Biplanar High-Speed Fluoroscopy of Pony Superficial Digital Flexor Tendon (SDFT)—An In Vivo Pilot Study
Abstract
1. Introduction
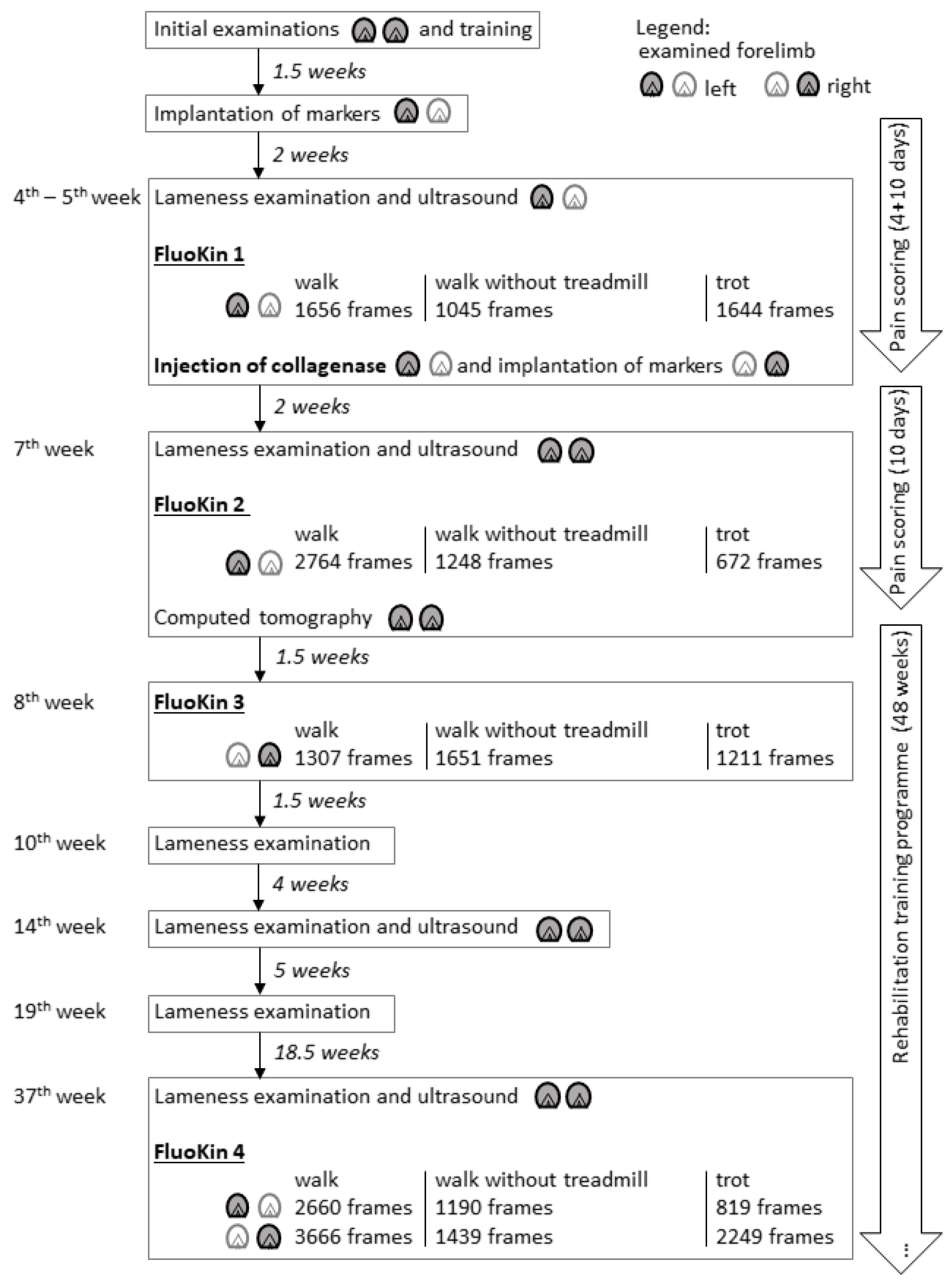
2. Materials and Methods
2.1. Study Design and Animal
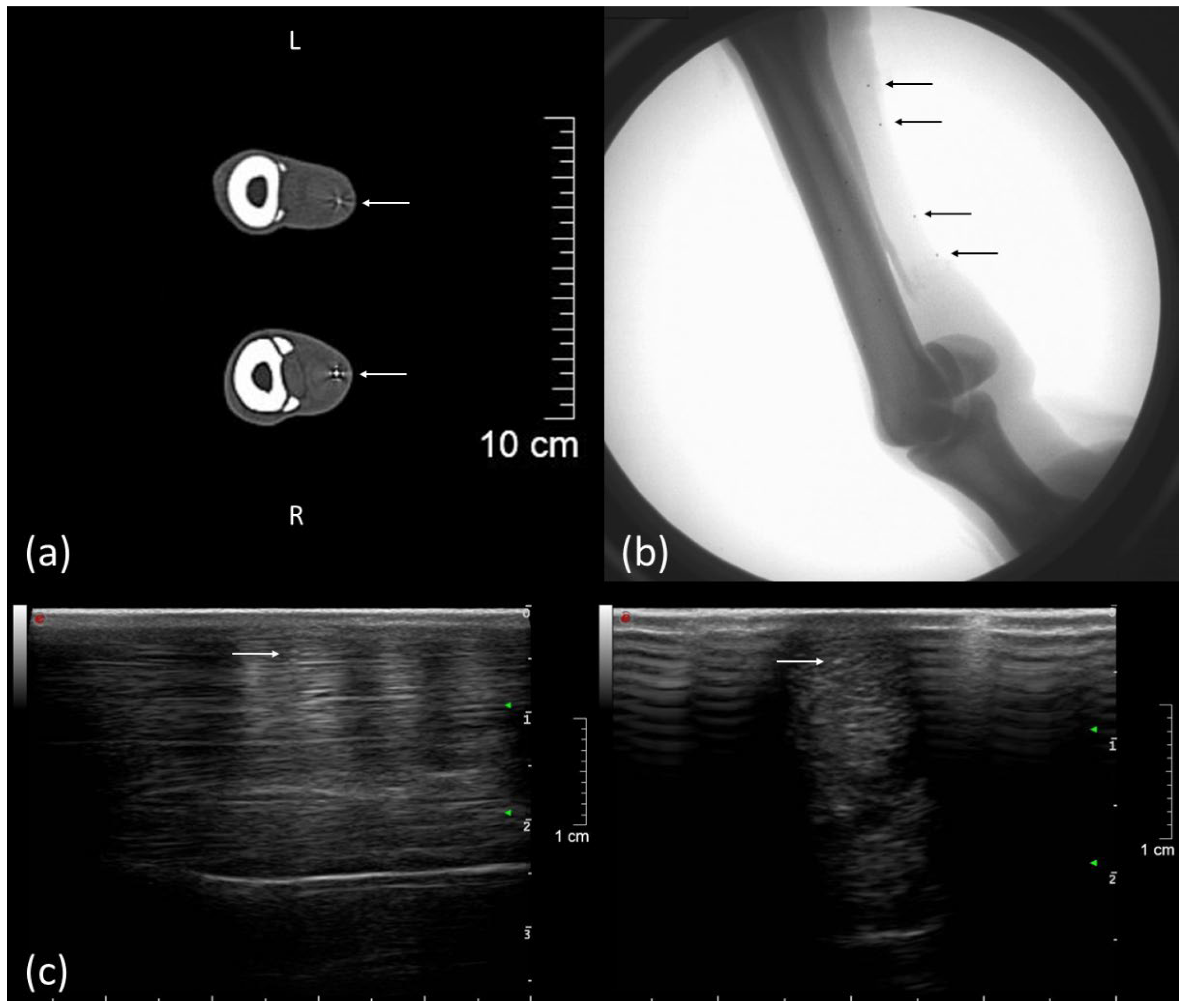
2.2. Implantation of Markers
2.3. Injection of Collagenase
2.4. Examinations and Treatment
2.5. Biplanar High-Speed Fluoroscopy
3. Results
3.1. Clinical Examination Including Ultrasound and Computed Tomography
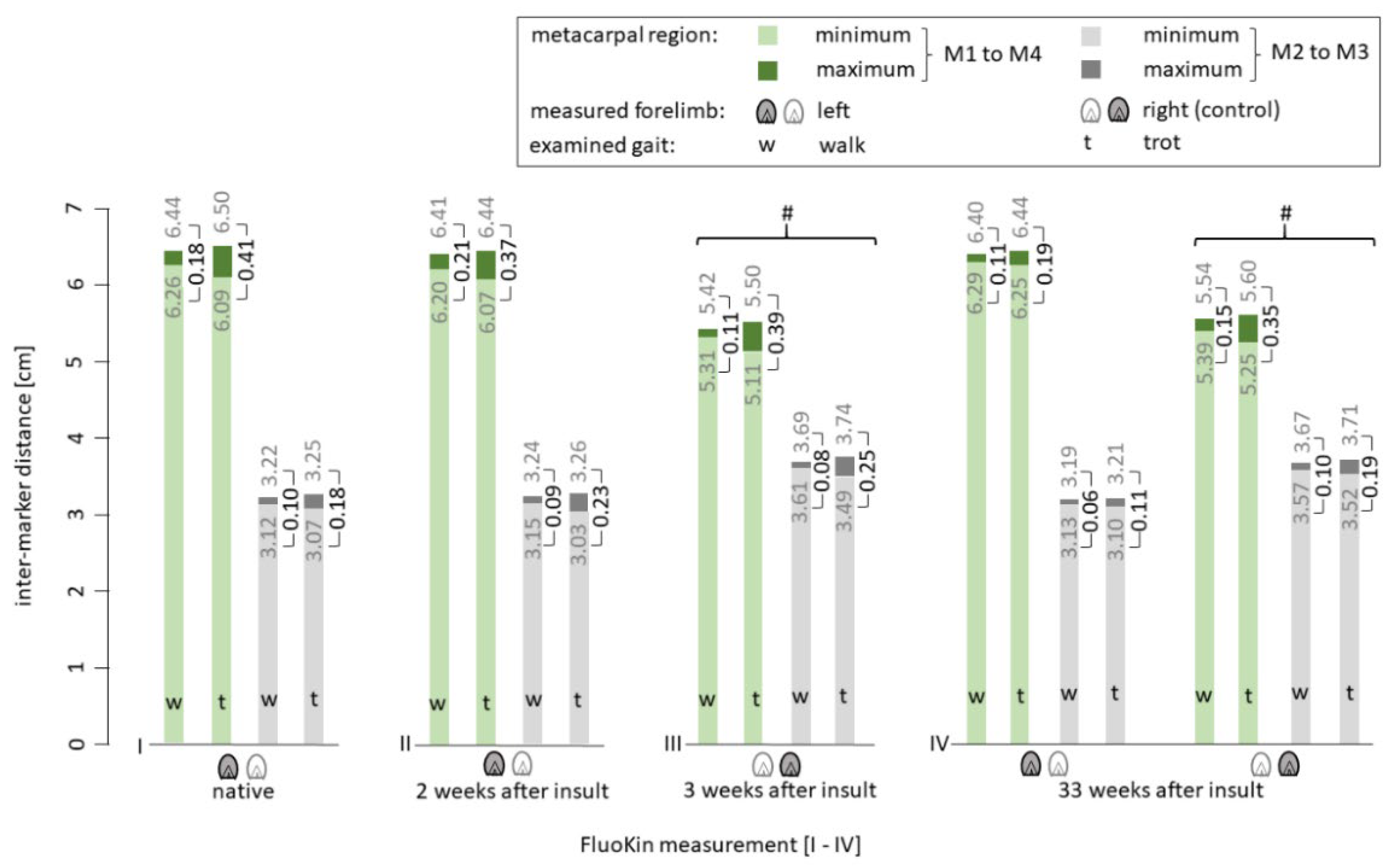
3.2. Biplanar High-Speed Fluoroscopy
4. Discussion
4.1. Clinical Examination
4.2. Ultrasound Examination
4.3. Biplanar High-Speed Fluoroscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, R.B.; Harkins, L.S.; Hammond, C.J.; Wood, J.L. Racehorse injuries, clinical problems and fatalities recorded on British racecourses from flat racing and National Hunt racing during 1996, 1997 and 1998. Equine Vet. J. 2001, 33, 478–486. [Google Scholar] [CrossRef]
- Smith, R.K.W.; Birch, H.L.; Goodman, S.; Heinegård, D.; Goodship, A.E. The influence of ageing and exercise on tendon growth and degeneration–Hypotheses for the initiation and prevention of strain-induced tendinopathies. Comp. Biochem. Physiol. Part. A Mol. Integr. Physiol. 2002, 133, 1039–1050. [Google Scholar] [CrossRef]
- Butcher, M.T.; Hermanson, J.W.; Ducharme, N.G.; Mitchell, L.M.; Soderholm, L.V.; Bertram, J.E.A. Superficial digital flexor tendon lesions in racehorses as a sequela to muscle fatigue: A preliminary study. Equine Vet. J. 2007, 39, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Patterson-Kane, J.C.; Firth, E.C. The pathobiology of exercise-induced superficial digital flexor tendon injury in Thoroughbred racehorses. Vet. J. 2009, 181, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Wollenman, P.; McMahon, P.J.; Knapp, S.; Ross, M.W. Lameness in the Polo Pony. In Diagnosis and Management of Lameness in the Horse, 2nd ed.; Ross, M.W., Dyson, S.J., Eds.; Elsevier/Saunders: St. Louis, MO, USA, 2011; pp. 1149–1164. [Google Scholar]
- Davis, C.S.; Smith, R.K.W. Diseases of tendon and ligament disorders. In Equine Surgery, 3rd ed.; Auer, J.A., Stick, J.A., Eds.; W.B. Saunders: St. Louis, MO, USA, 2006. [Google Scholar]
- Dyson, S.J. Medical management of superficial digital flexor tendonitis: A comparative study in 219 horses (1992–2000). Equine Vet. J. 2004, 36, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, C.T.; Clegg, P.D.; Birch, H.L. A review of tendon injury: Why is the equine superficial digital flexor tendon most at risk? Equine Vet. J. 2010, 42, 174–180. [Google Scholar] [CrossRef]
