Vet. 2012, 163, 116–122. [Google Scholar]
- Simon, D.; Schoenrock, D.; Nolte, I.; Baumgãrtner, W.; Barron, R.; Mischke, R. Cytologic examination of fine-needle aspirates from mammary gland tumors in the dog: Diagnostic accuracy with comparison to histopathology and association with postoperative outcome. Veter. Clin. Pathol. 2009, 38, 521–528. [Google Scholar] [CrossRef]
- Haziroglu, R.; Yardimci, B.; Aslan, S.; Yildirim, M.; Yumusak, N.; Beceriklisoy, H.; Agaoglu, R.; Küçükaslan, I. Cytological evaluation of canine mammary tumours with fine needle aspiration biopsy technique. Rev. Med. Vet. 2010, 161, 212–218. [Google Scholar]
- Schiller, A.B.; Tadros, T.S.; Birdsong, G.G.; Grossl, N.A. Cellular Dyscohesion in Fine-Needle Aspiration of Breast Carcinoma. Prognostic indicator for axillary lymph node metastases? Am. J. Clin. Pathol. 2001, 115, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.; Layne, G.; Shahan, C.; Zhang, J.; Wen, S.; Radis, S.; Richmond, B.; Partin, J.; Hazard, H. Comparison between Ultrasound and Pathologic Status of Axillary Lymph Nodes in Clinically Node-negative Breast Cancer Patients. Am. Surg. 2015, 81, 865–869. [Google Scholar] [CrossRef]
- Chocteau, F.; Abadie, J.; Loussouarn, D.; Nguyen, F. Proposal for a Histological Staging System of Mammary Carcinomas in Dogs and Cats. Part 1: Canine Mammary Carcinomas. Front. Veter. Sci. 2019, 6, 388. [Google Scholar] [CrossRef] [PubMed]
- Canadas, A.; França, M.; Pereira, C.; Vilaça, R.; Vilhena, H.; Tinoco, F.; Silva, M.J.; Ribeiro, J.; Medeiros, R.; Oliveira, P.; et al. Canine Mammary Tumors: Comparison of Classification and Grading Methods in a Survival Study. Veter. Pathol. 2018, 56, 208–219. [Google Scholar] [CrossRef] [PubMed]
- American Joint Committee on Cancer. Breast Cancer Staging, 7th ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Giuliano, A.E.; Connolly, J.L.; Edge, S.B.; Mittendorf, E.A.; Rugo, H.S.; Solin, L.J.; Weaver, D.L.; Winchester, D.J.; Hortobagyi, G.N. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 290–303. [Google Scholar] [CrossRef]
- Koh, J.; Kim, M.J. Introduction of a New Staging System of Breast Cancer for Radiologists: An Emphasis on the Prognostic Stage. Korean J. Radiol. 2019, 20, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Soler, M.; Dominguez, E.; Lucas, X.; Novellas, R.; Coelho, K.; Espada, Y.; Agut, A. Comparison between ultrasonographic findings of benign and malignant canine mammary gland tumours using B-mode, colour Doppler, power Doppler and spectral Doppler. Res. Veter. Sci. 2016, 107, 141–146. [Google Scholar] [CrossRef]
- Feliciano, M.A.R.; Uscategui, R.A.R.; Maronezi, M.C.; Simões, A.P.R.; Silva, P.; Gasser, B.; Pavan, L.; Carvalho, C.F.; Canola, J.C.; Vicente, W.R.R. Ultrasonography methods for predicting malignancy in canine mammary tumors. PLoS ONE 2017, 12, e0178143. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, M.A.R.; Ramirez, R.A.U.; Maronezi, M.C.; Maciel, G.S.; Avante, M.L.; Senhorello, I.L.S.; Mucédola, T.; Gasser, B.; Carvalho, C.F.; Vicente, W.R.R. Accuracy of four ultrasonography techniques in predicting histopathological classification of canine mammary carcinomas. Veter. Radiol. Ultrasound 2018, 59, 444–452. [Google Scholar] [CrossRef]
- Ranjkesh, M.; Hajibonabi, F.; Seifar, F.; Tarzamni, M.K.; Moradi, B.; Khamnian, Z. Diagnostic Value of Elastography, Strain Ratio, and Elasticity to B-Mode Ratio and Color Doppler Ultrasonography in Breast Lesions. Int. J. Gen. Med. 2020, ume 13, 215–224. [Google Scholar] [CrossRef]
- Stan, F.; Gudea, A.; Damian, A.; Gal, A.F.; Papuc, I.; Pop, A.R.; Martonos, C. Ultrasonographic Algorithm for the Assessment of Sentinel Lymph Nodes That Drain the Mammary Carcinomas in Female Dogs. Animals 2020, 10, 2366. [Google Scholar] [CrossRef]
- Hao, Y.; Ren, G.; Yang, W.; Zheng, W.; Wu, Y.; Li, W.; Li, X.; Li, Y.; Guo, X. Combination diagnosis with elastography strain ratio and molecular markers effectively improves the diagnosis rate of small breast cancer and lymph node metastasis. Quant. Imaging Med. Surg. 2020, 10, 678–691. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The Biology of Cancer: Metabolic Reprogramming Fuels Cell Growth and Proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, A.A.; Kasi, A. Genetics, Cancer Cell Cycle Phases. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563158/ (accessed on 30 December 2020).
