1. Introduction
The corneal surface is one of the zones with the highest density of nerve terminals, in which the density of corneal epithelial nerve terminals is 20–40 times that of nerve terminals in the dental pulp and 300–600 times that of cutaneous epithelial nerve terminals [
1,
2]. There are numerous sensory nerves and pain receptors on the corneal surface, which are mainly innervated by the long ciliary nerves derived from the ophthalmic branch of trigeminal nerves. Among them, the primary pain receptors are located in the epithelial layer, while the pressoreceptors are mainly located in the stromal layer [
3].
Corneal nerves not only possess the sensory function, but also provide various metabolic nutrition and support for the cornea, including regulating ion transport, the proliferation, differentiation, adhesion, and migration of cells and wound healing. Therefore, they are significant for maintaining the normal structure and function of the cornea [
4]. After corneal nerves are injured by various corneal operations, not only will the corneal sensation decrease, but also the corneal nutrition and metabolic disorders of different degrees may occur consequently [
5,
6]. The corneal nerve dysfunction can induce reduced mitosis of corneal epithelial cells, decreased adhesion activity between cells, decreased wound healing ability, and secondary neurotrophic corneal epithelial diseases, which are manifested as xerophthalmia, corneal infection, corneal ulcer, and so forth [
7]. Corneal penetrating surgery would cause the most serious damage to corneal nerves. After the penetrating incision is performed on the cornea at the corneoscleral limbus, corneal nerve trunk would be cut off, and the cornea near the incision would be free from the innervation, which makes the sensation become dull or disappear. The corneal sensation in corresponding zones would recover slowly. In animal experiments, it can be found that after the penetrating incision is performed on the corneal limbus of rabbits for 1–3 months, stromal corneal nerves could penetrate scar tissue and re-enter the corresponding parts of the cornea; however, the subepithelial nerve plexus and interepithelial nerve terminals are still missing or scarce 30 months after the operation [
8,
9,
10]. After the cornea is stimulated by penetrating surgery, nerve terminals will release some neurotransmitters, including acetylcholine (ACE), catecholamine (CA), substance P (SP), and calcitonin gene-related peptide (CGRP), which make the sensitivity of the corneal 100 times that of the conjunctiva. Corneal lesions (such as keratitis, corneal foreign bodies, corneal ulcers, etc.) in any depth can induce severe pain and blepharospasm [
11,
12,
13].
The canine cataract is a significant ophthalmic disease in clinic. Surgery is the only effective method in the treatment of canine cataract. The transparent corneal incision or scleral tunnel incision is usually applied in the process of cataract surgery. Currently, the transparent corneal incision is often performed in the direction of 12 o’clock in pet clinics [
14]. It is prone to the development of postoperative complications, such as corneal edema, delayed corneal epithelial healing, corneal endothelial dystrophy, decreased corneal perception, and decreased intraocular pressure [
15,
16,
17]. Partial symptoms are closely related to corneal nerve injury [
18,
19,
20]. Therefore, the occurrence of postoperative complications can be effectively reduced via exploring the difference of nerve distribution in different corneal positions of canines and selecting the zone with relatively few corneal nerve roots as the incision position. Furthermore, the corneal nerve distribution can provide a reasonable anatomical basis for the selection of incision position during the corneal penetrating surgery in veterinary clinical practice.
Currently, most understanding about corneal nerve morphology and distribution is obtained by corneal nerve staining, including gold chloride staining, acetylcholinesterase staining, and immunohistochemical staining [
21]. Gold chloride corneal nerve staining is a specific staining method for nerve terminals. There are various methods to stain corneal nerves with gold chloride reported in the literature [
22,
23,
24,
25]. The principle of gold chloride staining is generally considered as that gold ions in solution are reduced into gold particles in an acidic environment, which would attach to neurofibrils for color manifestation. The silver nitrate staining, gold chloride staining, and cholinesterase staining have been subjected to a comparison in the literature [
26]. It has been concluded that gold chloride staining can clearly manifest nerve fiber bundles and subepithelial nerve plexus in corneal parenchyma, as well as the extremely tenuous nerve fibers in corneal parenchyma [
27]. Besides, gold chloride staining can manifest corneal nerves in every layer of the cornea, especially the nerve terminals in the epithelial layer and stromal layer. However, gold chloride staining is limited by its unfavorable specificity, which induces failure in the discrimination of various corneal nerves. The objective of this study was to analyze the distribution patterns of corneal nerve roots at different locations. In order to avoid wrong or missing nerve roots, the gold chloride staining method with poor specificity was adopted, due to the fact that this method can stain nerves in the stromal layer and epithelial layer and manifest the extremely tenuous nerve fibers.