- Patterson-Kane, J.C.; Becker, D.L.; Rich, T. The pathogenesis of tendon microdamage in athletes: The horse as a natural model for basic cellular research. J. Comp. Pathol. 2012, 147, 227–247. [Google Scholar] [CrossRef]
- McCullagh, K.G.; Goodship, A.E.; Silver, I.A. Tendon injuries and their treatment in the horse. Vet. Rec. 1979, 105, 54–57. [Google Scholar] [CrossRef]
- Goodship, A.E.; Birch, H.L.; Wilson, A.M. The pathobiology and repair of tendon and ligament injury. Vet. Clin. N. Am. Equine Pract 1994, 10, 323–349. [Google Scholar] [CrossRef]
- Patterson-Kane, J.C.; Wilson, A.M.; Firth, E.C.; Parry, D.A.; Goodship, A.E. Exercise-related alterations in crimp morphology in the central regions of superficial digital flexor tendons from young thoroughbreds: A controlled study. Equine Vet. J. 1998, 30, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Dowling, B.A.; Dart, A.J.; Hodgson, D.R.; Smith, R.K. Superficial digital flexor tendonitis in the horse. Equine Vet. J. 2000, 32, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.R.; Nunamaker, D.M.; Butterweck, D.M. Application of a Hall-effect transducer for measurement of tendon strains in horses. Am. J. Vet. Res. 1989, 50, 1089–1095. [Google Scholar] [PubMed]
- Riemersma, D.J.; van den Bogert, A.J.; Jansen, M.O.; Schamhardt, H.C. Tendon strain in the forelimbs as a function of gait and ground characteristics and in vitro limb loading in ponies. Equine Vet. J. 1996, 28, 133–138. [Google Scholar] [CrossRef]
- Pourcelot, P.; Defontaine, M.; Ravary, B.; Lemâtre, M.; Crevier-Denoix, N. A non-invasive method of tendon force measurement. J. Biomech. 2005, 38, 2124–2129. [Google Scholar] [CrossRef] [PubMed]
- Duenwald, S.; Kobayashi, H.; Frisch, K.; Lakes, R.; Vanderby, R., Jr. Ultrasound echo is related to stress and strain in tendon. J. Biomech. 2011, 44, 424–429. [Google Scholar] [CrossRef]
- Vergari, C.; Ravary-Plumioën, B.; Evrard, D.; Laugier, P.; Mitton, D.; Pourcelot, P.; Crevier-Denoix, N. Axial speed of sound is related to tendon’s nonlinear elasticity. J. Biomech. 2012, 45, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Ellison, M.E.; Duenwald-Kuehl, S.E.; Forrest, L.J.; Vanderby, R., Jr.; Brounts, S.H. Reproducibility and feasibility of acoustoelastography in the superficial digital flexor tendons of clinically normal horses. Am. J. Vet. Res. 2014, 75, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Meershoek, L.S.; Lanovaz, J.L. Sensitivity analysis and application to trotting of a noninvasive method to calculate flexor tendon forces in the equine forelimb. Am. J. Vet. Res. 2001, 62, 1594–1598. [Google Scholar] [CrossRef] [PubMed]
- Crevier-Denoix, N.; Ruel, Y.; Dardillat, C.; Jerbi, H.; Sanaa, M.; Collobert-Laugier, C.; Ribot, X.; Denoix, J.M.; Pourcelot, P. Correlations between mean echogenicity and material properties of normal and diseased equine superficial digital flexor tendons: An in vitro segmental approach. J. Biomech. 2005, 38, 2212–2220. [Google Scholar] [CrossRef]
