Flow Cytometric Analysis of the Cytotoxic T-Cell Recall Response to Theileria parva in Cattle Following Vaccination by the Infection and Treatment Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Cell Lines
2.2. Generation of T. parva-Specific Effector T Cells
2.3. Flow Cytometric Cytotoxicity Assay
2.4. Data Analysis
3. Results
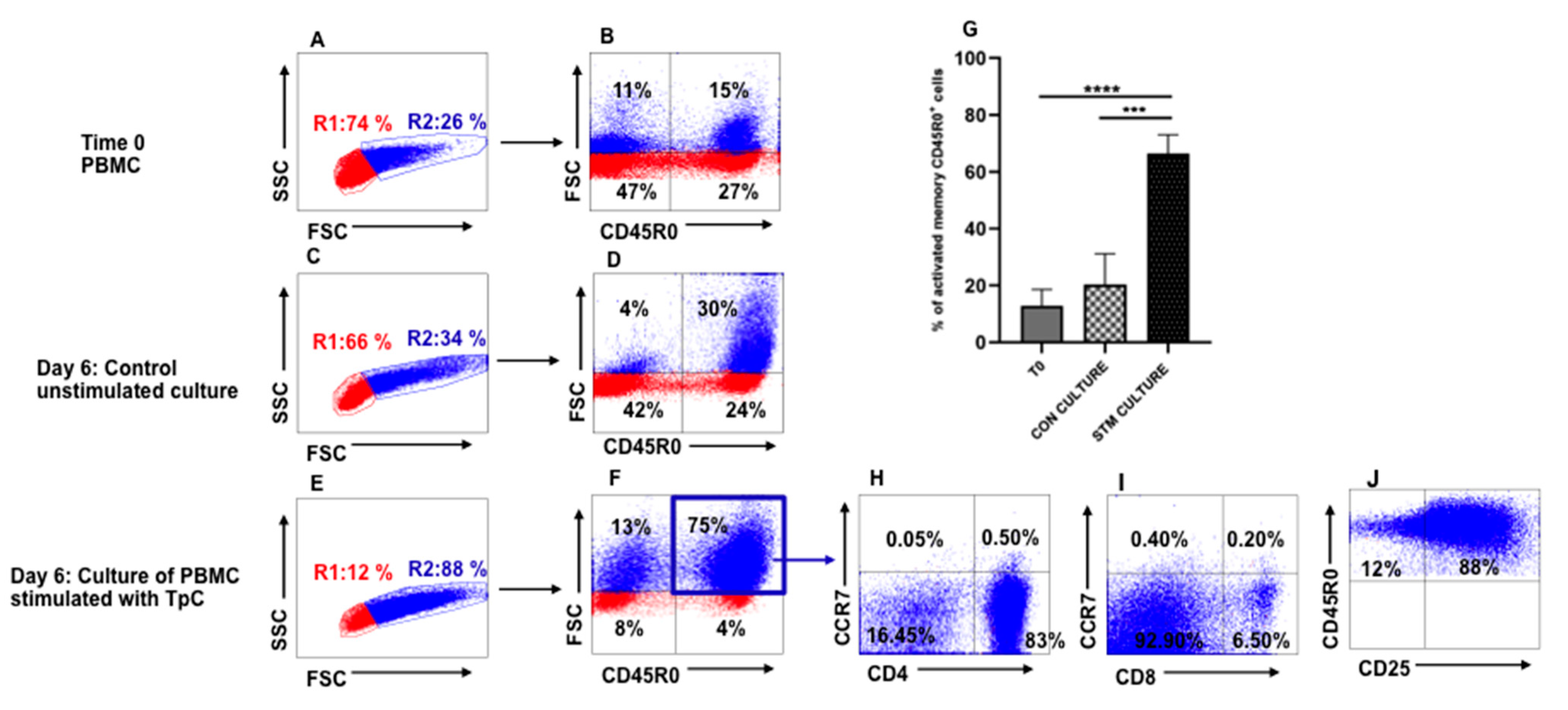
3.1. Recall Response to Stimulation of PBMC from T. parva-Immune Steers with Autologous T. parva-Infected Lymphocytes
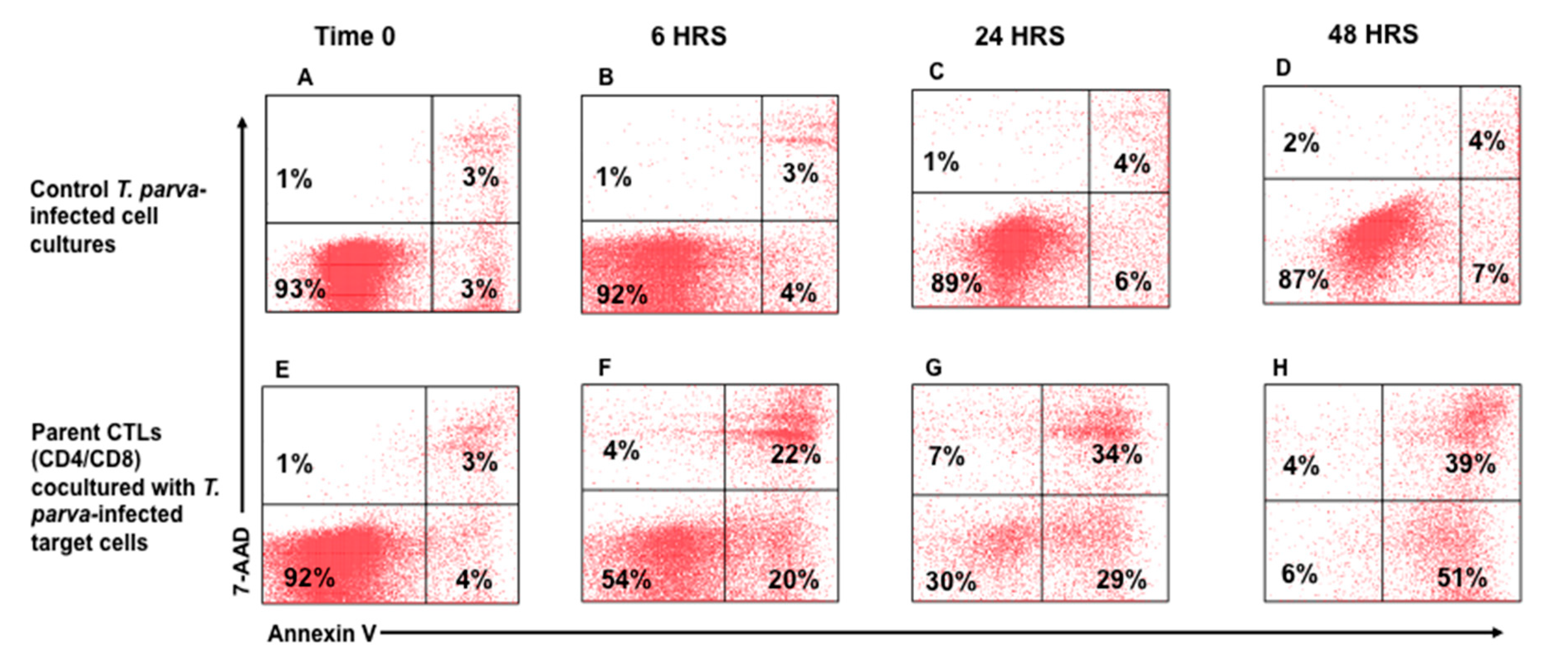
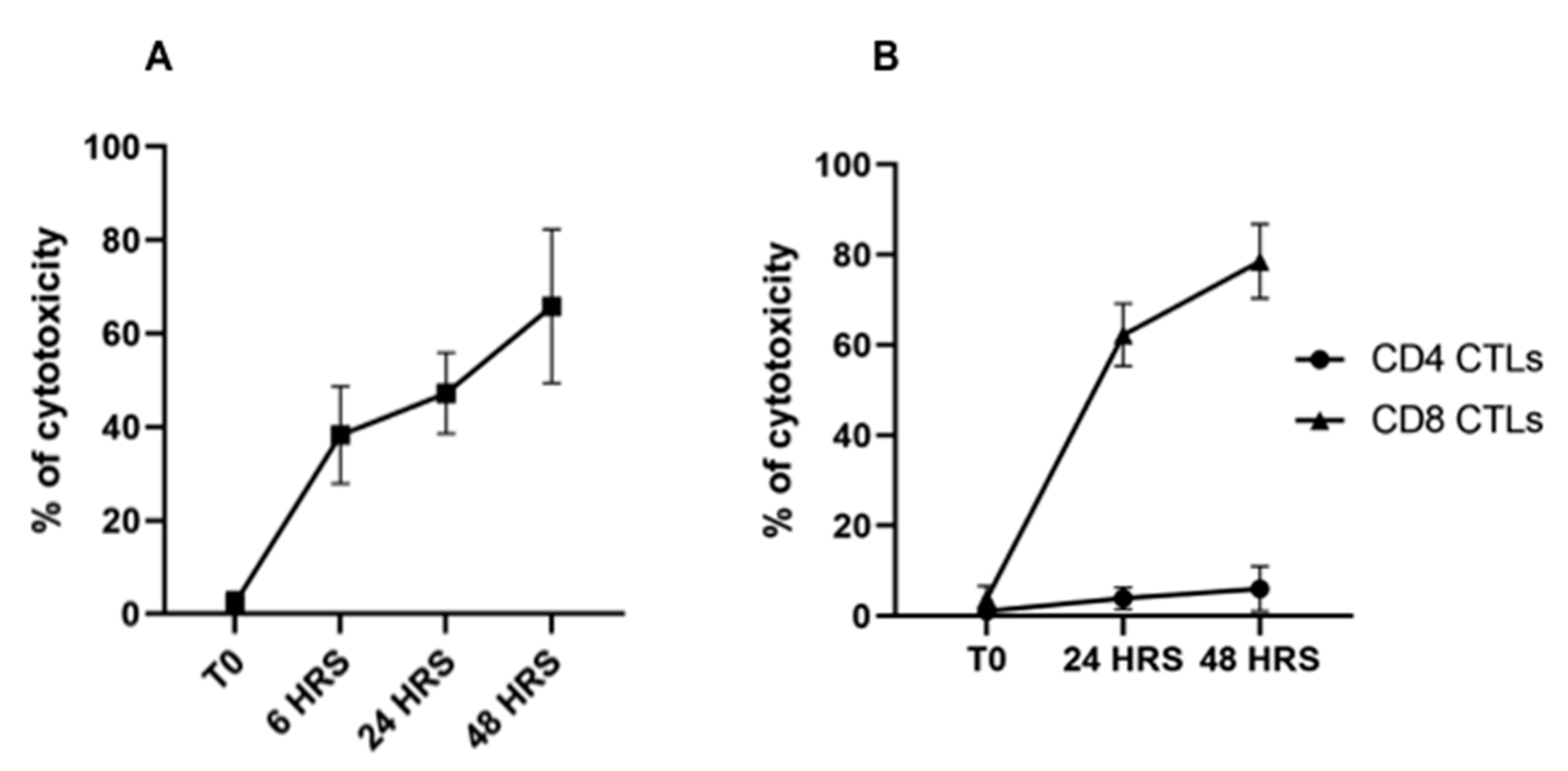
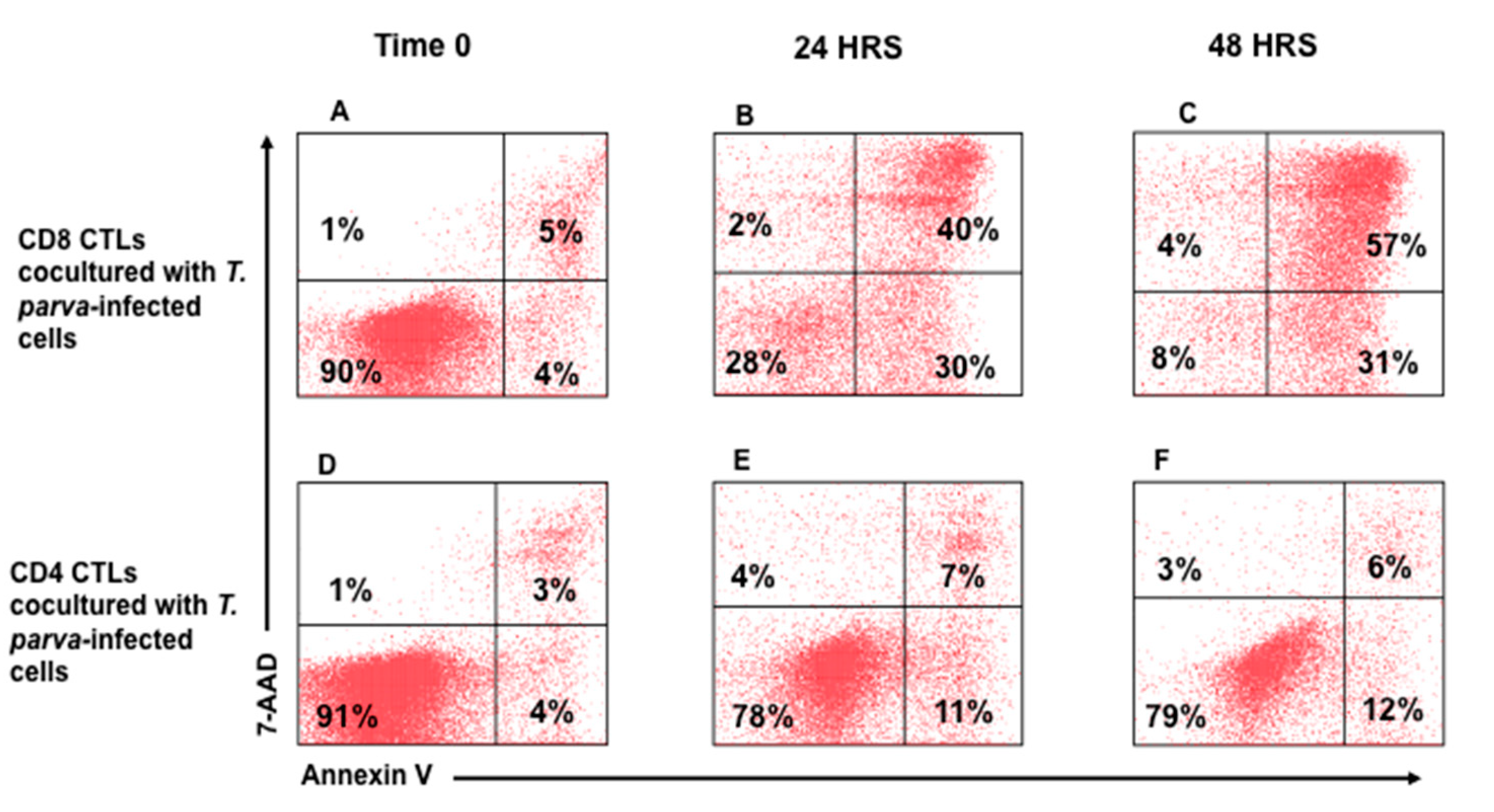
3.2. Flow Cytometric Detection of T-Cell Mediated Killing of Autologous, T. parva-Infected Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nene, V.; Kiara, H.; Lacasta, A.; Pelle, R.; Svitek, N.; Steinaa, L. The biology of Theileria parva and control of East Coast fever-Current status and future trends. Ticks Tick Borne Dis. 2016, 7, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.K.; Tilney, L.G. How individual cells develop from a syncytium: Merogony in Theileria parva (Apicomplexa). J. Cell Sci. 1992, 101 Pt 1, 109–123. [Google Scholar] [CrossRef]
- Tretina, K.; Gotia, H.T.; Mann, D.J.; Silva, J.C. Theileria-transformed bovine leukocytes have cancer hallmarks. Trends Parasitol. 2015, 31, 306–314. [Google Scholar] [CrossRef]
- Houston, E.F.; Taracha, E.L.; Brackenbury, L.; MacHugh, N.D.; McKeever, D.J.; Charleston, B.; Morrison, W.I. Infection of cattle with Theileria parva induces an early CD8 T cell response lacking appropriate effector function. Int. J. Parasitol. 2008, 38, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Bastos, R.G.; Sears, K.; Dinkel, K.D.; Knowles, D.P.; Fry, L.M. Changes in the Molecular and Functional Phenotype of Bovine Monocytes during Theileria parva Infection. Infect. Immun. 2019, 87. [Google Scholar] [CrossRef]