- Gao, X.; Leone, G.W.; Wang, H. Cyclin D-CDK4/6 functions in cancer. Adv. Cancer Res. 2020, 148, 147–169. [Google Scholar]
- Qie, S.; Diehl, J.A. Cyclin D1, cancer progression, and opportunities in cancer treatment. Klin. Wochenschr. 2016, 94, 1313–1326. [Google Scholar] [CrossRef]
- Roy, P.G.; Thompson, A.M. Cyclin D1 and breast cancer. Breast 2006, 15, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.S.; Han, H.S.; Hong, Y.C.; Lee, H.J.; Paik, N.-S. Prognostic value of combined analysis of cyclin D1 and estrogen receptor status in breast cancer patients. Pathol. Int. 2003, 53, 74–80. [Google Scholar] [CrossRef]
- Sfacteria, A.; Bertani, C.; Costantino, G.; Del Bue, M.; Paiardini, M.; Cervasi, B.; Piedimonte, A.; De Vico, G. Cyclin D1 Expression in Pre-cancerous and Cancerous Lesions of the Canine Mammary Gland. J. Comp. Pathol. 2003, 128, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Tateyama, S.; Rungsipipat, A.; Uchida, K.; Yamaguchi, R. Immunohistochemical Analysis of Cyclin A, Cyclin D1 and P53 in Mammary Tumors, Squamous Cell Carcinomas and Basal Cell Tumors of Dogs and Cats. J. Veter. Med Sci. 2000, 62, 743–750. [Google Scholar] [CrossRef]
- Möröy, T.; Geisen, C. Cyclin E. Int. J. Biochem. Cell Biol. 2004, 36, 1424–1439. [Google Scholar] [CrossRef]
- Teixeira, L.K.; Reed, S.I. Cyclin E Deregulation and Genomic Instability. Adv Exp Med Biol. 2017, 1042, 527–547. [Google Scholar] [CrossRef]
- Keyomarsi, K.; Tucker, S.L.; Buchholz, T.A.; Callister, M.; Ding, Y.; Hortobagyi, G.N.; Bedrosian, I.; Knickerbocker, C.; Toyofuku, W.; Lowe, M.; et al. Cyclin E and Survival in Patients with Breast Cancer. N. Engl. J. Med. 2002, 347, 1566–1575. [Google Scholar] [CrossRef]
- Hunt, K.K.; Karakas, C.; Ha, M.J.; Biernacka, A.; Yi, M.; Sahin, A.A.; Adjapong, O.; Hortobagyi, G.N.; Bondy, M.L.; Thompson, P.A.; et al. Cytoplasmic Cyclin E Predicts Recurrence in Patients with Breast Cancer. Clin. Cancer Res. 2017, 23, 2991–3002. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhang, J. Research Progress of PCNA in Reproductive System Diseases. Evid. Based Complement Altern. Med. 2021, 2021, 2391917. [Google Scholar] [CrossRef]
- Menon, S.S.; Guruvayoorappan, C.; Sakthivel, K.M.; Rasmi, R.R. Ki-67 protein as a tumour proliferation marker. Clin. Chim. Acta 2019, 491, 39–45. [Google Scholar] [CrossRef]
- Nowak, M.; Madej, J.A.; Pula, B.; Dziegiel, P.; Ciaputa, R. Expression of matrix metalloproteinase 2 (MMP-2), E-cadherin and Ki-67 in metastatic and non-metastatic canine mammary carcinomas. Ir. Veter. J. 2015, 69, 9. [Google Scholar] [CrossRef]
- Araújo, M.; Campos, L.; Damasceno, K.; Gamba, C.; Ferreira, E.; Cassali, G. HER-2, EGFR, Cox-2 and Ki67 expression in lymph node metastasis of canine mammary carcinomas: Association with clinical-pathological parameters and overall survival. Res. Veter. Sci. 2016, 106, 121–130. [Google Scholar] [CrossRef]
- Davey, M.G.; Hynes, S.O.; Kerin, M.J.; Miller, N.; Lowery, A.J. Ki-67 as a Prognostic Biomarker in Invasive Breast Cancer. Cancers 2021, 13, 4455. [Google Scholar] [CrossRef]
- Carvalho, M.I.; Pires, I.; Prada, J.; Lobo, L.; Queiroga, F.L. Ki-67 and PCNA Expression in Canine Mammary Tumors and Adjacent Nonneoplastic Mammary Glands. Veter. Pathol. 2016, 53, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Juríková, M.; Danihel, Ľ.; Polák, Š.; Varga, I. Ki67, PCNA, and MCM proteins: Markers of proliferation in the diagnosis of breast cancer. Acta Histochem. 2016, 118, 544–552. [Google Scholar] [CrossRef]
- Stuart-Harris, R.; Caldas, C.; Pinder, S.; Pharoah, P. Proliferation markers and survival in early breast cancer: A systematic review and meta-analysis of 85 studies in 32,825 patients. Breast 2008, 17, 323–334. [Google Scholar] [CrossRef]
- Aydogan, A.; Ozmen, O.; Haligur, M.; Sipahi, C.; Ileri, D.; Haligur, A. Immunohistochemical evaluation of bcl-2, ER-alpha, caspase -3, -8, -9, PCNA and Ki-67 expressions in canine mammary carcinomas. Biotech. Histochem. 2018, 93, 286–292. [Google Scholar] [CrossRef]
- Clavijo-Maldonado, A.; Ferreira, E.; Vargas-Hernández, C.; Páez, F.R. Canine mammary cancer. Veter. Stanica 2020, 51, 425–439. [Google Scholar] [CrossRef]
- Dutra, A.; Granja, N.; Schmitt, F.; Cassali, G. c-erbB-2 expression and nuclear pleomorphism in canine mammary tumors. Braz. J. Med Biol. Res. 2004, 37, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-L.; Huang, H.-M.; Liao, J.-W.; Wong, M.-L.; Chang, S.-C. Increased survival in dogs with malignant mammary tumours overexpressing HER-2 protein and detection of a silent single nucleotide polymorphism in the canine HER-2 gene. Veter. J. 2009, 180, 116–123. [Google Scholar] [CrossRef]
- Muhammadnejad, A.; Keyhani, E.; Mortazavi, P.; Behjati, F.; Haghdoost, I.S. Overexpression of HER-2/neu in Malignant Mammary Tumors: Translation of Clinicopathological Features from Dog to Human. Asian Pac. J. Cancer Prev. 2012, 13, 6415–6421. [Google Scholar] [CrossRef] [PubMed]
- Singer, J.; Weichselbaumer, M.; Stockner, T.; Mechtcheriakova, D.; Sobanov, Y.; Bajna, E.; Wrba, F.; Horvat, R.; Thalhammer, J.G.; Willmann, M.; et al. Comparative oncology: ErbB-1 and ErbB-2 homologues in canine cancer are susceptible to cetuximab and trastuzumab targeting. Mol. Immunol. 2012, 50, 200–209. [Google Scholar] [CrossRef]