- Brainerd, E.L.; Baier, D.B.; Gatesy, S.M.; Hedrick, T.L.; Metzger, K.A.; Gilbert, S.L.; Crisco, J.J. X-ray reconstruction of moving morphology (XROMM): Precision, accuracy and applications in comparative biomechanics research. J. Exp. Zool. A Ecol. Genet. Physiol. 2010, 313, 262–279. [Google Scholar] [CrossRef]
- Friden, T.; Ryd, L.; Lindstrand, A. Laxity and graft fixation after reconstruction of the anterior cruciate ligament. A roentgen stereophotogrammetric analysis of 11 patients. Acta Orthop. Scand. 1992, 63, 80–84. [Google Scholar] [CrossRef]
- Schepull, T.; Kvist, J.; Andersson, C.; Aspenberg, P. Mechanical properties during healing of Achilles tendon ruptures to predict final outcome: A pilot Roentgen stereophotogrammetric analysis in 10 patients. BMC Musculoskelet. Disord. 2007, 8, 116. [Google Scholar] [CrossRef]
- Cashman, P.M.M.; Baring, T.; Reilly, P.; Emery, R.J.H.; Amis, A.A. Measurement of migration of soft tissue by modified Roentgen stereophotogrammetric analysis (RSA): Validation of a new technique to monitor rotator cuff tears. J. Med. Eng. Technol. 2010, 34, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Marshall, N.E.; Keller, R.A.; Okoroha, K.; Guest, J.M.; Yu, C.; Muh, S.; Moutzouros, V. Radiostereometric Evaluation of Tendon Elongation After Distal Biceps Repair. Orthop. J. Sports Med. 2016, 4, 2325967116672620. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.C.; Reese, S.; Gerlach, K.; Böttcher, P.; Mülling, C.K.W. Cyclic tensile tests of Shetland pony superficial digital flexor tendon with an optimized cryo-clamp combined with biplanar high-speed fluoroscopy. BMC Vet. Res. 2021. accepted. [Google Scholar]
- Bussières, G.; Jacques, C.; Lainay, O.; Beauchamp, G.; Leblond, A.; Cadore, J.L.; Desmaizieres, L.M.; Cuvelliez, S.G.; Troncy, E. Development of a composite orthopaedic pain scale in horses. Res. Vet. Sci. 2008, 85, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.K.W.; McIlwraith, C.W. Consensus on equine tendon disease: Building on the 2007 Havemeyer symposium. Equine Vet. J. 2012, 44, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Dalla Costa, E.; Minero, M.; Lebelt, D.; Stucke, D.; Canali, E.; Leach, M.C. Development of the horse grimace scale (HGS) as a pain assessment tool in horses undergoing routine castration. PLoS ONE 2014, 9, e92281. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Reich, E.; Grund, S.; Mülling, C.K.W.; Geiger, S.M. Validation of 2 noninvasive, markerless reconstruction techniques in biplane high-speed fluoroscopy for 3-dimensional research of bovine distal limb kinematics. J. Dairy Sci. 2017, 100, 8372–8384. [Google Scholar] [CrossRef] [PubMed]