- Fry, L.M.; Schneider, D.A.; Frevert, C.W.; Nelson, D.D.; Morrison, W.I.; Knowles, D.P. East Coast Fever Caused by Theileria parva Is Characterized by Macrophage Activation Associated with Vasculitis and Respiratory Failure. PLoS ONE 2016, 11, e0156004. [Google Scholar] [CrossRef] [PubMed]
- Bastos, R.G.; Franceschi, V.; Tebaldi, G.; Connelley, T.; Morrison, W.I.; Knowles, D.P.; Donofrio, G.; Fry, L.M. Molecular and Antigenic Properties of Mammalian Cell-Expressed Theileria parva Antigen Tp9. Front. Immunol. 2019, 10, 897. [Google Scholar] [CrossRef]
- Bishop, R.P.; Odongo, D.; Ahmed, J.; Mwamuye, M.; Fry, L.M.; Knowles, D.P.; Nanteza, A.; Lubega, G.; Gwakisa, P.; Clausen, P.; et al. A review of recent research on Theileria parva: Implications for the infection and treatment vaccination method for control of East Coast fever. Transbound. Emerg. Dis. 2020, 67 (Suppl. 1), 56–67. [Google Scholar] [CrossRef]
- Patel, E.; Mwaura, S.; Kiara, H.; Morzaria, S.; Peters, A.; Toye, P. Production and dose determination of the Infection and Treatment Method (ITM) Muguga cocktail vaccine used to control East Coast fever in cattle. Ticks Tick Borne Dis. 2016, 7, 306–314. [Google Scholar] [CrossRef]
- Di Giulio, G.; Lynen, G.; Morzaria, S.; Oura, C.; Bishop, R. Live immunization against East Coast fever—Current status. Trends Parasitol. 2009, 25, 85–92. [Google Scholar] [CrossRef]
- McKeever, D.J.; Taracha, E.L.; Innes, E.L.; MacHugh, N.D.; Awino, E.; Goddeeris, B.M.; Morrison, W.I. Adoptive transfer of immunity to Theileria parva in the CD8+ fraction of responding efferent lymph. Proc. Natl. Acad. Sci. USA 1994, 91, 1959–1963. [Google Scholar] [CrossRef]
