Abstract
The use of cannabidiol (CBD) for animal species is an area of growing interest, for example for its anti-inflammatory and immuno-modulating properties, even though all of its biological effects are still not fully understood, especially in veterinary medicine. Therefore, the aim of this study was to investigate the anti-inflammatory and immuno-modulating properties of CBD for the first time directly in canine inflammatory response. We used an ex vivo model of LPS-stimulated whole dog blood. We stimulated the whole blood from healthy dogs with LPS 100 ng/mL for 24 h in the presence or not of CBD 50 and 100 μg/mL. We observed a reduction in IL-6 and TNF-α production from the group treated with CBD, but non-altered IL-10 levels. Moreover, we also observed from the CBD-treated group a reduction in Nf-κB and COX-2 expression. In conclusion, we demonstrated for the first time the anti-inflammatory and immuno-modulating properties of CBD directly in dogs’ immune cells, using a canine ex vivo inflammatory model. The results obtained from these studies encourage further studies to better understand the possible therapeutic role of CBD in veterinary medicine.
1. Introduction
Cannabidiol (CBD) is one of the major non-psychoactive plant (Cannabis sp.)-derived cannabinoids that has structural similarity to the primary psychotropic congener in cannabis, D9-tetrahydrocannabinol (THC). The use of cannabis for animal species is an area of growing interest, even if to date there are few studies that have characterized the biological effect of CBD in different animal species [1]. CBD is known to act as an anti-inflammatory and immunomodulant agent, and the effects of CBD on immune responses can involve both innate or adaptive responses [2]. Although there are only a few studies on the therapeutic use of CBD in dogs, the pharmacokinetics and tolerability of CBD has been characterized [3,4]. Recently, a pilot study conducted on dogs with naturally occurring osteoarthritis described the safety and efficacy of CBD for symptom relief of canine osteoarthritis-associated pain [5]. However, the immunomodulating, anti-inflammatory and other biological activities of CBD have been characterized much more often in human medicine than in veterinary medicine [6,7,8]. The aim of this study was to evaluate the effect of CBD directly in canine inflammatory response, and to achieve this aim, we used an ex vivo model where whole blood from healthy dogs was stimulated with LPS (100 ng/mL). Ex vivo whole blood stimulation is a reliable method to study the effect of immunomodulatory agents [9,10], as this method replicates the natural environment of immune cell response, preventing changes in phenotype that may occur with the isolation of the cells and minimize the volume of starting blood needed [11]. Thus, this model allowed us to characterize the effect of CBD on canine inflammatory response. As previously seen in other species, CBD is known to modulate cytokines’ secretion from immune cells and thus modulate several pathways in immune function [12,13]. We focused on the levels of the major regulating cytokines, IL-6, TNF-α and IL-10. IL-6 and TNF-α are the major pro-inflammatory cytokines driving inflammatory response and immune cell activation, and IL-10 is an anti-inflammatory cytokine and immune-regulating mediator [14]. Furthermore, we evaluated the effect of CBD on Nf-κB and COX-2 expression. Nf-κB is a key regulator of inflammatory response by regulating the expression of several pro-inflammatory mediators including COX-2. The finding of this study support the anti-inflammatory and immunomodulant effect of CBD in dogs’ immune cells, even though future studies are needed to better understand the biological activity of this compound in different species.
2. Materials and Methods
2.1. Canine Donors and Blood Collection
Whole blood samples were obtained from 6 healthy dogs (working lines of German Shepherd, aged between 2 and 10 years, 3 male and 3 female). An aliquot of heparinized blood was kindly donated with the owner’s informed consent from dogs undergoing routine blood tests. The dogs were healthy, based on clinical examinations performed by veterinarians and the results of complete blood cell counts. Blood samples were processed within 1 h of collection.
2.2. Whole Blood Culture and Stimulation
Whole blood culture and stimulation were performed as previously described [15]. Briefly, blood from each subject was diluted 1:2 with RPMI 1640 (Gibco, Life Technologies Corporation, Waltham, MA, USA) culture medium containing 200 U/mL penicillin and 200 mg/mL streptomycin. An aliquot of 500 μL of diluted whole blood from each subject was incubated (37 °C, 5% CO2) for 24 h with or without CBD (kanarescue 5%©). CBD, in the form of kanarescue compaun, was dissolved in DMSO (final concentration of 0.1% in assay media for all assays) and added directly to the media at the concentrations indicated as follow in duplicate:
- Control: whole blood + vehicle (DMSO 0.1%)
- LPS: whole blood + LPS 100 ng/mL + vehicle (DMSO 0.1%)
- CBD 50 μg: whole blood + LPS 100 ng/mL + CBD (kanarescue 5% in DMSO 0.1%) 50 μg/mL
- CBD 100 μg: whole blood + LPS 100 ng/mL CBD (kanarescue 5% in DMSO 0.1%) 100 μg/mL
After incubation, samples were centrifuged at 2000× g for 5 min at room temperature and processed as homogenous samples for each condition. In particular, the supernatants were harvested and stored immediately at −20 °C until further use, and cell pellets stored at −20 °C were immediately resuspended in appropriate lysis buffer and processed for rt-PCR and Western blot analysis as described above.