- Seung, B.-J.; Cho, S.-H.; Kim, S.-H.; Lim, H.-Y.; Sur, J.-H. Quantitative analysis of HER2 mRNA expression by RNA in situ hybridization in canine mammary gland tumors: Comparison with immunohistochemistry analysis. PLoS ONE 2020, 15, e0229031. [Google Scholar] [CrossRef]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.-T.; Zhou, T.-T.; Liu, B.; Bao, J.-K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Goldar, S.; Khaniani, M.S.; Derakhshan, S.M.; Baradaran, B. Molecular Mechanisms of Apoptosis and Roles in Cancer Development and Treatment. Asian Pac. J. Cancer Prev. 2015, 16, 2129–2144. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C. Mechanisms of Apoptosis. Am. J. Pathol. 2000, 157, 1415–1430. [Google Scholar] [CrossRef]
- Bensassi, F.; Gallerne, C.; El Dein, O.S.; Lemaire, C.; Hajlaoui, M.R.; Bacha, H. Involvement of mitochondria-mediated apoptosis in deoxynivalenol cytotoxicity. Food Chem. Toxicol. 2012, 50, 1680–1689. [Google Scholar] [CrossRef]
- Jin, Z.; El-Deiry, W.S. Overview of cell death signaling pathways. Cancer Biol. Ther. 2005, 4, 147–171. [Google Scholar] [CrossRef]
- Szegezdi, E.; Logue, S.; Gorman, A.; Samali, A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006, 7, 880–885. [Google Scholar] [CrossRef]
- Pu, X.; Storr, S.J.; Zhang, Y.; Rakha, E.A.; Green, A.R.; Ellis, I.O.; Martin, S.G. Caspase-3 and caspase-8 expression in breast cancer: Caspase-3 is associated with survival. Apoptosis 2016, 22, 357–368. [Google Scholar] [CrossRef]
- Rodrigues, H.; Carvalho, M.I.; Pires, I.; Prada, J.; Queiroga, F.L.; Information, R. Clinicopathological significance of caspase-3 and Ki-67 expression in canine mammary gland tumours. Acta Veter. Hung. 2016, 64, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Grzywnowicz, M.; Giannopoulos, K. Znaczenie receptora programowanej śmierci 1 oraz jego ligandów w układzie immunologicznym oraz nowotworach. Acta Haematol. Pol. 2012, 43, 132–145. [Google Scholar] [CrossRef]
- Huang, F.; Wang, B.; Zeng, J.; Sang, S.; Lei, J.; Lu, Y. MicroRNA-374b inhibits liver cancer progression via down regulating programmed cell death-1 expression on cytokine-induced killer cells. Oncol. Lett. 2018, 15, 4797–4804. [Google Scholar] [CrossRef]
- Riley, J.L. PD-1 signaling in primary T cells. Immunol. Rev. 2009, 229, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 Expression in Triple-Negative Breast Cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef]
- Shosu, K.; Sakurai, M.; Inoue, K.; Nakagawa, T.; Sakai, H.; Morimoto, M.; Okuda, M.; Noguchi, S.; Mizuno, T. Programmed Cell Death Ligand 1 Expression in Canine Cancer. In Vivo 2016, 30, 195–204. [Google Scholar]
- Maekawa, N.; Konnai, S.; Okagawa, T.; Nishimori, A.; Ikebuchi, R.; Izumi, Y.; Takagi, S.; Kagawa, Y.; Nakajima, C.; Suzuki, Y.; et al. Immunohistochemical Analysis of PD-L1 Expression in Canine Malignant Cancers and PD-1 Expression on Lymphocytes in Canine Oral Melanoma. PLoS ONE 2016, 11, e0157176. [Google Scholar] [CrossRef]
- Song, D.-W.; Ro, W.-B.; Park, H.-M. Evaluation of circulating PD-1 and PD-L1 as diagnostic biomarkers in dogs with tumors. J. Veter. Sci. 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Robins, H.; Alexe, G.; Harris, S.; Levine, A.J. The First Twenty-Five Years of p53 Research; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–25. [Google Scholar] [CrossRef]
- Vogelstein, B.; Lane, D.; Levine, A.J. Surfing the p53 network. Nature 2000, 408, 307–310. [Google Scholar] [CrossRef]
- Vousden, K.H.; Lu, X. Live or let die: The cell’s response to p53. Nat. Cancer 2002, 2, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yuan, Y.; Liu, G.; Wei, Y. P53 and Ki-67 as prognostic markers in triple-negative breast cancer patients. PLoS ONE 2017, 12, e0172324. [Google Scholar] [CrossRef] [PubMed]
- Klopfleisch, R.; Gruber, A. Differential expression of cell cycle regulators p21, p27 and p53 in metastasizing canine mammary adenocarcinomas versus normal mammary glands. Res. Veter. Sci. 2009, 87, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.F.; Maués, T.; Ramundo, M.S.; Figueiredo, A.M.S.; De Mello, M.F.V.; El-Jaick, K.B.; Ferreira, M.D.L.G.; Ferreira, A.M.R. TP53 gene expression levels and tumor aggressiveness in canine mammary carcinomas. J. Veter. Diagn. Investig. 2017, 29, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, B.; Bacci, B.; Angeli, C.; Benazzi, C.; Muscatello, L.V. p53, ER, and Ki67 Expression in Canine Mammary Carcinomas and Correlation With Pathological Variables and Prognosis. Veter. Pathol. 2021, 58, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Kaszak, I.; Witkowska-Piłaszewicz, O.; Niewiadomska, Z.; Dworecka-Kaszak, B.; Toka, F.N.; Jurka, P. Role of Cadherins in Cancer—A Review. Int. J. Mol. Sci. 2020, 21, 7624. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.; Lopes, C.; Carvalheira, J.; Santos, M.; Rutteman, G.; Gärtner, F. E-cadherin Expression in Canine Malignant Mammary Tumours: Relationship to Other Clinico-Pathological Variables. J. Comp. Pathol. 2006, 134, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yin, S.; Zhang, L.; Liu, W.; Chen, B. Prognostic value of reduced E-cadherin expression in breast cancer: A meta-analysis. Oncotarget 2017, 8, 16445–16455. [Google Scholar] [CrossRef] [PubMed]
