Prevalence of Reproductive Disorders including Mammary Tumors and Associated Mortality in Female Dogs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Survey of Reproductive Pathology including Mammary Tumors
2.3. Causes of Death
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
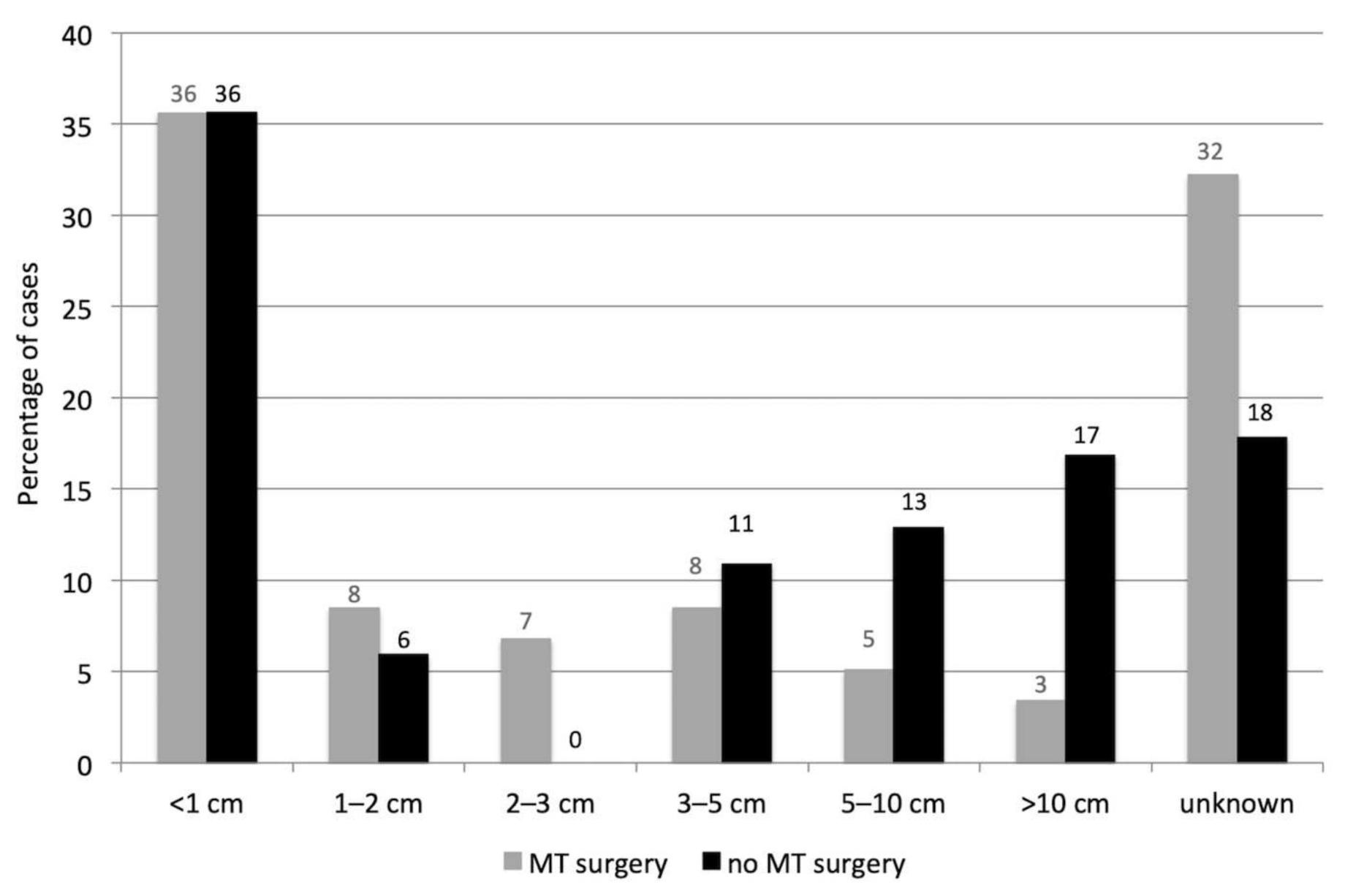
3.2. Reproductive Pathology including Mammary Tumors
3.3. Causes of Death
3.4. Survival Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Misdorp, W. Canine mammary tumours: Protective effect of late ovariectomy and stimulating effect of progestins. Vet. Q. 1988, 10, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Mulligan, R.M. Mammary cancer in the dog: A study of 120 cases. Am. J. Vet. Res. 1975, 36, 1391–1396. [Google Scholar]
- Schneider, R.; Dorn, C.R.; Taylor, D.O. Factors influencing canine mammary cancer development and postsurgical survival. J. Natl Cancer Inst. 1969, 43, 1249–1261. [Google Scholar] [PubMed]
- Beauvais, W.; Cardwell, J.M.; Brodbelt, D.C. The effect of neutering on the risk of mammary tumours in dogs- a systematic review. J. Small Anim. Pract. 2012, 53, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Belanger, J.M.; Bellumori, T.P.; Bannasch, D.L.; Famula, T.R.; Oberbauer, A.M. Correlation of neuter status and expression of heritable disorders. Canine Genet. Epidemiol. 2017, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Grüntzig, K.; Graf, R.; Boo, G.; Guscetti, F.; Hässig, M.; Axhausen, K.W.; Fabrikant, S.; Welle, M.; Meier, D.; Folkers, G.; et al. Swiss Canine Cancer Registry 1955-2008: Occurrence of the Most Common Tumour Diagnoses and Influence of Age, Breed, Body Size, Sex and Neutering Status on Tumour Development. J. Comp. Pathol. 2016, 155, 156–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, B.L.; Hart, L.A.; Thigpen, A.P.; Willits, N.H. Long-term health effects of neutering dogs: Comparison of Labrador Retrievers with Golden Retrievers. PLoS ONE 2014, 9, e102241. [Google Scholar] [CrossRef] [Green Version]
- Hart, B.L.; Hart, L.A.; Thigpen, A.P.; Willits, N.H. Neutering of German Shepherd Dogs: Associated joint disorders, cancers and urinary incontinence. Vet. Med. Sci. 2016, 2, 191–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres de la Riva, G.; Hart, B.L.; Farver, T.B.; Oberbauer, A.M.; Messam, L.L.; Willits, N.; Hart, L.A. Neutering dogs: Effects on joint disorders and cancers in golden retrievers. PLoS ONE 2013, 8, e55937. [Google Scholar] [CrossRef]
- Urfer, S.R.; Kaeberlein, M. Desexing Dogs: A Review of the Current Literature. Animals 2019, 9, 1086. [Google Scholar] [CrossRef] [Green Version]