2.3. ELISA Assays
Cytokine evaluation was performed in whole blood culture supernatant using a canine-specific ELISA kit according to the manufacturer’s protocols [16], for IL-6, IL-10 and TNF-α (Quantikine, R&D system). Briefly, 100 μL of the appropriate sample was added into the appropriate well of a 96-well plate and incubated at room temperature for 2 h. After washing, 200 µL of conjugate ab was added to each well and incubated at room temperature for 2 h. After washing, 120 μL of the substrate solution was added for 30′, and then 120 μL of the stop solution was added to each well. Finally, the plates were read at 450 nm. The lower limit detections were, respectively, 31.3, 15.6, and 7.8 pg/mL.
2.4. rt-PCR
Total RNA was isolated from cell pellets using the commercially available column kit RNeasy mini kit (Qiagen) according to the manufacturer’s protocols. Briefly, samples were lysed, and subsequently ethanol was added to obtain ideal binding conditions. The lysates obtained were loaded into the RNeasy silica membrane. RNA bound into the column and all contaminants were washed out. Pure, concentrated RNA was eluted in 50 μL of water. RNA was quantified with a spectrophotometer (NanoDrop, NanoReady Life Real, Hangzhou, China). Subsequently, first-strand cDNA synthesis was performed using RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo Scientific™, Rodano (MI), Italy) according to the manufacturer’s protocols and recommendations. The relative abundance of COX-2 was assessed by RT-PCR using QuantiTect SYBR Green PCR Kits (Qiagen, Milano, Italy) and QuantiTect primer Cf_PTGS2_1_SG (Qiagen) on a CFX-96 Touch Deep Well Real-Time PCR Detection System (Bio-Rad, Segrate (MI), Italy) using 1 μL of total cDNA. PCR conditions were as follows: initial denaturation at 95 °C for 15 min, followed by 45 cycles of amplification at 94 °C for 15 s and 72 °C for 30 s. Final extension at 60 °C for 60 s and holding at 4 °C were then performed. To provide precise quantification of the initial target in each PCR reaction, the amplification plot was examined, and the point of the early log phase of product accumulation was defined by assigning a fluorescence threshold above the background, defined as the threshold cycle number or Cq. Differences in the threshold cycle number were used to quantify the relative amount of the PCR targets contained within each tube with CFX Maestro Software (Bio-Rad). The relative expression of different amplicons was calculated by the delta-delta Cq (ΔΔCq) method and converted to the relative expression ratio (2−ΔΔCq) for statistical analysis. All canine data were normalized to the endogenous reference genes β-actin and GAPDH combined, respectively, Cf_ACTB1_1_SG and Cf_GAPDH_1_SG (QuantiTect primers, Qiagen). For each target gene, besides the biological replicates, three technical replicates were performed. Negative controls using RNA as a template were also included in all runs to test for the possible genomic DNA contamination of the samples.
2.5. Western Blot
Western blot analysis was performed as previously described in our previous studies with minor changes [17,18,19]. Briefly, protein concentrations were determined by the Bio-Rad protein assay method using bovine serum albumin as standard. Samples were heated at 100 °C for 5 min, and equal amounts of protein were separated on SDS-PAGE gel and transferred to PVDF membranes. The membranes were blocked with 1 × phosphate-buffered saline (PBS) and 5% (w/v) non- fat dried milk for 40 min at room temperature, and then blots were incubated with specific primary antibody Anti-Nf-κB p65 (1:1000, Cell Signalling) or anti-beta actin (1:1000, Abcam) in 1 × PBS, 5% w/v non-fat dried milk, and 0.1% Tween-20, and incubated at 4 °C overnight. After that, blots were incubated with the peroxidase-conjugated bovine anti-mouse IgG secondary antibody or peroxidase-conjugated goat anti-rabbit IgG (1:2000, Jackson Immuno Research) for 1 h at room temperature. The relative expression of the protein bands was detected with an enhanced chemiluminescence (ECL) system (Bio-Rad) and visualized with the Chemi Doc XRS (Bio-Rad, Segrate (MI), Italy). The bands were analyzed by using Image Lab 3.0 software (Bio-Rad, Segrate (MI), Italy) and standardized to the relevant housekeeping protein level. Results are expressed with the relative fold change to the control group.