- Geiger, S.M.; Reich, E.; Böttcher, P.; Grund, S.; Hagen, J. Validation of biplane high-speed fluoroscopy combined with two different noninvasive tracking methodologies for measuring in vivo distal limb kinematics of the horse. Equine Vet. J. 2018, 50, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Knoerlein, B.J.; Baier, D.B.; Gatesy, S.M.; Laurence-Chasen, J.D.; Brainerd, E.L. Validation of XMALab software for marker-based XROMM. J. Exp. Biol. 2016, 219, 3701–3711. [Google Scholar] [CrossRef]
- De Grauw, J.C.; van Loon, J.P.A.M. Systematic pain assessment in horses. Vet. J. 2016, 209, 14–22. [Google Scholar] [CrossRef]
- Van Loon, J.P.A.M.; van Dierendonck, M.C. Pain assessment in horses after orthopaedic surgery and with orthopaedic trauma. Vet. J. 2019, 246, 85–91. [Google Scholar] [CrossRef]
- Khan, K.M.; Cook, J.L.; Bonar, F.; Harcourt, P.; Astrom, M. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999, 27, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Sugg, K.B.; Lubardic, J.; Gumucio, J.P.; Mendias, C.L. Changes in macrophage phenotype and induction of epithelial-to-mesenchymal transition genes following acute Achilles tenotomy and repair. J. Orthop. Res. 2014, 32, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Killian, M.L.; Cavinatto, L.; Galatz, L.M.; Thomopoulos, S. The role of mechanobiology in tendon healing. J. Shoulder Elbow Surg. 2012, 21, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Alberius, P. Bone reactions to tantalum markers. A scanning electron microscopic study. Acta Anat. 1983, 115, 310–318. [Google Scholar] [CrossRef]
- Aronson, A.S.; Jonsson, N.; Alberius, P. Tantalum markers in radiography. An assessment of tissue reactions. Skeletal Radiol. 1985, 14, 207–211. [Google Scholar] [CrossRef]
- Roos, P.J.; Hull, M.L.; Howell, S.M. How cyclic loading affects the migration of radio-opaque markers attached to tendon grafts using a new method: A study using roentgen stereophotogrammetric analysis (RSA). J. Biomech. Eng. 2004, 126, 62–69. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goldin, B.; Block, W.D.; Pearson, J.R. Wound healing of tendon–I. Physical, mechanical and metabolic changes. J. Biomech. 1980, 13, 241–256. [Google Scholar] [CrossRef]
- Silver, I.A.; Brown, P.N.; Goodship, A.E.; Lanyon, L.E.; McCullagh, K.G.; Perry, G.C.; Williams, I.F. A clinical and experimental study of tendon injury, healing and treatment in the horse. Equine Vet. J. Suppl. 1983, 1, 1–43. [Google Scholar]