- Mwangi, D.M.; Honda, Y.; Graham, S.P.; Pelle, R.; Taracha, E.L.; Gachanja, J.; Nyanjui, J.K.; Bray, J.; Palmer, G.H.; Brown, W.C.; et al. Treatment of cattle with DNA-encoded Flt3L and GM-CSF prior to immunization with Theileria parva candidate vaccine antigens induces CD4 and CD8 T cell IFN-gamma responses but not CTL responses. Vet. Immunol. Immunopathol. 2011, 140, 244–251. [Google Scholar] [CrossRef]
- Graham, S.P.; Pelle, R.; Honda, Y.; Mwangi, D.M.; Tonukari, N.J.; Yamage, M.; Glew, E.J.; de Villiers, E.P.; Shah, T.; Bishop, R.; et al. Theileria parva candidate vaccine antigens recognized by immune bovine cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 2006, 103, 3286–3291. [Google Scholar] [CrossRef] [PubMed]
- Svitek, N.; Saya, R.; Awino, E.; Munyao, S.; Muriuki, R.; Njoroge, T.; Pelle, R.; Ndiwa, N.; Poole, J.; Gilbert, S.; et al. An Ad/MVA vectored Theileria parva antigen induces schizont-specific CD8(+) central memory T cells and confers partial protection against a lethal challenge. NPJ Vaccines 2018, 3, 35. [Google Scholar] [CrossRef]
- Fry, L.M.; Bastos, R.G.; Stone, B.C.; Williams, L.B.; Knowles, D.P.; Murphy, S.C. Gene gun DNA immunization of cattle induces humoral and CD4 T-cell-mediated immune responses against the Theileria parva polymorphic immunodominant molecule. Vaccine 2019, 37, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.J.; Cha, S.H.; Grimm, A.L.; Ajithdoss, D.; Rzepka, J.; Chung, G.; Yu, J.; Davis, W.C.; Ho, C.S. Pigs that recover from porcine reproduction and respiratory syndrome virus infection develop cytotoxic CD4+CD8+ and CD4+CD8- T-cells that kill virus infected cells. PLoS ONE 2018, 13, e0203482. [Google Scholar] [CrossRef]