2.6. Statistical Analysis
Each experiment included three independent replicates. The data resulting from all experiments are expressed as means ± SEM. Statistical differences between groups were compared using one-way ANOVA, followed by Tukey’s post hoc test for multiple comparisons, using GraphPad Prism version 9 (GraphPad Software Inc., La Jolla, CA, USA). A p-value of less than 0.05 was considered statistically significant [20,21].
3. Results
3.1. Effect of CBD on IL-6 and TNF-α Response in LPS Stimulated Whole Blood
Consistent with previous publications, we used an ex vivo model of inflammation where whole blood was stimulated with a canonical inflammatory stimulus such as LPS (100 ng/mL for 24 h) to induce an inflammatory response. Cytokines are the driving factors for inflammatory and immune response. Thus, we decided to evaluate the secretion of two of the major pro-inflammatory mediators, IL-6 and TNF-α, in the different experimental conditions used in this study. The ELISA test performed in the whole blood culture supernatant for IL-6 and TNF-α showed a significant increase in the secretion of these pro-inflammatory cytokines compared with the LPS-stimulated group, serving as our positive control group. As shown in Figure 1, when stimulation with LPS was performed in the presence of CBD (50 and 100 μg/mL) we observed a significant reduction in cytokines secretion. We observed a dose dependence effect of CBD; when compared to CBD 50 μg/mL, the dose of CBD 100 μg/mL produced a bigger inhibitory effect on IL-6 and TNF-α release in whole blood supernatant.
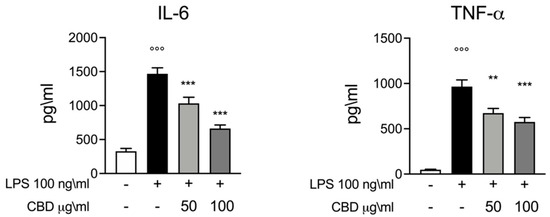
Figure 1.
ELISA assay for IL-6 and TNF-α whole blood culture supernatant 24 h after LPS 100 ng/mL stimulation. Data are presented as means ± SEM for each group. °°° p < 0.01 vs. control; ** p < 0.01 vs. LPS; *** p < 0.001 vs. LPS.
3.2. Effect of CBD on IL-10 in LPS Stimulated Whole Blood
Next, we evaluated the trend of IL-10 as a major anti-inflammatory cytokine. Furthermore, IL-10 plays a key role in the function regulation of several immune cells. Consistent with previous studies, the whole blood stimulation with LPS induced an increase in the levels of IL-10 compared to the control [15,22]. Interestingly, we found that CBD did not alter the levels of IL-10 (Figure 2); instead, we observed a slight trend in IL-10 levels after the treatment with CBD in a dose-dependent manner. As shown in Figure 2, we evaluated the TNF-α to IL-10 ratio to highlight a shift in inflammatory phenotype [22]. Our results show a significant increase in the TNF-α to IL-10 ratio induced by LPS, while the CBD produces a significant decrease in the TNF-α to IL-10 ratio in a dose-dependent manner.
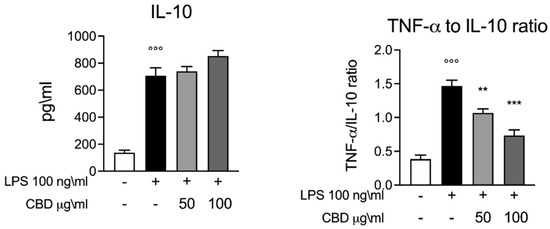
Figure 2.
ELISA assay for IL-10. Left panel, whole blood culture supernatant 24 h after LPS 100 ng/mL stimulation. Right panel, TNF-α to IL-10 ratio graph calculated based on the results of the ELISA assay. Data are presented as means ± SEM for each group. °°° p < 0.01 vs. control; ** p < 0.01 vs. LPS; *** p < 0.001 vs. LPS.