4. Discussion
In this study, it was found that the number of corneal nerve roots had no correlation with gender and age of canines. However, He et al. [
26] compared the number of human corneal nerve roots at different ages, in 2010. In the eye samples of donors aged 70 and over, it was found that the corneal nerve density decreased gradually with the increase of age. The author believes that aging reduces the number of central epithelial nerve terminals. In our study, the samples were mainly concentrated in canines aged from 8 months old to 3 years old. It is generally believed that canines under 1 year old are puppies and canines from 1 to 7 years old are adults. The results of this experiment show that there is no significant difference in the number of corneal nerve roots between adult canines and puppies. However, due to the limited age span of the samples, no corneal samples of elderly canines (>7 years old) were collected; thus, it is impossible to analyze the difference in the number of corneal nerve roots in aging canines. However, for the current sample, we believe that the number of corneal nerve roots will not change greatly during the transition from infancy to adulthood. At the same time, He et al. [
26] found that epithelial nerve density and terminal numbers were higher in the center of the cornea, rather than the periphery. In our study, it was found that although the distribution density of nerve terminals at the corneoscleral limbus of canines was low, it was the area of nerve root distribution. From this area, nerve terminals were sent out and interwoven in the center of cornea to form a high-density corneal nerve terminals network. This finding is consistent with the results of He et al. [
26].
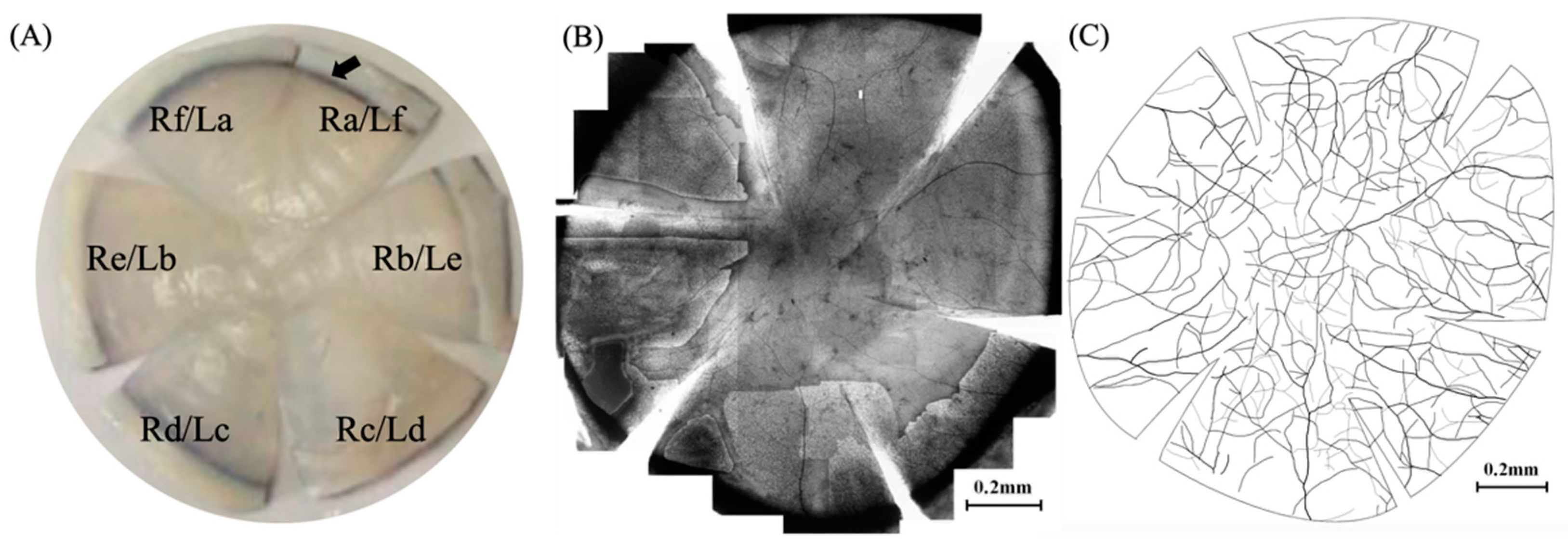
In addition, this paper adopts a six-quadrant zoning method for cornea, which is different from the four-quadrant zoning method proposed by Weigt et al. [
23] and He et al. [
26]. During the staining process, it could be observed that the staining quality around the incision in the direction of 12 o’clock for marking was higher than that in the contralateral side. Therefore, a short incision (1/4 radius length) was made along the radius in the direction of 12 o’clock when performing the initial marking in this direction, while long incisions (3/4 radius length) were made along the radius in the direction of 2 o’clock, 4 o’clock, 6 o’clock, 8 o’clock, and 10 o’clock, respectively, so that the cornea could be divided equally into six quadrants for the optimal staining results.
