Left Atrial Mural Thrombosis and Hemopericardium in a Dog with Myxomatous Mitral Valve Disease
Abstract
1. Introduction
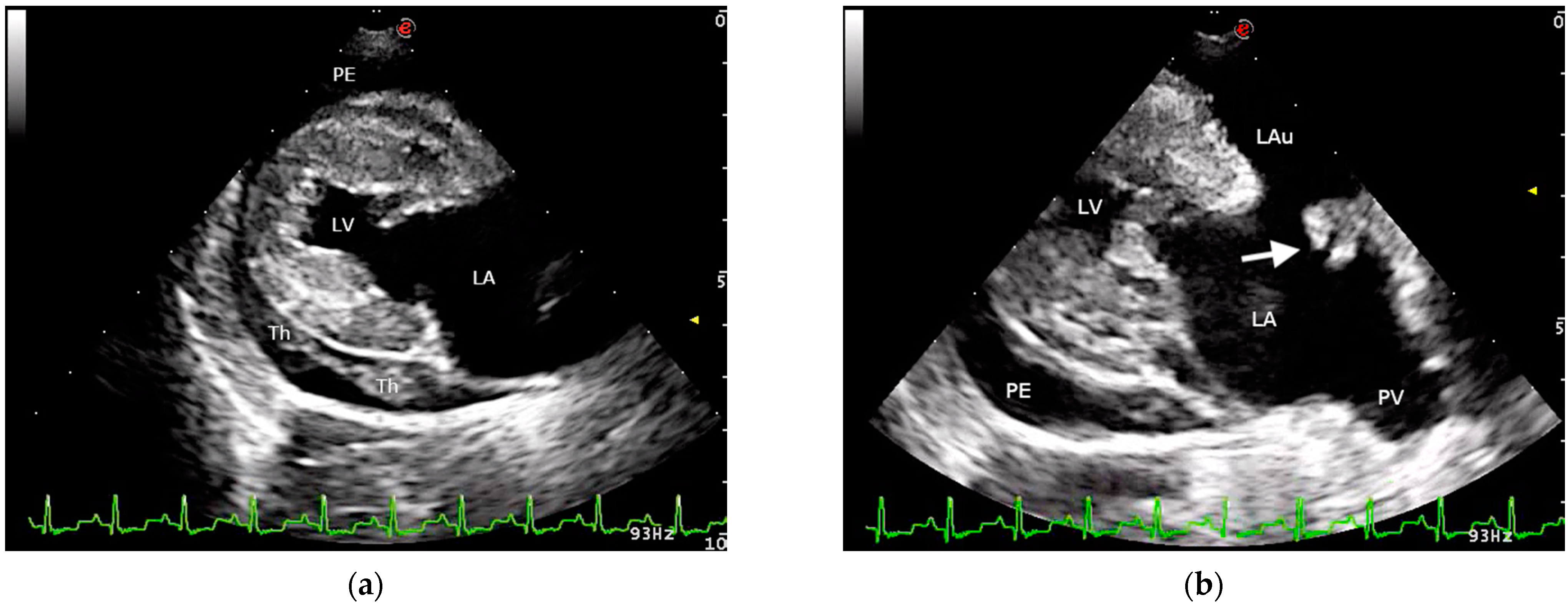
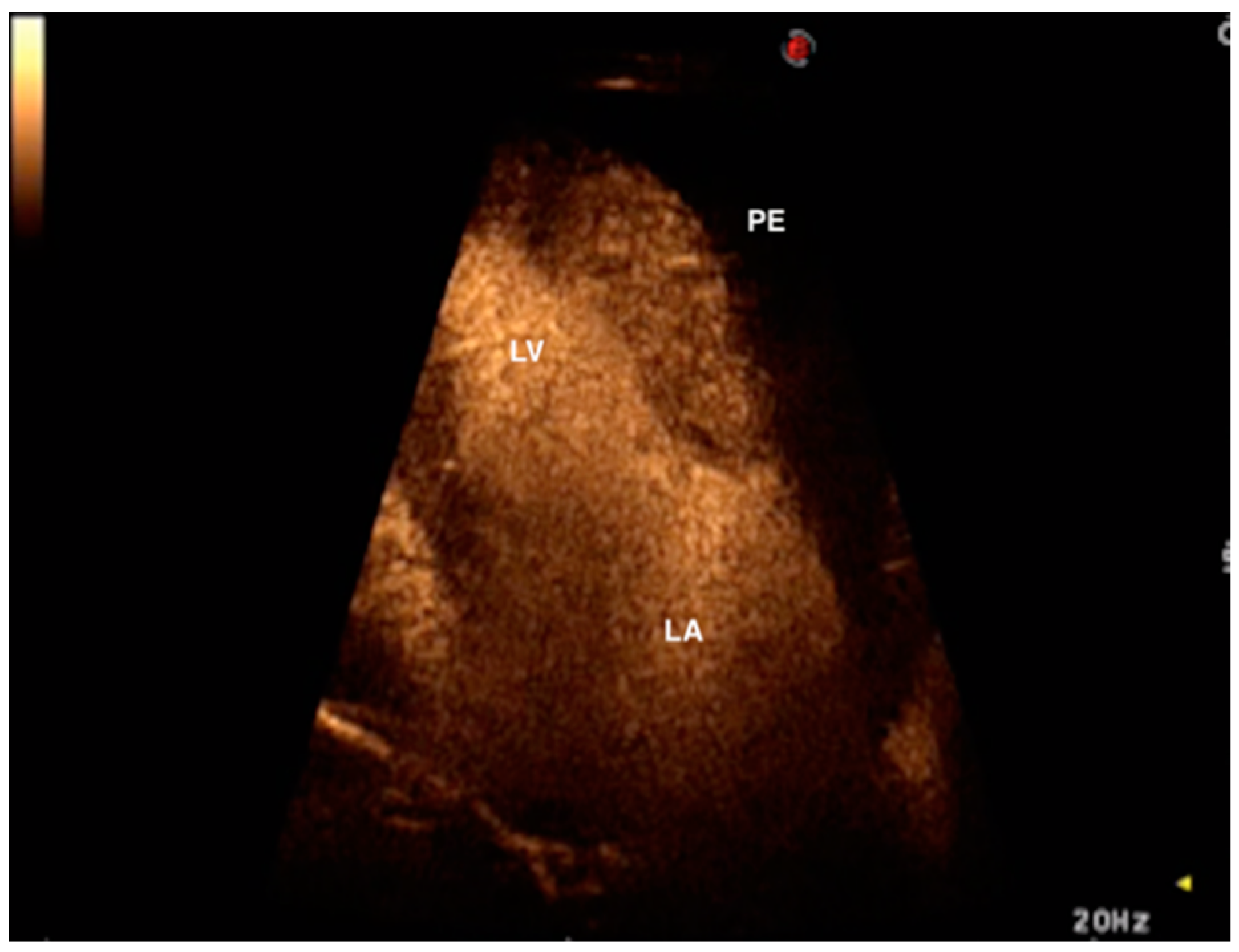
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Buchanan, J.W.; Kelly, A.M. Endocardial Splitting of the Left Atrium in the Dog with Hemorrhage and Hemopericardium. Vet. Radiol. 1964, 5, 28–38. [Google Scholar] [CrossRef]
- Buchanan, J.W. Spontaneous left atrial rupture in dogs. Adv. Exp. Med. Biol. 1972, 22, 315–334. [Google Scholar]
- Sadanaga, K.K.; MacDonald, M.J.; Buchanan, J.W. Echocardiography and Surgery in a Dog with Left Atrial Rupture and Hemopericardium. J. Vet. Intern. Med. 1990, 4, 216–221. [Google Scholar] [CrossRef]
- Prosek, R.; Sisson, D.D.; Oyama, M.A. What is your diagnosis? Pericardial effusion with a clot in the pericardial space likely caused by left atrial rupture secondary to mitral regurgitation. J. Am. Vet. Med. Assoc. 2003, 222, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Reineke, E.L.; Burkett, D.E.; Drobatz, K.J. Left atrial rupture in dogs: 14 cases (1990–2005). J. Vet. Emerg. Crit. Care 2008, 18, 158–164. [Google Scholar] [CrossRef]
- Fox, P.R. Pathology of myxomatous mitral valve disease in the dog. J. Vet. Cardiol. 2012, 14, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.K.; Tompkins, E.; Russell, N.J.; Zimmerman, S.A.; Yuhas, D.L.; Morrison, T.J.; Lesser, M.B. Left atrial rupture secondary to myxomatous mitral valve disease in 11 dogs. J. Am. Anim. Hosp. Assoc. 2014, 50, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Sleeper, M.M.; Maczuzak, M.E.; Bender, S.J. Myocardial infarct associated with a partial thickness left atrial tear in a dog with mitral insufficiency. J. Vet. Cardiol. 2015, 17, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Saura, D.; Florenciano, R.; de la Morena, G.; Soria, F.; Espinosa, M.D.; Arribas, J.M.; Valdés-Chávarri, M. Intraoperative myocardial rupture heralded by contrast echocardiography. J. Am. Soc. Echocardiogr. 2007, 20, 906. [Google Scholar] [CrossRef]
- Okabe, T.; Julien, H.M.; Kaliyadan, A.G.; Siu, H.; Marhefka, G.D. Prompt Recognition of Left Ventricular Free-Wall Rupture Aided by the Use of Contrast Echocardiography. Tex Heart Inst. J. 2015, 42, 474–478. [Google Scholar] [CrossRef]