3.3. Effect of CBD on Nf-κB and COX-2 Induction in LPS Stimulated Whole Blood
We also evaluated the effect of CBD on inflammatory pathways through the evaluation of Nf-κB and COX-2 expression levels. NF-κB activation has significant effects on the regulation of inflammatory response and in the transcriptional regulation of many pro-inflammatory cytokines, including TNF-α and IL-6. As is widely known, the LPS stimuli induced an increase in Nf-κB p-65 activation. Through Western blot analysis, we highlighted the expression of Nf-κB p-65; as shown in Figure 3, we observed a significantly increase in Nf-κB p-65 expression in the LPS group. According to the results for IL-6 and TNF-α (Figure 1), we observed a reduction in Nf-κB expression for the groups incubated with LPS and CBD (50 and 100 μg/mL) compared to the LPS-only group. Subsequently we evaluated the mRNA and protein levels for COX-2, which is known to be upregulated during inflammation. COX-2 plays a fundamental role in the inflammatory cascade, and thus in acute and chronic inflammation. Additionally, for COX-2, we observed significantly increased levels of both mRNA and protein for the LPS group, while the treatment with CBD (50 and 100 μg/mL) showed significantly lower COX-2 mRNA and protein expression compared to LPS. These results are in accordance with the results observed for Nf-κB and confirm an immune regulatory effect for CBD.
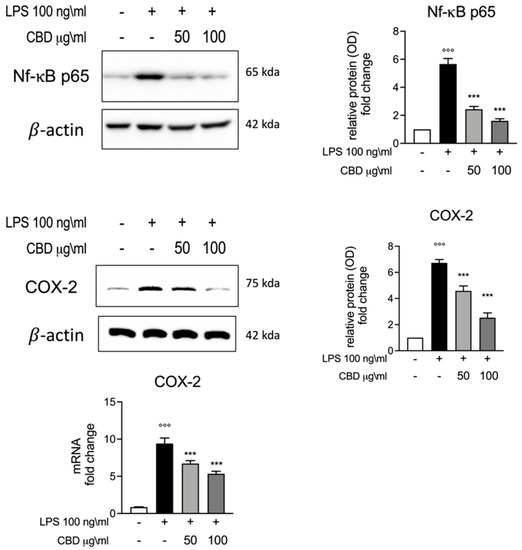
Figure 3.
Upper panels, Western blot analysis for Nf-κB expression and relative comparative fold change graph. Lower panel, Western blot and rt-PCR analysis for COX-2 protein and mRNA expression among the different experimental groups. Data are presented as means ± SEM for each group. °°° p < 0.01 vs. control; *** p < 0.001 vs. LPS.
4. Discussion
CBD is the major non-psychoactive component of Cannabis sp.; even though many of its biological effects are not clearly understood, recently, CBD was authorized in some countries for therapeutic purposes for several clinical conditions [3]. To date, only a few studies have assessed the therapeutic efficacy of CBD in dogs. CBD has been seen to be efficacious in reducing pain and joint inflammation signs in dogs with osteoarthritis [23], and in dogs with epilepsy [24]. As synthetized in a review by Della Rocca [3], despite the limited data on the pharmacological effects of CBD in dogs, the pharmacokinetics and tolerability of CBD and those of cannabis derivatives in general have been evaluated. Evidence supports the immunomodulant proprieties of CBD [25,26,27,28], but to date, there are no scientific data on the direct immunomodulatory effect of CBD in dogs. In fact, the biological activities of CBD have been characterized much more often in humans, with more clinical trials, than in other animal species [29,30,31]. Thus, the aim of this study was to assess the immunomodulatory and anti-inflammatory effect of CBD directly in dogs. Thus, we used an ex vivo model of whole dog blood from healthy dogs stimulated with LPS. This is a reliable model to study the effect of immune modulatory agents or immune response [32] in target species, and it has previously been demonstrated that this model is more representative of in vivo cytokine response patterns [33]. LPS as canonical inflammatory stimulus which is able to evoke a pro-inflammatory phenotype of immune cells [34]. Cytokines are responsible for the regulation of immunological and inflammatory processes, and play an important role in the proliferation and differentiation of lymphoid cells. Our experimental protocols consist of the stimulation of healthy dogs’ whole blood with LPS 100 ng/mL in the presence or not of CBD 50 and 100 μg/mL. We started by evaluating the levels of the pro-inflammatory cytokines IL-6 and TNF-α and of the anti-inflammatory IL-10 in the whole blood culture supernatant through ELISA assays. IL-6 and TNF-α play a key role in the physiological inflammatory response such as during infection, but also in several diseases where elevated levels of these cytokines are associated with a poor clinical outcome. IL-6 regulates both the innate and adaptive immune response [35], is a well-consolidated marker of inflammation, such as systemic inflammation, sepsis, and autoimmune disease [36], and is also a prognostic marker for canine critical care patients with acute internal disease [37]. Consistent