- Abdellrazeq, G.S.; Elnaggar, M.M.; Bannantine, J.P.; Park, K.T.; Souza, C.D.; Backer, B.; Hulubei, V.; Fry, L.M.; Khaliel, S.A.; Torky, H.A.; et al. A Mycobacterium avium subsp. paratuberculosis relA deletion mutant and a 35 kDa major membrane protein elicit development of cytotoxic T lymphocytes with ability to kill intracellular bacteria. Vet. Res. 2018, 49, 53. [Google Scholar] [CrossRef] [PubMed]
- Radley, D.E.; Brown, C.G.; Cunnigham, M.P.; Kimber, C.D.; Musisi, F.L.; Purnell, R.E.; Stagg, S.M. East Coast fever: Challenge if immunised cattle by prolonged exposure to infected ticks. Vet. Rec. 1975, 96, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.G.; Radley, D.E.; Cunningham, M.P.; Kirimi, I.M.; Morzaria, S.P.; Musoke, A.J. Immunization against East Coast fever (Theileria parva infection of cattle) by infection and treatment: Chemoprophylaxis with N-pyrrolidinomethyl tetracycline. Tropenmed. Parasitol. 1977, 28, 342–348. [Google Scholar]
- Young, A.S.; Leitch, B.L.; Dolan, T.T.; Mbogo, S.K.; Ndungu, S.G.; Grootenhuis, J.G.; De Castro, J.J. Evaluation of infection and treatment methods in immunization of improved cattle against theileriosis in an endemic area of Kenya. Vet Parasit. 1990, 35, 239–257. [Google Scholar] [CrossRef]
- Baldwin, C.L.; Black, S.J.; Brown, W.C.; Conrad, P.A.; Goddeeris, B.M.; Kinuthia, S.W.; Lalor, P.A.; MacHugh, N.D.; Morrison, W.I.; Morzaria, S.P. Bovine T cells, B cells, and null cells are transformed by the protozoan parasite Theileria parva. Infect. Immun. 1988, 56, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Goddeeris, B.M.; Morrison, W.I. Techniques for the generation, cloning, and characterization of bovine cytotoxic T cells specific for the protozoan Theileria parva. J. Tissue Cult. Methods 1988, 11, 101–110. [Google Scholar] [CrossRef]