- Gama, A.; Paredes, J.; Gärtner, F.; Alves, A.; Schmitt, F. Expression of E-cadherin, P-cadherin and β-catenin in canine malignant mammary tumours in relation to clinicopathological parameters, proliferation and survival. Veter. J. 2008, 177, 45–53. [Google Scholar] [CrossRef]
- da Rocha, A.A.; Carvalheira, J.; Gärtner, F. α-catenin, β-catenin and P-120-catenin immunoexpression in canine mammary tissues and their relationship with E-cadherin. Res. Veter. Sci. 2020, 130, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Ledecky, V.; Valencakova-Agyagosova, A.; Lepej, J.; Frischova, Z.; Hornak, S.; Nagy, V. Determination of carcinoembryonic antigen and cancer antigen values with the radioimmunoassay method in healthy females dogs. Vet. Med. 2013, 58, 277–283. [Google Scholar] [CrossRef]
- Di Gioia, D.; Blankenburg, I.; Nagel, D.; Heinemann, V.; Stieber, P. Tumor markers in the early detection of tumor recurrence in breast cancer patients: CA 125, CYFRA 21-1, HER2 shed antigen, LDH and CRP in combination with CEA and CA 15-3. Clin. Chim. Acta 2016, 461, 1–7. [Google Scholar] [CrossRef]
- Pedersen, A.C.; Sørensen, P.D.; Jacobsen, E.H.; Madsen, J.S.; Brandslund, I. Sensitivity of CA 15-3, CEA and serum HER2 in the early detection of recurrence of breast cancer. Clin. Chem. Lab. Med. (CCLM) 2013, 51, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Sun, X.; He, Y.; Liu, C.; Liu, H. Elevated Levels of Serum Tumor Markers CEA and CA15-3 Are Prognostic Parameters for Different Molecular Subtypes of Breast Cancer. PLoS ONE 2015, 10, e0133830. [Google Scholar] [CrossRef] [PubMed]
- Stieber, P.; Nagel, D.; Blankenburg, I.; Heinemann, V.; Untch, M.; Bauerfeind, I.; Di Gioia, D. Diagnostic efficacy of CA 15-3 and CEA in the early detection of metastatic breast cancer—A retrospective analysis of kinetics on 743 breast cancer patients. Clin. Chim. Acta 2015, 448, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Moazzezy, N.; Farahany, T.Z.; Oloomi, M.; Bouzari, S. Relationship between Preoperative Serum CA15-3 and CEA Levels and Clinicopathological Parameters in Breast Cancer. Asian Pac. J. Cancer Prev. 2014, 15, 1685–1688. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.; Lavalle, G.; Estrela-Lima, A.; de Faria, J.M.; Guimarães, J.; Dutra, A.P.; Ferreira, E.; de Sousa, L.; Rabelo, E.M.; da Costa, A.V.; et al. CA15.3, CEA and LDH in Dogs with Malignant Mammary Tumors. J. Veter. Intern. Med. 2012, 26, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Valencakova-Agyagosova, A.; Frischova, Z.; Sevcikova, Z.; Hajurka, J.; Lepej, J.; Szakallova, I.; Kredatusova, G.; Nagy, V.; Ledecky, V. Determination of carcinoembryonic antigen and cancer antigen (CA 15-3) in bitches with tumours on mammary gland: Preliminary report. Veter. Comp. Oncol. 2012, 12, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Senhorello, I.L.S.; Terra, E.M.; Sueiro, F.A.R.; Firmo, B.F.; Anai, L.A.; Goloni, C.; Canavari, I.C.; Ampuero, R.A.N.; Pereira, R.S.; Tinucci-Costa, M. Clinical value of carcinoembryonic antigen in mammary neoplasms of bitches. Veter. Comp. Oncol. 2019, 18, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, M.C.; Manuali, E.; Pacifico, E.; Ferri, C.; Romagnoli, M.; Mangili, V.; Fruganti, G. Cancer antigen 15/3: Possible diagnostic use in veterinary clinical oncology. Preliminary study. Veter. Res. Commun. 2010, 34, 103–106. [Google Scholar] [CrossRef]
- Manuali, E.; De Giuseppe, A.; Feliziani, F.; Forti, K.; Casciari, C.; Marchesi, M.C.; Pacifico, E.; Pawłowski, K.M.; Majchrzak, K.; Król, M. CA 15–3 cell lines and tissue expression in canine mammary cancer and the correlation between serum levels and tumour histological grade. BMC Veter. Res. 2012, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-H.; Lim, H.-Y.; Im, K.-S.; Shin, J.-I.; Kim, H.-W.; Sur, J.-H. Evaluation of Clinicopathological Characteristics and Oestrogen Receptor Gene Expression in Oestrogen Receptor-negative, Progesterone Receptor-positive Canine Mammary Carcinomas. J. Comp. Pathol. 2014, 151, 42–50. [Google Scholar] [CrossRef]
- Queiroga, F.L.; Pérez-Alenza, D.; Silvan, G.; Peña, L.; Illera, J.C. Positive correlation of steroid hormones and EGF in canine mammary cancer. J. Steroid Biochem. Mol. Biol. 2009, 115, 9–13. [Google Scholar] [CrossRef]
- Spoerri, M.; Guscetti, F.; Hartnack, S.; Boos, A.; Oei, C.; Balogh, O.; Nowaczyk, R.M.; Michel, E.; Reichler, I.M.; Kowalewski, M.P. Endocrine control of canine mammary neoplasms: Serum reproductive hormone levels and tissue expression of steroid hormone, prolactin and growth hormone receptors. BMC Veter. Res. 2015, 11, 235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Benavente, M.; Bianchi, C.; Aba, M. Expression of Oxytocin Receptors in Canine Mammary Tumours. J. Comp. Pathol. 2019, 170, 26–33. [Google Scholar] [CrossRef]
- Yip, C.-H.; Rhodes, A. Estrogen and progesterone receptors in breast cancer. Futur. Oncol. 2014, 10, 2293–2301. [Google Scholar] [CrossRef]