with previous studies, we found that LPS stimulation of canine whole blood induces a significant increase in the secretion of IL-6 and TNF-α [15]. Our results show that whole dog blood stimulated with LPS and treated with CBD 50 and 100 μg/mL had a significant lower level of IL-6 and TNF-α compared to LPS. These findings are in accordance with previous studies that assessed the immune modulatory effect of CBD in humans [28,38], due to the proprieties of CBD. To better understand the effect of CBD on dog immune cells, we decided to investigate the effect not only on the pro-inflammatory cytokines IL-6 and TNF-α, but also on IL-10 as an anti-inflammatory and immunoregulating cytokine. Interestingly, our results show that differently from IL-6 and TNF-α, CBD did not alter the levels of IL-10 in response to LPS; this effect of CBD has also been observed in previous studies [39,40]. In particular, we observed a slight trend for the upregulation of IL-10 by CBD—this can partially be explained by the combination of CBD treatment and LPS stimulation [41]. The balance between TNF-α and IL-10 is important for immune homeostasis maintenance; commonly, an increase in TNF-α is counterbalanced by a simultaneous increase in the anti-inflammatory cytokine IL-10, which suppresses the production of many activating and regulatory mediators [42,43]. Thus, we also evaluated the TNF-α to IL-10 ratio as a cytokine marker of the balance between key pro- and anti-inflammatory levels [12,18,40]. Our results show that LPS stimulation produces a significant increase in the TNF-α to IL-10 ratio in whole dog blood, while treatment with CBD is able to restore the TNF-α to IL-10 ratio compared to the control groups. To better investigate the effect of CBD during inflammation, we evaluate the expression levels of Nf-κB p-65. NF-κB regulates several events in the innate and adaptive immune response and serves as a key regulator of inflammatory responses.
NF-κB induces the expression of several pro-inflammatory genes, including those encoding cytokines and chemokines [44]. Our results show that treatment with CBD 50 and 100 μg/ mL reduced Nf-κB p65 expression during stimulation with LPS. COX-2 is a key enzyme in the synthesis of prostaglandins from arachidonic acid and plays a key role in inflammatory response. Its expressions is known to be upregulated in response to certain stimuli, including growth factors and pro-inflammatory cytokines [45]. Our results show that stimulation of LPS in healthy whole dog blood induced a significant increase in COX-2 mRNA, while the treatment with CBD 50 and 100 μg/mL reduced the increased COX-2 mRNA induced by LPS. This effect on COX-2 can be explained by the results observed for IL-6, TNF-α and Nf-κB.
5. Limitations of the Study
Due to the novelty of this preliminary study, there are some limitations which should be pointed out. Although some limited studies published previously show that CBD is well-tolerated in different species in vivo, the lack of more accurate studies on the cytotoxic effects of this compound, if present, and in particular on the effects on different cell types means that there is no indication as to whether the doses used in this study could fall within a therapeutic range. A limitation of this study is the limited range of CBD concentrations used, and the limited sample size in terms of the dog breeds used. Furthermore, with regard to the sources and preparation of CBD, they are very different from each other, both in terms of composition and the concentration of CBD, which is often not well titrated. In this study, we refer to CBD as originating from a commercial preparation available for dogs, such as kanarescue 5%.
6. Conclusions
In conclusion, in this study, we provide the first evidence of the immunomodulatory effect of CBD in stimulated whole dog blood. Our results demonstrate that CBD was able to reduce the inflammatory response induced by LPS. In particular, we found that CBD was able to modulate the cytokine production stimulated by LPS and thus reduced the inflammatory response, as confirmed by Nf-κB and COX-2 reduction in the group treated with CBD 50 and 100 μg/mL. Due to the lack of scientific data on the use of CBD in dogs, our results obtained in an ex vivo canine model encourage further studies to better understand the possible therapeutic role of CBD in veterinary medicine.