- Urfer, S.R.; Wang, M.; Yang, M.; Lund, E.M.; Lefebvre, S.L. Risk Factors Associated with Lifespan in Pet Dogs Evaluated in Primary Care Veterinary Hospitals. J. Am. Anim Hosp. Assoc. 2019, 55, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Zink, M.C.; Farhoody, P.; Elser, S.E.; Ruffini, L.D.; Gibbons, T.A.; Rieger, R.H. Evaluation of the risk and age of onset of cancer and behavioral disorders in gonadectomized Vizslas. J. Am. Vet. Med. Assoc. 2014, 244, 309–319. [Google Scholar] [CrossRef]
- Kristiansen, V.M.; Nødtvedt, A.; Breen, A.M.; Langeland, M.; Teige, J.; Goldschmidt, M.; Jonasdottir, T.J.; Grotmol, T.; Sørenmo, K. Effect of ovariohysterectomy at the time of tumor removal in dogs with benign mammary tumors and hyperplastic lesions: A randomized controlled clinical trial. J. Vet. Intern. Med. 2013, 27, 935–942. [Google Scholar] [CrossRef]
- Stratmann, N.; Failing, K.; Richter, A.; Wehrend, A. Mammary tumor recurrence in bitches after regional mastectomy. Vet. Surg. 2008, 37, 82–86. [Google Scholar] [CrossRef] [PubMed]
- MacEwen, E.G.; Hayes, A.A.; Harvey, H.J.; Patnaik, A.K.; Mooney, S.; Passe, S. Prognostic factors for feline mammary tumors. J. Am. Vet. Med. Assoc. 1984, 185, 201–204. [Google Scholar]
- McNeill, C.J.; Sorenmo, K.U.; Shofer, F.S.; Gibeon, L.; Durham, A.C.; Barber, L.G.; Baez, J.L.; Overley, B. Evaluation of adjuvant doxorubicin-based chemotherapy for the treatment of feline mammary carcinoma. J. Vet. Intern. Med. 2009, 23, 123–129. [Google Scholar] [CrossRef]
- Gemignani, F.; Mayhew, P.D.; Giuffrida, M.A.; Palaigos, J.; Runge, J.J.; Holt, D.E.; Robertson, N.A.; Seguin, B.; Walker, M.; Singh, A.; et al. Association of surgical approach with complication rate, progression-free survival time, and disease-specific survival time in cats with mammary adenocarcinoma: 107 cases (1991–2014). J. Am. Vet. Med. Assoc. 2018, 252, 1393–1402. [Google Scholar] [CrossRef] [Green Version]
- Taylor, G.N.; Shabestari, L.; Williams, J.; Mays, C.W.; Angus, W.; McFarland, S. Mammary neoplasia in a closed beagle colony. Cancer Res. 1976, 36, 2740–2743. [Google Scholar]
- Byron, J.K.; Taylor, K.H.; Phillips, G.S.; Stahl, M.S. Urethral Sphincter Mechanism Incompetence in 163 Neutered Female Dogs: Diagnosis, Treatment, and Relationship of Weight and Age at Neuter to Development of Disease. J. Vet. Intern. Med. 2017, 31, 442–448. [Google Scholar] [CrossRef] [Green Version]