- Enwemeka, C.S. Inflammation, cellularity, and fibrillogenesis in regenerating tendon: Implications for tendon rehabilitation. Phys. Ther. 1989, 69, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Denoix, J.M. Functional anatomy of tendons and ligaments in the distal limbs (manus and pes). Vet. Clin. N. Am. Equine Pract. 1994, 10, 273–322. [Google Scholar] [CrossRef]
- Watkins, J.P.; Auer, J.A.; Gay, S.; Morgan, S.J. Healing of surgically created defects in the equine superficial digital flexor tendon: Collagen-type transformation and tissue morphologic reorganization. Am. J. Vet. Res. 1985, 46, 2091–2096. [Google Scholar]
- Van den Boom, R.; Wilmink, J.M.; O’Kane, S.; Wood, J.; Ferguson, M.W.J. Transforming growth factor-beta levels during second- intention healing are related to the different course of wound contraction in horses and ponies. Wound Repair Regen. 2002, 10, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Wilmink, J.M.; van Herten, J.; van Weeren, P.R.; Barneveld, A. Retrospective study of primary intention healing and sequestrum formation in horses compared to ponies under clinical circumstances. Equine Vet. J. 2002, 34, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Pickersgill, C.H.; Marr, C.M.; Reid, S.W. Repeatability of diagnostic ultrasonography in the assessment of the equine superficial digital flexor tendon. Equine Vet. J. 2001, 33, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Karlin, W.M.; Stewart, A.A.; Durgam, S.S.; Naughton, J.F.; O’Dell-Anderson, K.J.; Stewart, M.C. Evaluation of experimentally induced injury to the superficial digital flexor tendon in horses by use of low-field magnetic resonance imaging and ultrasonography. Am. J. Vet. Res. 2011, 72, 791–798. [Google Scholar] [CrossRef]
- Dakin, S.G.; Jespers, K.; Warner, S.; O’Hara, L.K.; Dudhia, J.; Goodship, A.E.; Wilson, A.M.; Smith, R.K.W. The relationship between in vivo limb and in vitro tendon mechanics after injury: A potential novel clinical tool for monitoring tendon repair. Equine Vet. J. 2011, 43, 418–423. [Google Scholar] [CrossRef]
- Crevier, N.; Pourcelot, P.; Denoix, J.-M.; Geiger, D.; Bortolussi, C.; Ribot, X.; Sanaa, M. Segmental variations of in vitro mechanical properties in equine superficial digital flexor tendons. Am. J. Vet. Res. 1996, 57, 1111–1117. [Google Scholar]
- Solomon, L.B.; Callary, S.A. Emerging ideas: Soft tissue applications of radiostereometric analysis. Clin. Orthop. Relat. Res. 2011, 469, 1512–1516. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.K.; Hull, M.L.; Howell, S.M. Migration of radio-opaque markers injected into tendon grafts: A study using roentgen stereophotogrammetric analysis (RSA). J. Biomech. Eng. 2005, 127, 887–890. [Google Scholar] [CrossRef][Green Version]