- Caivano, D.; Birettoni, F.; Bufalari, A.; De Monte, V.; Angeli, G.; Giorgi, M.E.; Patata, V.; Porciello, F. Contrast-enhanced ultrasonographic findings in three dogs with lung lobe torsion. J. Vet. Med. Sci. 2016, 78, 427–430. [Google Scholar] [CrossRef][Green Version]
- Usechak, P.J.; Bright, J.M.; Day, T.K. Thrombotic complications associated with atrial fibrillation in three dogs. J. Vet. Cardiol. 2012, 14, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Ballocco, I.; Pinna Parpaglia, M.L.; Corda, F.; Serra, G.; Corda, A. Left atrial thrombosis secondary to blunt cardiac injury in two dogs. Vet. Rec. Case Rep. 2019, 7, e000803. [Google Scholar] [CrossRef]
- Caivano, D.; Birettoni, F.; Giorgi, M.E.; Porciello, F. What is your diagnosis? Intracardiac thrombus. J. Am. Vet. Med. Assoc. 2014, 245, 1003–1005. [Google Scholar] [CrossRef]
- Berg, R.J.; Wingfield, W.E.; Hoopes, P.J. Idiopathic hemorrhagic pericardial effusion in eight dogs. J. Am. Vet. Med. Assoc. 1984, 185, 988–992. [Google Scholar] [PubMed]
- Ribas, T.; Pipe-Martin, H.; Kim, K.S.; Leissinger, M.K.; Bauer, R.W.; Grasperge, B.J.; Grooters, A.M.; Sutton, D.A.; Pariaut, R. Fungal myocarditis and pericardial effusion secondary to Inonotus tropicalis (phylum Basidiomycota) in a dog. J. Vet. Cardiol. 2015, 17, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Caivano, D.; Birettoni, F.; Marchesi, M.C.; Moretti, G.; Corda, A.; Petrescu, V.F.; Porciello, F.; Bufalari, A. Septic Pericarditis and Cardiac Tamponade Caused by Migrating Intrathoracic Grass Awn in an English Setter Dog. J. Vet. Med. 2019, 74, 82–87. [Google Scholar]
- Berg, R.; Wingfield, W. Pericardial effusion in the dog: A review of 42 cases. J. Am. Anim. Hosp. Assoc. 1984, 20, 721–730. [Google Scholar]
- Dunning, D.; Monnet, E.; Orton, E.C.; Salman, M.D. Analysis of prognostic indicators for dogs with pericardial effusion: 46 cases (1985—1996). J. Am. Vet. Med. Assoc. 1998, 212, 1276–1280. [Google Scholar]
- Guglielmini, C.; Diana, A.; Santarelli, G.; Torbidone, A.; Di Tommaso, M.; Baron Toaldo, M.; Cipone, M. Accuracy of radiographic vertebral heart score and sphericity index in the detection of pericardial effusion in dogs. J. Am. Vet. Med. Assoc. 2012, 241, 1048–1055. [Google Scholar] [CrossRef]