- De Bleser, B.; Brodbelt, D.C.; Gregory, N.G.; Martinez, T.A. The association between acquired urinary sphincter mechanism incompetence in bitches and early spaying: A case-control study. Vet. J. 2011, 187, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.E.; Thrusfield, M.V. Association in bitches between breed, size, neutering and docking, and acquired urinary incontinence due to incompetence of the urethral sphincter mechanism. Vet. Rec. 1993, 133, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Spain, C.V.; Scarlett, J.M.; Houpt, K.A. Long-term risks and benefits of early-age gonadectomy in dogs. J. Am. Vet. Med. Assoc. 2004, 224, 380–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egenvall, A.; Hagman, R.; Bonnett, B.N.; Hedhammar, A.; Olson, P.; Lagerstedt, A.S. Breed risk of pyometra in insured dogs in Sweden. J. Vet. Intern. Med. 2001, 15, 530–538. [Google Scholar] [CrossRef]
- Hagman, R. Pyometra in Small Animals. Vet. Clin. N. Am. Small Anim Pract. 2018, 48, 639–661. [Google Scholar] [CrossRef] [PubMed]
- Jitpean, S.; Hagman, R.; Ström Holst, B.; Höglund, O.V.; Pettersson, A.; Egenvall, A. Breed variations in the incidence of pyometra and mammary tumours in Swedish dogs. Reprod. Domest. Anim. 2012, 47 (Suppl. 6), 347–350. [Google Scholar] [CrossRef]
- Salas, Y.; Márquez, A.; Diaz, D.; Romero, L. Epidemiological Study of Mammary Tumors in Female Dogs Diagnosed during the Period 2002-2012: A Growing Animal Health Problem. PLoS ONE 2015, 10, e0127381. [Google Scholar] [CrossRef] [Green Version]
- Vascellari, M.; Capello, K.; Carminato, A.; Zanardello, C.; Baioni, E.; Mutinelli, F. Incidence of mammary tumors in the canine population living in the Veneto region (Northeastern Italy): Risk factors and similarities to human breast cancer. Prev Vet. Med. 2016, 126, 183–189. [Google Scholar] [CrossRef]
- Thrusfield, M.V.; Holt, P.E.; Muirhead, R.H. Acquired urinary incontinence in bitches: Its incidence and relationship to neutering practices. J. Small Anim. Pract. 1998, 39, 559–566. [Google Scholar] [CrossRef]
- Stöcklin-Gautschi, N.M.; Hässig, M.; Reichler, I.M.; Hubler, M.; Arnold, S. The relationship of urinary incontinence to early spaying in bitches. J. Reprod. Fertil Suppl. 2001, 57, 233–236. [Google Scholar] [PubMed]
- Chocteau, F.; Abadie, J.; Loussouarn, D.; Nguyen, F. Proposal for a Histological Staging System of Mammary Carcinomas in Dogs and Cats. Part 1: Canine Mammary Carcinomas. Front. Vet. Sci. 2019, 6, 388. [Google Scholar] [CrossRef] [Green Version]