- Rohwedder, T.; Fischer, M.S.; Böttcher, P. In vivo fluoroscopic kinematography of dynamic radio-ulnar incongruence in dogs. Open Vet. J. 2017, 7, 221–228. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rohwedder, T.; Fischer, M.S.; Böttcher, P. In vivo axial humero-ulnar rotation in normal and dysplastic canine elbow joints. Tierarztl. Prax. Ausg. K Kleintiere. Heimtiere. 2018, 46, 83–89. [Google Scholar] [CrossRef]
- Flanigan, P.; Kshettry, V.R.; Benzel, E.C. World War II, tantalum, and the evolution of modern cranioplasty technique. Neurosurg. Focus 2014, 36, E22. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.M.; Whitton, R.C.; Kawcak, C.E.; Stover, S.M.; Pandy, M.G. Relationship between muscle forces, joint loading and utilization of elastic strain energy in equine locomotion. J. Exp. Biol 2010, 213, 3998–4009. [Google Scholar] [CrossRef] [PubMed]
- Williams, I.F.; McCullagh, K.G.; Goodship, A.E.; Silver, I.A. Studies on the pathogenesis of equine tendonitis following collagenase injury. Res. Vet. Sci. 1984, 36, 326–338. [Google Scholar] [CrossRef]
- Wilmink, J.; Wilson, A.M.; Goodship, A.E. Functional significance of the morphology and micromechanics of collagen fibres in relation to partial rupture of the superficial digital flexor tendon in racehorses. Res. Vet. Sci. 1992, 53, 354–359. [Google Scholar] [CrossRef]
- Nabeshima, Y.; Grood, E.S.; Sakurai, A.; Herman, J.H. Uniaxial tension inhibits tendon collagen degradation by collagenase in vitro. J. Orthop. Res. 1996, 14, 123–130. [Google Scholar] [CrossRef]
- Oloumi, M.M.; Vosough, D.; Derakhshanfar, A.; Nematollahi, M.H. The healing potential of plantago lanceolata ointment on collagenase-induced tendinitis in burros (Equus asinus). J. Equine Vet. Sci. 2011, 31, 470–474. [Google Scholar] [CrossRef]
- Clayton, H.M.; Schamhardt, H.C.; Willemen, M.A.; Lanovaz, J.L.; Colborne, G.R. Kinematics and ground reaction forces in horses with superficial digital flexor tendinitis. Am. J. Vet. Res. 2000, 61, 191–196. [Google Scholar] [CrossRef]
- Clayton, H.M.; Schamhardt, H.C.; Willemen, M.A.; Lanovaz, J.L.; Colborne, G.R. Net joint moments and joint powers in horses with superficial digital flexor tendinitis. Am. J. Vet. Res. 2000, 61, 197–201. [Google Scholar] [CrossRef]
- Clayton, H.M.; Willemen, M.A.; Lanovaz, J.L.; Schamhardt, H.C. Effects of a Heel Wedge in Horses with Superficial Digital Flexor Tendinitis. Vet. Comp. Orthop. Traumatol. 2000, 13, 1–8. [Google Scholar] [CrossRef]