- Timmermans-Sprang, E.P.M.; Gracanin, A.; Mol, J.A. Molecular Signaling of Progesterone, Growth Hormone, Wnt, and HER in Mammary Glands of Dogs, Rodents, and Humans: New Treatment Target Identification. Front. Veter. Sci. 2017, 4, 53. [Google Scholar] [CrossRef]
- Hilton, H.N.; Clarke, C.L.; Graham, J.D. Estrogen and progesterone signalling in the normal breast and its implications for cancer development. Mol. Cell. Endocrinol. 2017, 466, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Mainenti, M.; Rasotto, R.; Carnier, P.; Zappulli, V. Oestrogen and progesterone receptor expression in subtypes of canine mammary tumours in intact and ovariectomised dogs. Veter. J. 2014, 202, 62–68. [Google Scholar] [CrossRef]
- Millanta, F.; Calandrella, M.; Bari, G.; Niccolini, M.; Vannozzi, I.; Poli, A. Comparison of steroid receptor expression in normal, dysplastic, and neoplastic canine and feline mammary tissues. Res. Veter. Sci. 2005, 79, 225–232. [Google Scholar] [CrossRef]
- Nieto, A.; Peña, L.; Pérez-Alenza, M.D.; Sánchez, M.A.; Flores, J.M.; Castaño, M. Immunohistologic Detection of Estrogen Receptor Alpha in Canine Mammary Tumors: Clinical and Pathologic Associations and Prognostic Significance. Veter. Pathol. 2000, 37, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Sorenmo, K.U.; Durham, A.C.; Kristiansen, V.; Pena, L.; Goldschmidt, M.H.; Stefanovski, D. Developing and testing prognostic bio-scoring systems for canine mammary gland carcinomas. Veter. Comp. Oncol. 2019, 17, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Terzaghi, L.; Banco, B.; Groppetti, D.; Dall’Acqua, P.C.; Giudice, C.; Pecile, A.; Grieco, V.; Lodde, V.; Luciano, A.M. Progesterone receptor membrane component 1 (PGRMC1) expression in canine mammary tumors: A preliminary study. Res. Veter. Sci. 2020, 132, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Tavares, W.L.; E Lavalle, G.; Figueiredo, M.S.; Souza, A.G.; Bertagnolli, A.C.; AB Viana, F.; Paes, P.R.; A Carneiro, R.; Cavalcanti, G.A.; Melo, M.M.; et al. Evaluation of adverse effects in tamoxifen exposed healthy female dogs. Acta Veter. Scand. 2010, 52, 67. [Google Scholar] [CrossRef]
- Fiehn, O. Combining Genomics, Metabolome Analysis, and Biochemical Modelling to Understand Metabolic Networks. Comp. Funct. Genom. 2001, 2, 155–168. [Google Scholar] [CrossRef]
- Bujak, R.; Struck-Lewicka, W.; Markuszewski, M.J.; Kaliszan, R. Metabolomics for laboratory diagnostics. J. Pharm. Biomed. Anal. 2015, 113, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Armitage, E.G.; Ciborowski, M. Applications of Metabolomics in Cancer Studies. Adv. Exp. Med. Biol. 2017, 965, 209–234. [Google Scholar] [CrossRef]
- Woo, H.M.; Kim, K.M.; Choi, M.H.; Jung, B.H.; Lee, J.; Kong, G.; Nam, S.J.; Kim, S.; Bai, S.W.; Chung, B.C. Mass spectrometry based metabolomic approaches in urinary biomarker study of women’s cancers. Clin. Chim. Acta 2009, 400, 63–69. [Google Scholar] [CrossRef]
- Budczies, J.; Denkert, C.; Müller, B.M.; Brockmöller, S.F.; Klauschen, F.; Györffy, B.; Dietel, M.; Richter-Ehrenstein, C.; Marten, U.; Salek, R.M.; et al. Remodeling of central metabolism in invasive breast cancer compared to normal breast tissue—A GC-TOFMS based metabolomics study. BMC Genom. 2012, 13, 334. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, S.; Liu, L.; Gowda, G.N.; Bonney, P.; Stewart, J.; Knapp, D.W.; Raftery, D. NMR-based metabolomics study of canine bladder cancer. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2012, 1822, 1807–1814. [Google Scholar] [CrossRef]
- Valko-Rokytovská, M.; Očenáš, P.; Salayová, A.; Titková, R.; Kostecká, Z. Specific urinary metabolites in canine mammary gland tumors. J. Veter. Sci. 2020, 21, e23. [Google Scholar] [CrossRef]
- Sitter, B.; Bathen, T.F.; Singstad, T.E.; Fjøsne, H.E.; Lundgren, S.; Halgunset, J.; Gribbestad, I.S. Quantification of metabolites in breast cancer patients with different clinical prognosis using HR MAS MR spectroscopy. NMR Biomed. 2010, 23, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Guler, E.N. Gene Expression Profiling in Breast Cancer and Its Effect on Therapy Selection in Early-Stage Breast Cancer. Eur. J. Breast Health 2017, 13, 168–174. [Google Scholar] [CrossRef]
- Prat, A.; Pineda, E.; Adamo, B.; Galván, P.; Fernández, A.; Gaba, L.; Díez, M.; Viladot, M.; Arance, A.; Muñoz, M. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 2015, 24, S26–S35. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.F.M.; Al-Amodi, H.S.A.B. Exploitation of Gene Expression and Cancer Biomarkers in Paving the Path to Era of Personalized Medicine. Genom. Proteom. Bioinform. 2017, 15, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Pawłowski, K.M.; Maciejewski, H.; Majchrzak, K.; Dolka, I.; A Mol, J.; Motyl, T.; Król, M. Five markers useful for the distinction of canine mammary malignancy. BMC Veter. Res. 2013, 9, 138. [Google Scholar] [CrossRef]
- Varallo, G.R.; Gelaleti, G.B.; Maschio-Signorini, L.B.; Moschetta, M.G.; Lopes, J.R.; De Nardi, A.B.; Tinucci-Costa, M.; Rocha, R.M.; De Campos Zuccari, D.A.P. Prognostic phenotypic classification for canine mammary tumors. Oncol. Lett. 2019, 18, 6545–6553. [Google Scholar] [CrossRef]