The canine corneal diameter is 14–18 mm, the average number of nerve roots is 24.43, and the average density of nerve roots at the corneoscleral limbus is 0.43–0.56 roots/mm (total number of nerve roots/πd). Based on the distribution patterns of canine corneal nerves, the nerve fibers of corneal nerve roots of canines emanated from the corneoscleral limbus to the central cornea. When a transparent corneal incision is made, the incision will be made on the cornea within about 1 mm to the corneoscleral limbus in a direction perpendicular to the radius of the cornea. The corneal nerves in the corresponding area could be cut off properly, which will directly damage the nerve roots at the corneoscleral limbus and the corneal nerve terminals, including the nerve branches between epithelial cells in the superficial layer and the nerve plexus in the corneal stromal layer. Even the overlapping branches extending from other regions of the corneal nerve would also be cut off [
30]. In contrast, a scleral tunnel incision will be made parallel to the corneoscleral limbus from approximately 2.5 mm posterior to the corneoscleral limbus to a depth of 1/2 scleral thickness. The scleral tunnel knife will be adopted to peel forward along the scleral arc to the posterior corneoscleral limbus. Subsequently, the knife will be slightly elevated to avoid the location of the nerve roots at the corneoscleral limbus and peel forward along the corneal arc to 1 mm within the transparent cornea. Then, the puncture knife will be employed to puncture the anterior chamber. The depth of the tunnel knife in the cornea is approximately flush with the position of the corneal stromal layer. At this point, the dense nerve plexus within the stromal layer of the cornea will be partially cut off, while the nerve plexus in the superficial stromal layer will not be cut off. Besides, there are still nerve terminals from other zones of the cornea distributed in the corneal counterpart with the scleral tunnel incision. Therefore, it can be observed clinically that although the postoperative corneal sensation is diminished, it is not as significant as in the transparent corneal incision [
18].
Currently, the incision length for the implantation of the soft intraocular lens is about 3.2 mm, and that the implantation of the rigid intraocular lens is about 5.5 mm in canine clinical cataract surgery. As per the density of nerve roots at the corneoscleral limbus (0.43–0.56 roots/mm), an average of 1.38–1.79 roots and 2.37–3.08 roots would be cut off, respectively, in these two common surgical incisions. Although the number of nerve roots cut for these two incisions is not large compared with the total number in the cornea (24.43), corneal nerves would intersect and anastomose with each other, which can partially compensate the cut region of nerve roots. However, even if the injury degree is small, more methods shall be taken to further reduce the injury degree of corneal nerves caused by corneal penetrating incision.