- Meershoek, L.S.; Lanovaz, J.L.; Schamhardt, H.C.; Clayton, H.M. Calculated forelimb flexor tendon forces in horses with experimentally induced superficial digital flexor tendinitis and the effects of application of heel wedges. Am. J. Vet. Res. 2002, 63, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Lustgarten, M.; Redding, W.R.; Labens, R.; Davis, W.; Daniel, T.M.; Griffith, E.; Seiler, G.S. Elastographic evaluation of naturally occuring tendon and ligament injuries of the equine distal limb. Vet. Radiol. Ultrasound 2015, 56, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Bukowiecki, C.F.; Bramlage, L.R.; Gabel, A.A. In vitro strength of the suspensory apparatus in training and resting horses. Vet. Surg. 1987, 16, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.C. Mechanobiology of tendon. J. Biomech. 2006, 39, 1563–1582. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.K.W.; Goodship, A.E. The effect of early training and the adaptation and conditioning of skeletal tissues. Vet. Clin. N. Am. Equine Pract 2008, 24, 37–51. [Google Scholar] [CrossRef]
- Narici, M.V.; Maganaris, C.N. Adaptability of elderly human muscles and tendons to increased loading. J. Anat. 2006, 208, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Fouré, A.; Nordez, A.; Cornu, C. Plyometric training effects on Achilles tendon stiffness and dissipative properties. J. Appl. Physiol. 2010, 109, 849–854. [Google Scholar] [CrossRef]
- Kubo, K.; Ikebukuro, T.; Maki, A.; Yata, H.; Tsunoda, N. Time course of changes in the human Achilles tendon properties and metabolism during training and detraining in vivo. Eur. J. Appl. Physiol. 2012, 112, 2679–2691. [Google Scholar] [CrossRef] [PubMed]
- Lochner, F.K.; Milne, D.W.; Mills, E.J.; Groom, J.J. In vivo and in vitro measurement of tendon strain in the horse. Am. J. Vet. Res. 1980, 41, 1929–1937. [Google Scholar] [PubMed]
- Johnson, G.A.; Livesay, G.A.; Woo, S.L.; Rajagopal, K.R. A single integral finite strain viscoelastic model of ligaments and tendons. J. Biomech. Eng. 1996, 118, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Williams, I.F.; Heaton, A.; McCullagh, K.G. Cell morphology and collagen types in equine tendon scar. Res. Vet. Sci 1980, 28, 302–310. [Google Scholar] [CrossRef]
- Parry, D.A.; Barnes, G.R.; Craig, A.S. A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proc. R Soc. Lond. B Biol. Sci. 1978, 203, 305–321. [Google Scholar] [CrossRef]
- Diamant, J.; Keller, A.; Baer, E.; Litt, M.; Arridge, R.G. Collagen; ultrastructure and its relation to mechanical properties as a function of ageing. Proc. R Soc. Lond. B Biol. Sci. 1972, 180, 293–315. [Google Scholar] [CrossRef] [PubMed]
- Stashak, T.S.; Theoret, C. Equine Wound Management; Veterinary Wound Management Society/V.W.M.S.: Ames, IA, USA, 2008; ISBN 978-0813812236. [Google Scholar]
- Crevier-Denoix, N.; Collobert, C.; Pourcelot, P.; Denoix, J.M.; Sanaa, M.; Geiger, D.; Bernard, N.; Ribot, X.; Bortolussi, C.; Bousseau, B. Mechanical properties of pathological equine superficial digital flexor tendons. Equine Vet. J. Suppl. 1997, 23–26. [Google Scholar] [CrossRef]