Author Contributions
Conceptualization and validation R.C. and C.D.I.; Data curation and formal analysis M.C. and D.I.; Investigation and writing—original draft preparation E.G., A.F.P., R.S. and R.F.; Methology and and formal analysis P.L. and R.D.; Supervision and writing—review and editing R.D.P. and S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Informed consent was obtained from all subjects involved in the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data obtained from this study are included into the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ritter, S.; Zadik-Weiss, L.; Almogi-Hazan, O.; Or, R. Cannabis, One Health, and Veterinary Medicine: Cannabinoids’ Role in Public Health, Food Safety, and Translational Medicine. Rambam Maimonides Med. J. 2020, 11. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef]
- Della Rocca, G.; Di Salvo, A. Hemp in Veterinary Medicine: From Feed to Drug. Front. Vet. Sci. 2020, 7, 387. [Google Scholar] [CrossRef]
- Vaughn, D.M.; Paulionis, L.J.; Kulpa, J.E. Randomized, placebo-controlled, 28-day safety and pharmacokinetics evaluation of repeated oral cannabidiol administration in healthy dogs. Am. J. Vet. Res. 2021, 82, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Mejia, S.; Duerr, F.M.; Griffenhagen, G.; McGrath, S. Evaluation of the Effect of Cannabidiol on Naturally Occurring Osteoarthritis-Associated Pain: A Pilot Study in Dogs. J. Am. Anim. Hosp. Assoc. 2021, 57, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.J.; Rupasinghe, H.P.V. Cannabidiol-based natural health products for companion animals: Recent advances in the management of anxiety, pain, and inflammation. Res. Vet. Sci. 2021, 140, 38–46. [Google Scholar] [CrossRef]
- Pagano, S.; Coniglio, M.; Valenti, C.; Federici, M.I.; Lombardo, G.; Cianetti, S.; Marinucci, L. Biological effects of Cannabidiol on normal human healthy cell populations: Systematic review of the literature. Biomed. Pharmacother. 2020, 132, 110728. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, L.; Butcher, B.; Henson, J.D.; Rutolo, D.; Hall, S. A pilot safety, tolerability and pharmacokinetic study of an oro-buccal administered cannabidiol-dominant anti-inflammatory formulation in healthy individuals: A randomized placebo-controlled single-blinded study. Inflammopharmacology 2021. [Google Scholar] [CrossRef]
- Spierenburg, E.A.J.; Portengen, L.; Smit, L.A.M.; Krop, E.J.M.; Hylkema, M.N.; Rijkers, G.T.; Heederik, D.; Wouters, I.M. Stability of individual LPS-induced ex vivo cytokine release in a whole blood assay over a five-year interval. J. Immunol. Methods 2018, 460, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Burnsides, C.; Corry, J.; Alexander, J.; Balint, C.; Cosmar, D.; Phillips, G.; Marketon, J.I. Ex vivo stimulation of whole blood as a means to determine glucocorticoid sensitivity. J. Inflamm. Res. 2012, 5, 89–97. [Google Scholar] [CrossRef][Green Version]
- Kathrani, A.; Hall, E. A preliminary study assessing cytokine production following ex vivo stimulation of whole blood with diet in dogs with chronic enteropathy. BMC Vet. Res. 2019, 15, 185. [Google Scholar] [CrossRef] [PubMed]
- Peyravian, N.; Deo, S.; Daunert, S.; Jimenez, J.J. Cannabidiol as a Novel Therapeutic for Immune Modulation. Immunotargets Ther. 2020, 9, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.L.; Springs, A.E.; Kaminski, N.E. The profile of immune modulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T cells (NFAT). Biochem. Pharmacol. 2008, 76, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Angelone, D.F.; Wessels, M.R.; Coughlin, M.; Suter, E.E.; Valentini, P.; Kalish, L.A.; Levy, O. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-alpha production in vitro and in vivo. Pediatr. Res. 2006, 60, 205–209. [Google Scholar] [CrossRef]