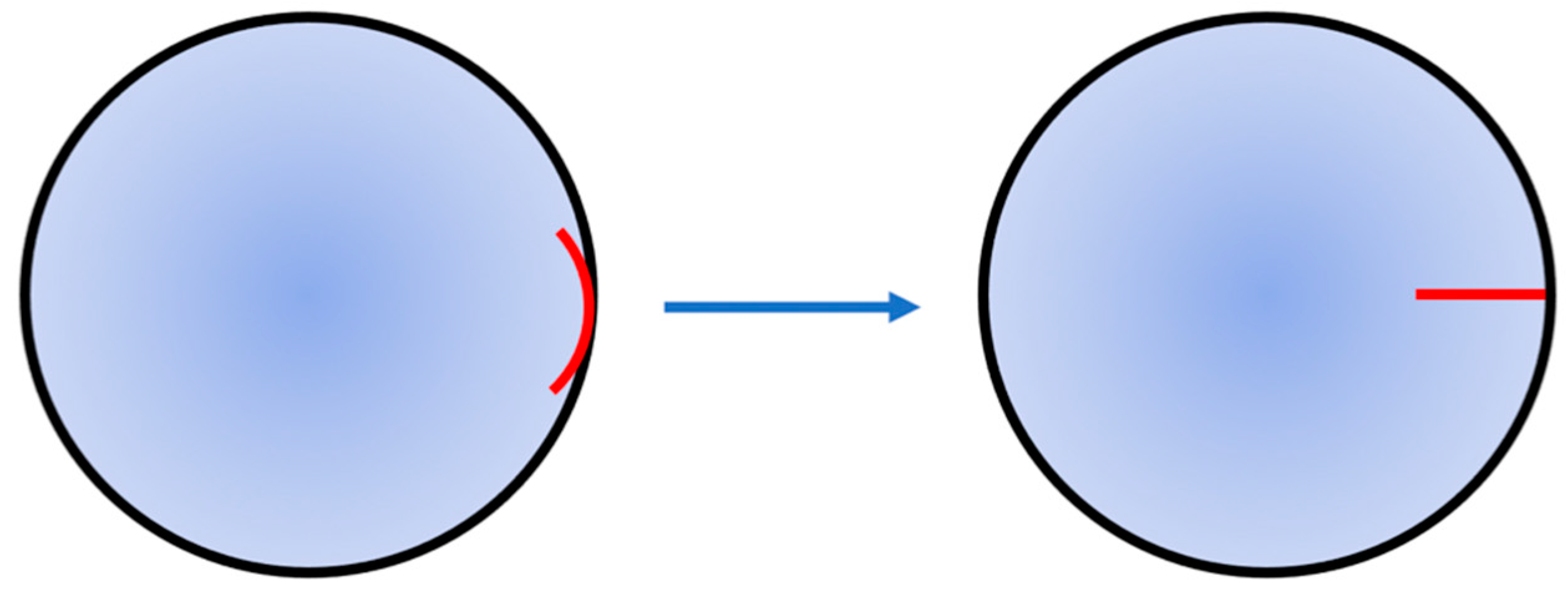
As revealed by the results of this study, the nerve roots in all zones of the canine cornea are evenly and symmetrically distributed. Therefore, there is no obvious difference in the injury degree, no matter which zone of the corneoscleral limbus is selected as the incision position. However, different incision directions would directly affect the injury degree of nerve roots. It can be maintained that the injury degree of nerve roots can be reduced to the lowest when the incision is made along the corneal radius. In the surgery that does not require the direction of corneal incision, the surgical approach of corneal penetrating surgery is adjusted from the direction perpendicular to radius to that along the radius (
Figure 3), which can avoid the position of nerve roots at the corneoscleral limbus and reduce the injury degree of corneal nerves caused by corneal penetrating surgery. Although the incision along the radius may cut off the nerve fiber branches inside the cornea, it can protect the nerve roots at the corneoscleral limbus and minimize the injury degree of corneal nerves. Therefore, in order to reduce the postoperative complications caused by excessive transection of corneal nerves, it is a new idea worth exploring to adjust the incision direction of corneal penetrating surgery.