- Hussain, S.; Saxena, S.; Shrivastava, S.; Mohanty, A.K.; Kumar, S.; Singh, R.J.; Kumar, A.; Wani, S.A.; Gandham, R.K.; Kumar, N.; et al. Gene expression profiling of spontaneously occurring canine mammary tumours: Insight into gene networks and pathways linked to cancer pathogenesis. PLoS ONE 2018, 13, e0208656. [Google Scholar] [CrossRef]
- Thumser-Henner, P.; Nytko, K.J.; Bley, C.R. Mutations of BRCA2 in canine mammary tumors and their targeting potential in clinical therapy. BMC Veter. Res. 2020, 16, 30. [Google Scholar] [CrossRef]
- Ozmen, O.; Kul, S.; Risvanli, A.; Ozalp, G.; Sabuncu, A.; Kul, O. Somatic SNPs of the BRCA2 gene at the fragments encoding RAD51 binding sites of canine mammary tumors. Veter. Comp. Oncol. 2017, 15, 1479–1486. [Google Scholar] [CrossRef]
- Maués, T.; El-Jaick, K.B.; Costa, F.B.; Araujo, G.E.F.; Soares, M.V.G.; Moreira, A.S.; Ferreira, M.L.G.; Ferreira, A.M.R. Common germline haplotypes and genotypes identified in BRCA2 exon 11 of dogs with mammary tumours and histopathological analyses. Veter. Comp. Oncol. 2018, 16, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Canadas, A.; Santos, M.; Nogueira, A.; Assis, J.; Gomes, M.; Lemos, C.; Medeiros, R.; Dias-Pereira, P. Canine mammary tumor risk is associated with polymorphisms in RAD51 and STK11 genes. J. Veter. Diagn. Investig. 2018, 30, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Fish, E.J.; Irizarry, K.J.; DeInnocentes, P.; Ellis, C.J.; Prasad, N.; Moss, A.G.; Bird, R.C. Malignant canine mammary epithelial cells shed exosomes containing differentially expressed microRNA that regulate oncogenic networks. BMC Cancer 2018, 18, 832. [Google Scholar] [CrossRef]
- Aggarwal, T.; Wadhwa, R.; Gupta, R.; Paudel, K.R.; Collet, T.; Chellappan, D.K.; Gupta, G.; Perumalsamy, H.; Mehta, M.; Satija, S.; et al. MicroRNAs as Biomarker for Breast Cancer. Endocrine. Metab. Immune Disord. Drug Targets 2020, 20, 1597–1610. [Google Scholar] [CrossRef]
- Wang, H.; Tan, Z.; Hu, H.; Liu, H.; Wu, T.; Zheng, C.; Wang, X.; Luo, Z.; Wang, J.; Liu, S.; et al. microRNA-21 promotes breast cancer proliferation and metastasis by targeting LZTFL1. BMC Cancer 2019, 19, 738. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-J.; Lee, K.-H.; Nam, A.-R.; Cho, J.-Y. Genome-Wide Methylation Profiling in Canine Mammary Tumor Reveals miRNA Candidates Associated with Human Breast Cancer. Cancers 2019, 11, 1466. [Google Scholar] [CrossRef] [PubMed]
- Bulkowska, M.; Rybicka, A.; Senses, K.M.; Ulewicz, K.; Witt, K.; Szymanska, J.; Taciak, B.; Klopfleisch, R.; Hellmén, E.; Dolka, I.; et al. MicroRNA expression patterns in canine mammary cancer show significant differences between metastatic and non-metastatic tumours. BMC Cancer 2017, 17, 728. [Google Scholar] [CrossRef]
- Heishima, K.; Ichikawa, Y.; Yoshida, K.; Iwasaki, R.; Sakai, H.; Nakagawa, T.; Tanaka, Y.; Hoshino, Y.; Okamura, Y.; Murakami, M.; et al. Circulating microRNA-214 and -126 as potential biomarkers for canine neoplastic disease. Sci. Rep. 2017, 7, 2301. [Google Scholar] [CrossRef]
- Ramadan, E.S.; Salem, N.Y.; Emam, I.A.; AbdElKader, N.A.; Farghali, H.A.; Khattab, M.S. MicroRNA-21 expression, serum tumor markers, and immunohistochemistry in canine mammary tumors. Veter. Res. Commun. 2021, 46, 377–388. [Google Scholar] [CrossRef]
- Lee, K.-H.; Park, H.-M.; Son, K.-H.; Shin, T.-J.; Cho, J.-Y. Transcriptome Signatures of Canine Mammary Gland Tumors and Its Comparison to Human Breast Cancers. Cancers 2018, 10, 317. [Google Scholar] [CrossRef]
- Kim, K.-K.; Seung, B.-J.; Kim, D.; Park, H.-M.; Lee, S.; Song, D.-W.; Lee, G.; Cheong, J.-H.; Nam, H.; Sur, J.-H.; et al. Whole-exome and whole-transcriptome sequencing of canine mammary gland tumors. Sci. Data 2019, 6, 147. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-M.; Yang, I.S.; Seung, B.-J.; Lee, S.; Kim, D.; Ha, Y.-J.; Seo, M.-K.; Kim, K.-K.; Kim, H.S.; Cheong, J.-H.; et al. Cross-species oncogenic signatures of breast cancer in canine mammary tumors. Nat. Commun. 2020, 11, 3616. [Google Scholar] [CrossRef] [PubMed]
- Seung, B.-J.; Lim, H.-Y.; Shin, J.-I.; Kim, H.-W.; Cho, S.-H.; Kim, S.-H.; Sur, J.-H. CD204-Expressing Tumor-Associated Macrophages Are Associated with Malignant, High-Grade, and Hormone Receptor–Negative Canine Mammary Gland Tumors. Veter. Pathol. 2018, 55, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Panni, R.Z.; Linehan, D.C.; DeNardo, D.G. Targeting tumor-infiltrating macrophages to combat cancer. Immunotherapy 2013, 5, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- de Souza, T.A.; de Campos, C.B.; De Biasi Bassani Goncalves, A.; Nunes, F.C.; Monteiro, L.N.; de Oliveira Vasconcelos, R.; Cassali, G.D. Relationship between the inflammatory tumor microenvironment and different histologic types of canine mammary tumors. Res. Vet. Sci. 2018, 119, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.N.; dos Reis, D.C.; Salgado, B.S.; Cassali, G.D. Clinical significance and prognostic role of tumor-associated macrophages infiltration according to histologic location in canine mammary carcinomas. Res. Veter. Sci. 2020, 135, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, M.S.; Brandi, A.; de Oliveira Matos Prado, J.K.; Elias, F.; Dalmolin, F.; Lainetti, P.D.F.; Prado, M.C.M.; Leis-Filho, A.F.; Fonseca-Alves, C.E. Tumor-infiltrating CD4+ and CD8+ lymphocytes and macrophages are associated with prognostic factors in triple-negative canine mammary complex type carcinoma. Res. Veter. Sci. 2019, 126, 29–36. [Google Scholar] [CrossRef]