- Fowler, B.L.; Axiak, S.M.; DeClue, A.E. Blunted pathogen-associated molecular pattern motif induced TNF, IL-6 and IL-10 production from whole blood in dogs with lymphoma. Vet. Immunol. Immunopathol. 2011, 144, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; D’Amico, R.; Cordaro, M.; Crupi, R.; Mandalari, G.; Impellizzeri, D.; et al. The Role of Cashew (Anacardium occidentale L.) Nuts on an Experimental Model of Painful Degenerative Joint Disease. Antioxidants 2020, 9, 511. [Google Scholar] [CrossRef]
- Sarr, O.; Dyck, D.J.; Mutch, D.M. [Letter to the Editor] Protein phosphorylation status is preserved following dual RNA and protein extraction using the Qiagen RNeasy Mini Kit. BioTechniques 2016, 61, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Siracusa, R.; Impellizzeri, D.; D’Amico, R.; Peritore, A.F.; Crupi, R.; Gugliandolo, E.; Fusco, R.; Di Paola, R.; Schievano, C.; et al. Safety and efficacy of a new micronized formulation of the ALIAmide palmitoylglucosamine in preclinical models of inflammation and osteoarthritis pain. Arthritis. Res. Ther. 2019, 21, 254. [Google Scholar] [CrossRef]
- Esposito, E.; Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Siracusa, R.; Gugliandolo, E.; Crupi, R.; Cuzzocrea, S. A new co-micronized composite containing palmitoylethanolamide and polydatin shows superior oral efficacy compared to their association in a rat paw model of carrageenan-induced inflammation. Eur. J. Pharmacol. 2016, 782, 107–118. [Google Scholar] [CrossRef]
- Di Paola, R.; Fusco, R.; Gugliandolo, E.; D’Amico, R.; Campolo, M.; Latteri, S.; Carughi, A.; Mandalari, G.; Cuzzocrea, S. The Antioxidant Activity of Pistachios Reduces Cardiac Tissue Injury of Acute Ischemia/Reperfusion (I/R) in Diabetic Streptozotocin (STZ)-Induced Hyperglycaemic Rats. Front. Pharmacol. 2018, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Siracusa, R.; D’Amico, R.; Peritore, A.F.; Cordaro, M.; Gugliandolo, E.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Melatonin Plus Folic Acid Treatment Ameliorates Reserpine-Induced Fibromyalgia: An Evaluation of Pain, Oxidative Stress, and Inflammation. Antioxidants 2019, 8, 628. [Google Scholar] [CrossRef]
- Jaffey, J.A.; Amorim, J.; DeClue, A.E. Effect of calcitriol on in vitro whole blood cytokine production in critically ill dogs. Vet. J. 2018, 236, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Gamble, L.J.; Boesch, J.M.; Frye, C.W.; Schwark, W.S.; Mann, S.; Wolfe, L.; Brown, H.; Berthelsen, E.S.; Wakshlag, J.J. Pharmacokinetics, Safety, and Clinical Efficacy of Cannabidiol Treatment in Osteoarthritic Dogs. Front. Vet. Sci. 2018, 5, 165. [Google Scholar] [CrossRef]
- McGrath, S.; Bartner, L.R.; Rao, S.; Packer, R.A.; Gustafson, D.L. Randomized blinded controlled clinical trial to assess the effect of oral cannabidiol administration in addition to conventional antiepileptic treatment on seizure frequency in dogs with intractable idiopathic epilepsy. J. Am. Vet. Med. Assoc. 2019, 254, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Tanner, C.; Mechoulam, R.; Pertwee, R.G.; Ross, R.A. Inhibition of human neutrophil chemotaxis by endogenous cannabinoids and phytocannabinoids: Evidence for a site distinct from CB1 and CB2. Mol. Pharmacol. 2008, 73, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Lodzki, M.; Godin, B.; Rakou, L.; Mechoulam, R.; Gallily, R.; Touitou, E. Cannabidiol-transdermal delivery and anti-inflammatory effect in a murine model. J. Control. Release. 2003, 93, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Jan, T.R.; Su, S.T.; Wu, H.Y.; Liao, M.H. Suppressive effects of cannabidiol on antigen-specific antibody production and functional activity of splenocytes in ovalbumin-sensitized BALB/c mice. Int. Immunopharmacol. 2007, 7, 773–780. [Google Scholar] [CrossRef]
- Nichols, J.M.; Kaplan, B.L.F. Immune Responses Regulated by Cannabidiol. Cannabis Cannabinoid Res. 2020, 5, 12–31. [Google Scholar] [CrossRef]
- Bebee, B.; Taylor, D.M.; Bourke, E.; Pollack, K.; Foster, L.; Ching, M.; Wong, A. The CANBACK trial: A randomised, controlled clinical trial of oral cannabidiol for people presenting to the emergency department with acute low back pain. Med. J. Aust. 2021, 214, 370–375. [Google Scholar] [CrossRef]