- Sorenmo, K.U.; Kristiansen, V.M.; Cofone, M.A.; Shofer, F.S.; Breen, A.M.; Langeland, M.; Mongil, C.M.; Grondahl, A.M.; Teige, J.; Goldschmidt, M.H. Canine mammary gland tumours; a histological continuum from benign to malignant; clinical and histopathological evidence. Vet. Comp. Oncol. 2009, 7, 162–172. [Google Scholar] [CrossRef]
- Chang, S.C.; Chang, C.C.; Chang, T.J.; Wong, M.L. Prognostic factors associated with survival two years after surgery in dogs with malignant mammary tumors: 79 cases (1998-2002). J. Am. Vet. Med. Assoc. 2005, 227, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Peña, L.; De Andrés, P.J.; Clemente, M.; Cuesta, P.; Pérez-Alenza, M.D. Prognostic value of histological grading in noninflammatory canine mammary carcinomas in a prospective study with two-year follow-up: Relationship with clinical and histological characteristics. Vet. Pathol. 2013, 50, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, F.; Peña, L.; Ibisch, C.; Loussouarn, D.; Gama, A.; Rieder, N.; Belousov, A.; Campone, M.; Abadie, J. Canine invasive mammary carcinomas as models of human breast cancer. Part 1: Natural history and prognostic factors. Breast Cancer Res. Treat. 2018, 167, 635–648. [Google Scholar] [CrossRef] [Green Version]
- Kristiansen, V.M.; Peña, L.; Díez Córdova, L.; Illera, J.C.; Skjerve, E.; Breen, A.M.; Cofone, M.A.; Langeland, M.; Teige, J.; Goldschmidt, M.; et al. Effect of Ovariohysterectomy at the Time of Tumor Removal in Dogs with Mammary Carcinomas: A Randomized Controlled Trial. J. Vet. Intern. Med. 2016, 30, 230–241. [Google Scholar] [CrossRef] [Green Version]
- Tran, C.M.; Moore, A.S.; Frimberger, A.E. Surgical treatment of mammary carcinomas in dogs with or without postoperative chemotherapy. Vet. Comp. Oncol. 2016, 14, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.M.; Creevy, K.E.; Promislow, D.E. Reproductive capability is associated with lifespan and cause of death in companion dogs. PLoS ONE 2013, 8, e61082. [Google Scholar] [CrossRef] [Green Version]
- Kent, M.S.; Burton, J.H.; Dank, G.; Bannasch, D.L.; Rebhun, R.B. Association of cancer-related mortality, age and gonadectomy in golden retriever dogs at a veterinary academic center (1989–2016). PLoS ONE 2018, 13, e0192578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorenmo, K.U.; Durham, A.C.; Radaelli, E.; Kristiansen, V.; Peña, L.; Goldschmidt, M.H.; Stefanovski, D. The estrogen effect; clinical and histopathological evidence of dichotomous influences in dogs with spontaneous mammary carcinomas. PLoS ONE 2019, 14, e0224504. [Google Scholar] [CrossRef] [Green Version]