- Storset, A.K.; Kulberg, S.; Berg, I.; Boysen, P.; Hope, J.C.; Dissen, E. NKp46 defines a subset of bovine leukocytes with natural killer cell characteristics. Eur. J. Immunol. 2004, 34, 669–676. [Google Scholar] [CrossRef]
- Allen, A.J.; Park, K.T.; Barrington, G.M.; Hamilton, M.J.; Davis, W.C. Development of a bovine ileal cannulation model to study the immune response and mechanisms of pathogenesis paratuberculosis. Clin. Vaccine Immunol. 2009, 16, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Packard, B.Z.; Telford, W.G.; Komoriya, A.; Henkart, P.A. Granzyme B activity in target cells detects attack by cytotoxic lymphocytes. J. Immunol. 2007, 179, 3812–3820. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.G.; Donnenberg, V.S.; Donnenberg, A.D.; Gooding, W.; Whiteside, T.L. A novel multiparametric flow cytometry-based cytotoxicity assay simultaneously immunophenotypes effector cells: Comparisons to a 4 h 51Cr-release assay. J. Immunol. Methods 2007, 325, 51–66. [Google Scholar] [CrossRef]
- Morrison, W.I.; Goddeeris, B.M.; Brown, W.C.; Baldwin, C.L.; Teale, A.J. Theileria parva in cattle: Characterization of infected lymphocytes and the immune responses they provoke. Vet. Immunol. Immunopathol. 1989, 20, 213–237. [Google Scholar] [CrossRef]
- Baldwin, C.; Goddeeris, B.M.; Morrison, W.I. Bovine helper T-cell clones specific for lymphocytes infected with Theileria parva (Muguga). Parasite Immunol. 1987, 9, 499–513. [Google Scholar] [CrossRef]
- Baldwin, C.; Iams, K.P.; Brown, W.C.; Grab, D.J. Theileria parva: CD4+ helper and cytotoxic T-cell clones react with a schizont-derived antigen associated with the surface of Theileria parva-infected lymphocytes. Exp. Parasitol. 1992, 75, 19–30. [Google Scholar] [CrossRef]
- Brown, W.C.; Lonsdale-Eccles, J.D.; Demartini, J.C.; Grab, D.J. Recognition of soluble Theileria parva antigen by bovine helper T cell clones: Characterization and partial purification of the antigen. J Immunol. 1990, 144, 271–277. [Google Scholar]