- De Briyne, N.; Holmes, D.; Sandler, I.; Stiles, E.; Szymanski, D.; Moody, S.; Neumann, S.; Anadon, A. Cannabis, Cannabidiol Oils and Tetrahydrocannabinol-What Do Veterinarians Need to Know? Animals 2021, 11, 892. [Google Scholar] [CrossRef]
- Mlost, J.; Bryk, M.; Starowicz, K. Cannabidiol for Pain Treatment: Focus on Pharmacology and Mechanism of Action. Int. J. Mol. Sci. 2020, 21, 8870. [Google Scholar] [CrossRef]
- Tran, T.A.T.; Grievink, H.W.; Lipinska, K.; Kluft, C.; Burggraaf, J.; Moerland, M.; Tasev, D.; Malone, K.E. Whole blood assay as a model for in vitro evaluation of inflammasome activation and subsequent caspase-mediated interleukin-1 beta release. PLoS ONE 2019, 14, e0214999. [Google Scholar] [CrossRef]
- Schmitz, S.; Henrich, M.; Neiger, R.; Werling, D.; Allenspach, K. Comparison of TNFalpha responses induced by Toll-like receptor ligands and probiotic Enterococcus faecium in whole blood and peripheral blood mononuclear cells of healthy dogs. Vet. Immunol. Immunopathol. 2013, 153, 170–174. [Google Scholar] [CrossRef]
- Messerer, D.A.C.; Vidoni, L.; Erber, M.; Stratmann, A.E.P.; Bauer, J.M.; Braun, C.K.; Hug, S.; Adler, A.; Nilsson Ekdahl, K.; Nilsson, B.; et al. Animal-Free Human Whole Blood Sepsis Model to Study Changes in Innate Immunity. Front. Immunol. 2020, 11, 571992. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- McGeough, M.D.; Pena, C.A.; Mueller, J.L.; Pociask, D.A.; Broderick, L.; Hoffman, H.M.; Brydges, S.D. Cutting edge: IL-6 is a marker of inflammation with no direct role in inflammasome-mediated mouse models. J. Immunol. 2012, 189, 2707–2711. [Google Scholar] [CrossRef] [PubMed]
- Schuttler, J.; Neumann, S. Interleukin-6 as a prognostic marker in dogs in an intensive care unit. Vet. Clin. Pathol. 2015, 44, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Szekely, Y.; Ingbir, M.; Bentur, O.S.; Hochner, O.; Porat, R. Natural cannabinoids suppress the cytokine storm in sepsis-like in vitro model. Eur. Cytokine Netw. 2020, 31, 50–58. [Google Scholar] [CrossRef]
- Vuolo, F.; Petronilho, F.; Sonai, B.; Ritter, C.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Dal-Pizzol, F. Evaluation of Serum Cytokines Levels and the Role of Cannabidiol Treatment in Animal Model of Asthma. Mediators. Inflamm. 2015, 2015, 538670. [Google Scholar] [CrossRef]
- Verrico, C.D.; Wesson, S.; Konduri, V.; Hofferek, C.J.; Vazquez-Perez, J.; Blair, E.; Dunner, K., Jr.; Salimpour, P.; Decker, W.K.; Halpert, M.M. A randomized, double-blind, placebo-controlled study of daily cannabidiol for the treatment of canine osteoarthritis pain. Pain 2020, 161, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Joffre, J.; Yeh, C.C.; Wong, E.; Thete, M.; Xu, F.; Zlatanova, I.; Lloyd, E.; Kobzik, L.; Legrand, M.; Hellman, J. Activation of CB1R Promotes Lipopolysaccharide-Induced IL-10 Secretion by Monocytic Myeloid-Derived Suppressive Cells and Reduces Acute Inflammation and Organ Injury. J. Immunol. 2020, 204, 3339–3350. [Google Scholar] [CrossRef] [PubMed]
- Shmarina, G.V.; Pukhalsky, A.L.; Kokarovtseva, S.N.; Pukhalskaya, D.A.; Shabalova, L.A.; Kapranov, N.I.; Kashirskaja, N.J. Tumor necrosis factor-alpha/interleukin-10 balance in normal and cystic fibrosis children. Mediators. Inflamm. 2001, 10, 191–197. [Google Scholar] [CrossRef]
- Winger, E.E.; Reed, J.L.; Ashoush, S.; El-Toukhy, T.; Ahuja, S.; Taranissi, M. Degree of TNF-alpha/IL-10 cytokine elevation correlates with IVF success rates in women undergoing treatment with Adalimumab (Humira) and IVIG. Am. J. Reprod. Immunol. 2011, 65, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.Y.; Argyle, S.A.; Kamida, A.; Morrison, K.O.; Argyle, D.J. The long-acting COX-2 inhibitor mavacoxib (Trocoxil) has anti-proliferative and pro-apoptotic effects on canine cancer cell lines and cancer stem cells in vitro. BMC Vet. Res. 2014, 10, 184. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).