| Breed | Total Cohort (n = 599) | Intact Females (n = 306) | ES Females (n = 50) | LS Females (n = 243) |
|---|---|---|---|---|
| American Staffordshire Terrier | 6 (1.0%) | 2 (0.6%) | 1 (0.5%) | 3 (1.2%) |
| Beagle | 9 (1.5%) | 5 (1.6%) | 0 | 4 (1.6%) |
| Beauceron | 12 (2.0%) | 6 (2.0%) | 3 (1.5%) | 3 (1.2%) |
| Belgian Shepherd | 16 (2.7%) | 6 (2.0%) | 0 | 10 (4.1%) |
| Bernese Mountain Dog | 3 (0.5%) | 2 (0.6%) | 0 | 1 (0.4%) |
| Bichon | 24 (4.0%) | 13 (4.2%) | 2 (1.0%) | 9 (3.7%) |
| Border Collie | 12 (2.0%) | 5 (1.6%) | 0 | 7 (2.9%) |
| Boxer | 16 (2.7%) | 3 (1.0%) | 3 (1.5%) | 10 (4.1%) |
| Brittany fawn dog | 5 (0.8%) | 3 (1.0%) | 0 | 2 (0.8%) |
| Brittany Spaniel | 38 (6.3%) | 23 (7.5%) | 1 (0.5%) | 14 (5.8%) |
| Cavalier King Charles | 11 (1.8%) | 3 (1.0%) | 0 | 8 (3.3%) |
| Cocker spaniel | 14 (2.3%) | 11 (3.6%) | 1 (0.5%) | 2 (0.8%) |
| Collie | 8 (1.3%) | 3 (1.0%) | 1 (0.5%) | 4 (1.6%) |
| Continental Toy Spaniel | 7 (1.2%) | 3 (1.0%) | 0 | 4 (1.6%) |
| Coton de Tuléar | 5 (0.8%) | 4 (1.3%) | 0 | 1 (0.4%) |
| Cross-bred dogs | 118 (19.7%) | 55 (18.0%) | 18 (9.0%) | 45 (18.5%) |
| Dachshund | 26 (4.3%) | 16 (5.2%) | 0 | 10 (4.1%) |
| German Shepherd | 15 (2.5%) | 4 (1.3%) | 1 (0.5%) | 10 (4.1%) |
| German Shorthaired Pointer | 4 (0.7%) | 3 (1.0%) | 0 | 1 (0.4%) |
| Golden Retriever | 16 (2.7%) | 6 (2.0%) | 0 | 10 (4.1%) |
| Labrador Retriever | 58 (9.7%) | 27 (8.8%) | 10 (5.0%) | 21 (8.6%) |
| Lhassa Apso | 6 (1.0%) | 3 (1.0%) | 0 | 3 (1.2%) |
| Newfoundland dog | 4 (0.7%) | 4 (1.3%) | 0 | 0 |
| Pekingese dog | 3 (0.5%) | 1 (0.3%) | 0 | 2 (0.8%) |
| Pinscher | 10 (1.7%) | 2 (0.6%) | 3 (1.5%) | 5 (2.1%) |
| Poodle | 32 (5.3%) | 18 (5.9%) | 3 (1.5%) | 11 (4.5%) |
| Pyrenean Mountain dog | 3 (0.5%) | 3 (1.0%) | 0 | 0 |
| Rottweiler | 21 (3.5%) | 11 (3.6%) | 0 | 10 (4.1%) |
| Setter | 17 (2.8%) | 14 (4.6%) | 0 | 3 (1.2%) |
| Shih Tzu | 12 (2.0%) | 6 (2.0%) | 1 (0.5%) | 5 (2.1%) |
| St. Bernard | 3 (0.5%) | 0 | 0 | 3 (1.2%) |
| West Highland White Terrier | 3 (0.5%) | 2 (0.6%) | 0 | 1 (0.4%) |
| Yorkshire Terrier | 33 (5.5%) | 22 (7.2%) | 1 (0.5%) | 10 (4.1%) |
| Other breeds (*) | 29 (4.8%) | 17 (5.6%) | 1 (0.5%) | 11 (4.5%) |
| Total | 599 (100%) |
| Causes of Death | ES Group (n = 31) | LS Group (n = 123) | Intact Eemales (n = 139) | |
|---|---|---|---|---|
| Non-Mammary Cancer (n = 48) | 10 (32.3%) | 21 (17.1%) | 17 (12.2%) | |
| Lymphoma (n = 7) | 2 (6.5%) | 2 (1.6%) | 3 (2.2%) | |
| Liver cancer (n = 5) | 2 (6.5%) | 2 (1.6%) | 1 (0.7%) | |
| Oral cancer (n = 5) | 1 (3.2%) | 3 (2.4%) | 1 (0.7%) | |
| Spleen malignancy (n = 4) | 2 (6.5%) | 2 (1.6%) | 0 | |