- Daubenberger, C.A.; Taracha, E.L.; Gaidulis, L.; Davis, W.C.; McKeever, D.J. Bovine gammadelta T-cell responses to the intracellular protozoan parasite Theileria parva. Infect Immun. 1999, 67, 2241–2249. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W.I.; Goddeeris, B.M.; Teale, A.J.; Groocock, C.M.; Kemp, S.J.; Stagg, D.A. Cytotoxic T cells elicited in cattle challenged with Theileria parva (Muguga): Evidence for restriction by class I MHC determinants and parasite strain specificity. Parasite Immunol. 1987, 9, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Zaritskaya, L.; Shurin, M.R.; Sayers, T.J.; Malyguine, A.M. New flow cytometric assays for monitoring cell-mediated cytotoxicity. Expert Rev. Vaccines 2010, 9, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Staska, L.M.; Davies, C.J.; Brown, W.C.; McGuire, T.C.; Suarez, C.E.; Park, J.Y.; Mathison, B.A.; Abbott, J.R.; Baszler, T.V. Identification of vaccine candidate peptides in the NcSRS2 surface protein of Neospora caninum by using CD4+ cytotoxic T lymphocytes and gamma interferon-secreting T lymphocytes of infected holstein cattle. Infect. Immun. 2005, 73, 1321–1329. [Google Scholar] [CrossRef]
- Staska, L.M.; McGuire, T.C.; Davies, C.J.; Lewin, H.A.; Baszler, T.V. Neospora caninum-infected cattle develop parasite-specific CD4+ cytotoxic T lymphocytes. Infect. Immun. 2003, 71, 3272–3279. [Google Scholar] [CrossRef]
- Curiel, T.J.; Krug, E.C.; Purner, M.B.; Poignard, P.; Berens, R.L. Cloned human CD4+ cytotoxic T lymphocytes specific for Toxoplasma gondii lyse tachyzoite-infected target cells. J. Immunol. 1993, 151, 2024–2031. [Google Scholar]