- Carvalho, M.I.; Pires, I.; Prada, J.; Gregório, H.; Lobo, L.; Queiroga, F.L. Intratumoral FoxP3 expression is associated with angiogenesis and prognosis in malignant canine mammary tumors. Veter. Immunol. Immunopathol. 2016, 178, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Nakazawa, M.; Uchida, M.; Yoshitake, R.; Nakagawa, T.; Nishimura, R.; Miyamoto, R.; Bonkobara, M.; Yonezawa, T.; Momoi, Y. Foxp3+ Regulatory T Cells Associated with CCL17/CCR4 Expression in Carcinomas of Dogs. Veter. Pathol. 2020, 57, 497–506. [Google Scholar] [CrossRef]
- Karayannopoulou, M.; Anagnostou, T.; Margariti, A.; Kostakis, C.; Kritsepi-Konstantinou, M.; Psalla, D.; Savvas, I. Evaluation of blood T-lymphocyte subpopulations involved in host cellular immunity in dogs with mammary cancer. Veter. Immunol. Immunopathol. 2017, 186, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Rogez, B.; Pascal, Q.; Bobillier, A.; Machuron, F.; Lagadec, C.; Tierny, D.; Le Bourhis, X.; Chopin, V. CD44 and CD24 Expression and Prognostic Significance in Canine Mammary Tumors. Veter. Pathol. 2018, 56, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Zmigrodzka, M.; Rzepecka, A.; Krzyzowska, M.; Witkowska-Pilaszewicz, O.; Cywinska, A.; Winnicka, A. The cyclooxygenase-2/prostaglandin E2 pathway and its role in the pathogenesis of human and dog hematological malignancies. J. Physiol. Pharmacol. 2018, 69, 653–661. [Google Scholar] [CrossRef]
- Carvalho, M.I.; Pires, I.; Prada, J.; Pinto, C.; Gregório, H.; Cogliati, B.; Queiroga, F.L. Assessing the Interleukin 35 Immuno-expression in Malignant Canine Mammary Tumors: Association with Clinicopathological Parameters and Prognosis. Anti-Cancer Res. 2019, 39, 2077–2083. [Google Scholar] [CrossRef]
- Carvalho, M.I.; Pires, I.; Prada, J.; Raposo, T.P.; Gregório, H.; Lobo, L.; Queiroga, F.L. High COX -2 expression is associated with increased angiogenesis, proliferation and tumoural inflammatory infiltrate in canine malignant mammary tumours: A multivariate survival study. Veter. Comp. Oncol. 2016, 15, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Raposo, T.P.; Pires, I.; Prada, J.; Queiroga, F.L.; Argyle, D.J. Exploring new biomarkers in the tumour microenvironment of canine inflammatory mammary tumours. Veter. Comp. Oncol. 2016, 15, 655–666. [Google Scholar] [CrossRef]
- Anadol, E.; Saglam, A.S.Y.; Gultiken, N.; Karakas, K.; Alcigir, E.; Alkan, H.; Kanca, H. Expression of iNOS, COX-2 and VEGF in canine mammary tumours and non-neoplastic mammary glands: Association with clinicopathological features and tumour grade. Acta Veter. Hung. 2017, 65, 382–393. [Google Scholar] [CrossRef]
- Gray, M.; Meehan, J.; Martinez-Perez, C.; Kay, C.; Turnbull, A.K.; Morrison, L.R.; Pang, L.Y.; Argyle, D. Naturally-Occurring Canine Mammary Tumors as a Translational Model for Human Breast Cancer. Front. Oncol. 2020, 10, 617. [Google Scholar] [CrossRef]
- Dos Reis, D.C.; Damasceno, K.A.; De Campos, C.B.; Veloso, E.S.; Pêgas, G.R.A.; Kraemer, L.R.; Rodrigues, M.A.; Mattos, M.; Gomes, D.A.; Campos, P.P.; et al. Versican and Tumor-Associated Macrophages Promotes Tumor Progression and Metastasis in Canine and Murine Models of Breast Carcinoma. Front. Oncol. 2019, 9, 577. [Google Scholar] [CrossRef]
- Andaluz, A.; Yeste, M.; Rodríguez-Gil, J.E.; Rigau, T.; García, F.; del Alamo, M.M.R. Pro-inflammatory cytokines: Useful markers for the diagnosis of canine mammary tumours? Veter. J. 2016, 210, 92–94. [Google Scholar] [CrossRef]
- Carvalho, M.I.; Bianchini, R.; Fazekas-Singer, J.; Herrmann, I.; Flickinger, I.; Thalhammer, J.G.; Pires, I.; Jensen-Jarolim, E.; Queiroga. F.L. Bidirectional Regulation of COX-2 Expression Between Cancer Cells and Macrophages. Anticancer Res. 2018, 38, 2811–2817. [Google Scholar] [CrossRef]
- Bujak, J.K.; Szopa, I.M.; Pingwara, R.; Kruczyk, O.; Krzemińska, N.; Mucha, J.; Majchrzak-Kuligowska, K. The Expression of Selected Factors Related to T Lymphocyte Activity in Canine Mammary Tumors. Int. J. Mol. Sci. 2020, 21, 2292. [Google Scholar] [CrossRef]
- Hussain, S.; Saxena, S.; Shrivastava, S.; Arora, R.; Singh, R.J.; Jena, S.C.; Kumar, N.; Sharma, A.; Sahoo, M.; Tiwari, A.K.; et al. Multiplexed Autoantibody Signature for Serological Detection of Canine Mammary Tumours. Sci. Rep. 2018, 8, 15785. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.V.S.; Bhardwaj, R.; Mahajan, K.; Kashyap, N.; Kumar, A.; Verma, R. The overexpression of Hsp90B1 is associated with tumorigenesis of canine mammary glands. Mol. Cell. Biochem. 2017, 440, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Birdi, R.; Kumar, B.V.S.; Gupta, K.; Kashyap, N.; Kumar, A. Circulating level of heat shock protein 27 is elevated in dogs with mammary tumors. 3 Biotech. 2019, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Witkowska-Piłaszewicz, O.D.; Żmigrodzka, M.; Winnicka, A.; Miśkiewicz, A.; Strzelec, K.; Cywińska, A. Serum amyloid A in equine health and disease. Equine Veter. J. 2018, 51, 293–298. [Google Scholar] [CrossRef]