| Hemangiosarcoma (n = 3) | 0 | 2 (1.6%) | 1 (0.7%) | |
| Lung cancer (n = 3) | 2 (6.5%) | 1 (0.8%) | 0 | |
| Soft tissue sarcoma (n = 3) | 0 | 3 (2.4%) | 0 | |
| Pancreatic cancer (n = 2) | 0 | 1 (0.8%) | 1 (0.7%) | |
| Osteosarcoma (n = 2) | 0 | 2 (1.6%) | 0 | |
| Other sites (n = 14) 1 | 1 (3.2%) | 3 (2.4%) | 10 (7.2%) | |
| Reproductive pathology (n = 68) | 0 | 14 (11.4%) | 54 (38.8%) | |
| Mammary cancer (n = 53) | 0 | 11 (8.9%) | 42 (30.2%) | |
| Pyometra (n = 12) | 0 | 2 (1.6%) | 10 (7.2%) | |
| Ovarian carcinoma (n = 3) | 0 | 1 (0.8%) | 2 (1.4%) | |
| Other causes (n = 177) | 21 (67.7%) | 88 (71.5%) | 68 (48.9%) | |
| Locomotor disability (n = 46) 2 | 6 (19.4%) | 22 (17.9%) | 18 (12.9%) | |
| Renal failure (n = 20) | 2 (6.5%) | 11 (8.9%) | 7 (5.0%) | |
| Heart failure (n = 15) | 1 (3.3%) | 12 (9.8%) | 2 (1.4%) | |
| Traffic accident (n = 11) | 1 (3.3%) | 4 (3.3%) | 6 (4.3%) | |
| Hepatic disorders (n = 9) 3 | 1 (3.3%) | 5 (4.1%) | 3 (2.2%) | |
| Endocrine diseases (n = 8) 4 | 2 (6.5%) | 2 (1.6%) | 4 (2.9%) | |
| Respiratory failure (n = 5) | 1 (3.3%) | 2 (1.6%) | 2 (1.4%) | |
| Pancreatitis (n = 5) | 0 | 4 (3.3%) | 1 (0.7%) | |
| Glaucoma (n = 3) | 1 (3.3%) | 2 (1.6%) | 0 | |
| Aggressiveness (n = 3) | 0 | 0 | 3 (2.2%) | |
| Miscellaneous 5 (n = 52) | 6 (19.4%) | 24 (19.5%) | 22 (15.8%) | |
| Variables | HR | 95% CI | p |
|---|---|---|---|
| Age at the diagnosis (years) | 1.26 | 1.10–1.46 | 0.0014 |
| Neutered females versus intact | 0.33 | 0.15–0.71 | 0.0049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beaudu-Lange, C.; Larrat, S.; Lange, E.; Lecoq, K.; Nguyen, F. Prevalence of Reproductive Disorders including Mammary Tumors and Associated Mortality in Female Dogs. Vet. Sci. 2021, 8, 184. https://doi.org/10.3390/vetsci8090184
Beaudu-Lange C, Larrat S, Lange E, Lecoq K, Nguyen F. Prevalence of Reproductive Disorders including Mammary Tumors and Associated Mortality in Female Dogs. Veterinary Sciences. 2021; 8(9):184. https://doi.org/10.3390/vetsci8090184
Chicago/Turabian StyleBeaudu-Lange, Claire, Sylvain Larrat, Emmanuel Lange, Kevin Lecoq, and Frédérique Nguyen. 2021. "Prevalence of Reproductive Disorders including Mammary Tumors and Associated Mortality in Female Dogs" Veterinary Sciences 8, no. 9: 184. https://doi.org/10.3390/vetsci8090184
APA StyleBeaudu-Lange, C., Larrat, S., Lange, E., Lecoq, K., & Nguyen, F. (2021). Prevalence of Reproductive Disorders including Mammary Tumors and Associated Mortality in Female Dogs. Veterinary Sciences, 8(9), 184. https://doi.org/10.3390/vetsci8090184