- Purner, M.B.; Berens, R.L.; Nash, P.B.; van Linden, A.; Ross, E.; Kruse, C.; Krug, E.C.; Curiel, T.J. CD4-mediated and CD8-mediated cytotoxic and proliferative immune responses to Toxoplasma gondii in seropositive humans. Infect. Immun. 1996, 64, 4330–4338. [Google Scholar] [CrossRef]
- Overgaard, N.H.; Jung, J.; Steptoe, R.J.; Wells, J.W. CD4+/CD8+ double-positive T cells: More than just a developmental stage? J. Leukoc. Biol. 2015, 97, 31–48. [Google Scholar] [CrossRef]
- Taracha, E.L.N.; Awino, E.; McKeever, D.J. Distinct CD4+ T cell helper requirements in Theileria parva-immune and -naive bovine CTL precursors. J. Immunol. 1997, 159, 4539–4545. [Google Scholar]
- Franceschi, V.; Mahmoud, A.H.; Abdellrazeq, G.S.; Tebaldi, G.; Macchi, F.; Russo, L.; Fry, L.M.; Elnaggar, M.M.; Bannantine, J.P.; Park, K.T.; et al. Capacity to Elicit Cytotoxic CD8 T Cell Activity Against Mycobacterium avium subsp. paratuberculosis Is Retained in a Vaccine Candidate 35 kDa Peptide Modified for Expression in Mammalian Cells. Front. Immunol. 2019, 10, 2859. [Google Scholar] [CrossRef]
- Abdellrazeq, G.S.; Fry, L.M.; Elnaggar, M.M.; Bannantine, J.P.; Schneider, D.A.; Chamberlin, W.M.; Mahmoud, A.H.A.; Park, K.T.; Hulubei, V.; Davis, W.C. Simultaneous cognate epitope recognition by bovine CD4 and CD8 T cells is essential for primary expansion of antigen-specific cytotoxic T-cells following ex vivo stimulation with a candidate Mycobacterium avium subsp. paratuberculosis peptide vaccine. Vaccine 2020. [Google Scholar] [CrossRef] [PubMed]

| Monoclonal or Secondary Antibody | Antibody Isotype | Antibody Specificity | Antibody Supplier |
|---|---|---|---|
| ILA11A | IgG2a | Bovine CD4 | WSUMAC, USA |
| 7C2B | IgG2a | Bovine CD8 | WSUMAC, USA |
| CACT80C | IgG1 | Bovine CD8 | WSUMAC, USA |
| ILA116A | IgG1 | Bovine CD45R0 | WSUMAC, USA |