- Szczubiał, M.; Dabrowski, R.; Łopuszyński, W.; Bochniarz, M.; Krawczyk, M. Changes in serum neopterin and C-reactive protein concentrations in female dogs with mammary gland tumours. Pol. J. Vet. Sci. 2018, 21, 691–696. [Google Scholar] [CrossRef]
- Li, Q.; Kim, Y.-S.; An, J.-H.; Kwon, J.-A.; Han, S.-H.; Song, W.-J.; Youn, H.-Y. Anti-tumor effects of rivoceranib against canine melanoma and mammary gland tumour in vitro and in vivo mouse xenograft models. BMC Veter. Res. 2021, 17, 338. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, G.; Alonso-Diez, A.; Pérez-Alenza, D.; Peña, L. From Conventional to Precision Therapy in Canine Mammary Cancer: A Comprehensive Review. Front. Veter. Sci. 2021, 8, 1–33. [Google Scholar] [CrossRef]
| Biomarker/Biomarkers | Function | Main Finding | References |
|---|---|---|---|
| cycylin D1, cyclin E1, | Cell cycle | cyclin D1 overexpression is commonly related with metastasis and shorter life expectancy | [43,44,45,46,47,48,49,50,51,52] |
| Ki-67, PCNA | Proliferation | expression of Ki-67 and PCNA positively correlates with cancer progression | [53,54,55,56,57,58,59,60] |
| HER-2 | Proliferation | there is a correlation between HER-2 expression and tumor mitotic index, high histological grade, and tumor size | [62,63,64,65,66,67] |
| PD-1 | Apoptosis | most canine mammary tumor show expression of the PD-L1 | [68,69,70,71,72,73,74,75,76,77,78,79,80,81,82] |
| p53 | Apoptosis | p53 expression was connected with high proliferative activity and high histological grade | [83,84,85,86,87,88,89,90] |
| E-cadherin | Cell adhesion | downregulation of E-cadherin expression is connected with increased tumor growth and disease spreading, tumor malignancy, the aggressiveness of metastases, and short life expectancy | [91,92,93,94,95] |
| CEA, CA 15-3 | Cell adhesion | both positively correlate with the presence of metastases, tumor dimension, and histological grade, it is recommended for them to be evaluated together | [96,97,98,99,100,101,102,103,104,105,106] |
| ER, PR | Hormone receptors | the expression of ER or PR was more common in non-malignant tumors, and usually was connected with better clinical outcome | [107,108,109,110,111,112,113,114,115,116,117,118,119] |
| 5-hydroxyindolacetic acid, serotonin, indoxyl sulphate, and kynurenic acid, 3,4-dihydroxy-L-phenylalanine and epinephrine | Metabolites of tyrosine and tryptophan | detected in samples of the urine in CMTs patients | [125,126] |
| HYAL-1 | Gene encoding lysosomal hyaluronidase | a possible biomarker linked with cell growth, migration, invasion, and angiogenesis | [132] |
| BRCA1, BRCA2 | Genes involved in DNA repair | The 97.9% of dogs with CMT had one up to three genetic variations out of the seven | [134,135,136,137] |
| miR-214 and miR-126 | miRNA | remarkably up-regulated in serum of bitches with mammary carcinoma | [143] |
| miR-18a | miRNA | seemed to be a good prognostic factor | [139] |
| miR-21 | miRNA | be more sensitive than other commonly used markers | [144] |
| CD204+ macrophages | Inflammatory cell infiltration | In dogs with III grade CMTs, the number of CD204-positive macrophages was greater than in grades I and II | [151] |
| LT CD3+, LTCD4+ | Inflammatory cell infiltration | high CD3+ and CD4+ cells in the tumor tissue correlate with shorter survival time | [152] |
| LT FoxP3+ | Inflammatory cell infiltration | a big number of intratumoral FoxP3+ cells was related to defective differentiation of tumors, high histological grade of malignancy, and increased angiogenesis, thus guarded prognosis | [153] |
| COX-2 | Inflammatory markers | increased expression is correlated with the histological grade of malignancy | [161] |
| VEGF, TNF-α, TGF-β1 | pro-inflammatory and pro-tumoral cytokines and chemokines | CMT cytokines expression seems to be a marker of canine mammary tumors malignancy | [162] |
| MCP-1, MCP-2, PDGF-BB, RANTES, SCF | pro-inflammatory cytokines | expressed in tumors with higher aggressiveness | [164] |
| IL-35 | interleukin | connected with worse overall survival by increased histological grade of malignancy, mitotic index, neoplastic intravascular emboli, and lymph node metastasis | [165] |
| CXCR3, CCR2, IL-4, IL-12p40 | pro-inflammatory cytokines and interleukines | cytokines connected with T lymphocyte activity are upregulated | [166] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaszak, I.; Witkowska-Piłaszewicz, O.; Domrazek, K.; Jurka, P. The Novel Diagnostic Techniques and Biomarkers of Canine Mammary Tumors. Vet. Sci. 2022, 9, 526. https://doi.org/10.3390/vetsci9100526
Kaszak I, Witkowska-Piłaszewicz O, Domrazek K, Jurka P. The Novel Diagnostic Techniques and Biomarkers of Canine Mammary Tumors. Veterinary Sciences. 2022; 9(10):526. https://doi.org/10.3390/vetsci9100526
Chicago/Turabian StyleKaszak, Ilona, Olga Witkowska-Piłaszewicz, Kinga Domrazek, and Piotr Jurka. 2022. "The Novel Diagnostic Techniques and Biomarkers of Canine Mammary Tumors" Veterinary Sciences 9, no. 10: 526. https://doi.org/10.3390/vetsci9100526
APA StyleKaszak, I., Witkowska-Piłaszewicz, O., Domrazek, K., & Jurka, P. (2022). The Novel Diagnostic Techniques and Biomarkers of Canine Mammary Tumors. Veterinary Sciences, 9(10), 526. https://doi.org/10.3390/vetsci9100526