- Buchner, H.H.; Savelberg, H.H.; Schamhardt, H.C.; Merkens, H.W.; Barneveld, A. Kinematics of treadmill versus overground locomotion in horses. Vet. Q. 1994, 16 (Suppl. S2), S87–S90. [Google Scholar] [CrossRef]
- Back, W.; Schamhardt, H.C.; van Weeren, P.R.; Barneveld, A. A comparison between the trot of pony and horse foals to characterize equine locomotion at young age. Equine Vet. J. Suppl. 1999, 31, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Crevier-Denoix, N.; Ravary-Plumiöen, B.; Vergari, C.; Camus, M.; Holden-Douilly, L.; Falala, S.; Jerbi, H.; Desquilbet, L.; Chateau, H.; Denoix, J.M.; et al. Comparison of superficial digital flexor tendon loading on asphalt and sand in horses at the walk and trot. Vet. J. 2013, 198 (Suppl. S1), e130–e136. [Google Scholar] [CrossRef] [PubMed]



| Animal | Specimen | Strain | |||
|---|---|---|---|---|---|
| During Walk | During Trot | Rupture | |||
| Riemersma et al. 1985 | ponies | whole SDFT | 1.74% | ||
| Riemersma et al. 1988 | ponies | whole SDFT | 2.3% | ||
| Stephens et al. 1989 | horses | whole SDFT | 3.3% | ||
| Jansen et al. 1993 | ponies | whole SDFT | 3.5% | ||
| Crevier et al. 1996 | horses | metacarpal segments | proximal 4.78%, mid 5.05%, distal 4.43% | ||
| Riemersma et al. 1996 | ponies | whole SDFT | 2.19% | 4.15% | |
| Butcher et al. 2007 | horses | whole SDFT | 3.6% | 5.6% | |
| Lawson et al. 2007 | horses | whole SDFT | 6.71% | 8.46% | |
| This study | pony | metacarpal region of the SDFT | 2.86% mid: 3.13% | 6.78% mid: 6.06% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, F.C.; Gerlach, K.; Geiger, S.M.; Gittel, C.; Böttcher, P.; Mülling, C.K.W. Biplanar High-Speed Fluoroscopy of Pony Superficial Digital Flexor Tendon (SDFT)—An In Vivo Pilot Study. Vet. Sci. 2021, 8, 92. https://doi.org/10.3390/vetsci8060092
Wagner FC, Gerlach K, Geiger SM, Gittel C, Böttcher P, Mülling CKW. Biplanar High-Speed Fluoroscopy of Pony Superficial Digital Flexor Tendon (SDFT)—An In Vivo Pilot Study. Veterinary Sciences. 2021; 8(6):92. https://doi.org/10.3390/vetsci8060092
Chicago/Turabian StyleWagner, Franziska C., Kerstin Gerlach, Sandra M. Geiger, Claudia Gittel, Peter Böttcher, and Christoph K. W. Mülling. 2021. "Biplanar High-Speed Fluoroscopy of Pony Superficial Digital Flexor Tendon (SDFT)—An In Vivo Pilot Study" Veterinary Sciences 8, no. 6: 92. https://doi.org/10.3390/vetsci8060092
APA StyleWagner, F. C., Gerlach, K., Geiger, S. M., Gittel, C., Böttcher, P., & Mülling, C. K. W. (2021). Biplanar High-Speed Fluoroscopy of Pony Superficial Digital Flexor Tendon (SDFT)—An In Vivo Pilot Study. Veterinary Sciences, 8(6), 92. https://doi.org/10.3390/vetsci8060092