| CACT116A | IgG1 | Bovine CD25 | WSUMAC, USA |
| GB21A | IgG2b | Bovine γδ TCR | WSUMAC, USA |
| 3D12 | IgG2a | Human CCR7 | BD Pharmingen, USA |
| AKS8 | IgG2a | Bovine CD335 | Norwegian University of Life Sciences [23] |
| Goat anti-mouse | IgG2a PE.CY5.5 | ThermoFisher, USA | |
| Goat anti-mouse | IgG1 Alexa Fluor 647 | ThermoFisher, USA | |
| Goat anti-mouse | IgG1 FITC | ThermoFisher, USA | |
| Goat anti-mouse | IgG3 FITC | Southern Biotech, USA | |
| Goat anti-rat | IgG FITC | Southern Biotech, USA | |
| Goat anti-mouse | IgG2b PE | Southern Biotech, USA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elnaggar, M.M.; Knowles, D.P.; Davis, W.C.; Fry, L.M. Flow Cytometric Analysis of the Cytotoxic T-Cell Recall Response to Theileria parva in Cattle Following Vaccination by the Infection and Treatment Method. Vet. Sci. 2021, 8, 114. https://doi.org/10.3390/vetsci8060114
Elnaggar MM, Knowles DP, Davis WC, Fry LM. Flow Cytometric Analysis of the Cytotoxic T-Cell Recall Response to Theileria parva in Cattle Following Vaccination by the Infection and Treatment Method. Veterinary Sciences. 2021; 8(6):114. https://doi.org/10.3390/vetsci8060114
Chicago/Turabian StyleElnaggar, Mahmoud M., Donald P. Knowles, William C. Davis, and Lindsay M. Fry. 2021. "Flow Cytometric Analysis of the Cytotoxic T-Cell Recall Response to Theileria parva in Cattle Following Vaccination by the Infection and Treatment Method" Veterinary Sciences 8, no. 6: 114. https://doi.org/10.3390/vetsci8060114
APA StyleElnaggar, M. M., Knowles, D. P., Davis, W. C., & Fry, L. M. (2021). Flow Cytometric Analysis of the Cytotoxic T-Cell Recall Response to Theileria parva in Cattle Following Vaccination by the Infection and Treatment Method. Veterinary Sciences, 8(6), 114. https://doi.org/10.3390/vetsci8060114