- Peddle, G.D.; Buchanan, J.W. Acquired atrial septal defects secondary to rupture of the atrial septum in dogs with degenerative mitral valve disease. J. Vet. Cardiol. 2010, 12, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Lake-Bakaar, G.A.; Mok, M.Y.; Kittleson, M.D. Fossa ovalis tear causing right to left shunting in a Cavalier King Charles Spaniel. J. Vet. Cardiol. 2012, 14, 541–545. [Google Scholar] [CrossRef]
- Ware, W.A.; Hopper, D.L. Cardiac tumors in dogs: 1982–1995. J. Vet. Intern. Med. 1999, 13, 95–103. [Google Scholar] [PubMed]
- Crosara, S.; Ljungvall, I.; Margiocco, M.L.; Häggström, J.; Tarducci, A.; Borgarelli, M. Use of contrast echocardiography for quantitative and qualitative evaluation of myocardial perfusion and pulmonary transit time in healthy dogs. Am. J. Vet. Res. 2012, 73, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Goggs, R.; Bacek, L.; Bianco, D.; Koenigshof, A.; Li, R.H.L. Consensus on the rational use of antithrombotics in veterinary critical care (CURATIVE): Domain 2-defining rational therapeutic usage. J. Vet. Emerg. Crit. Care 2019, 29, 49–59. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caivano, D.; Marchesi, M.C.; Birettoni, F.; Lepri, E.; Porciello, F. Left Atrial Mural Thrombosis and Hemopericardium in a Dog with Myxomatous Mitral Valve Disease. Vet. Sci. 2021, 8, 112. https://doi.org/10.3390/vetsci8060112
Caivano D, Marchesi MC, Birettoni F, Lepri E, Porciello F. Left Atrial Mural Thrombosis and Hemopericardium in a Dog with Myxomatous Mitral Valve Disease. Veterinary Sciences. 2021; 8(6):112. https://doi.org/10.3390/vetsci8060112
Chicago/Turabian StyleCaivano, Domenico, Maria Chiara Marchesi, Francesco Birettoni, Elvio Lepri, and Francesco Porciello. 2021. "Left Atrial Mural Thrombosis and Hemopericardium in a Dog with Myxomatous Mitral Valve Disease" Veterinary Sciences 8, no. 6: 112. https://doi.org/10.3390/vetsci8060112
APA StyleCaivano, D., Marchesi, M. C., Birettoni, F., Lepri, E., & Porciello, F. (2021). Left Atrial Mural Thrombosis and Hemopericardium in a Dog with Myxomatous Mitral Valve Disease. Veterinary Sciences, 8(6), 112. https://doi.org/10.3390/vetsci8060112





