The Therapeutic Effects of Oral Intake of Hydrogen Rich Water on Cutaneous Wound Healing in Dogs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dogs
2.2. Creation of Surgical Wounds
2.3. Examination of the Skin Wound
2.4. Statistical Analysis
3. Results
3.1. Wound Healing
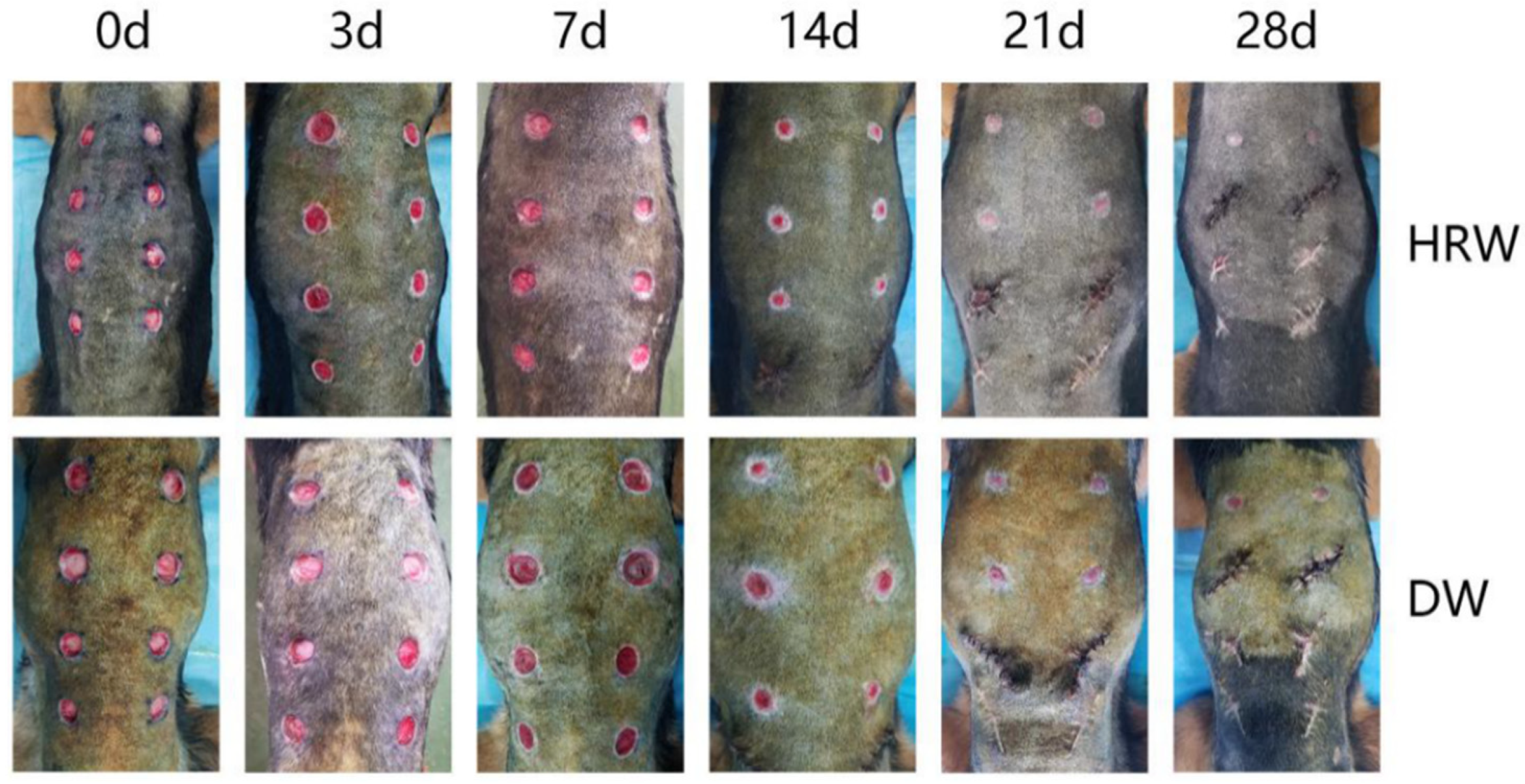
3.1.1. Clinical Wound Evaluation
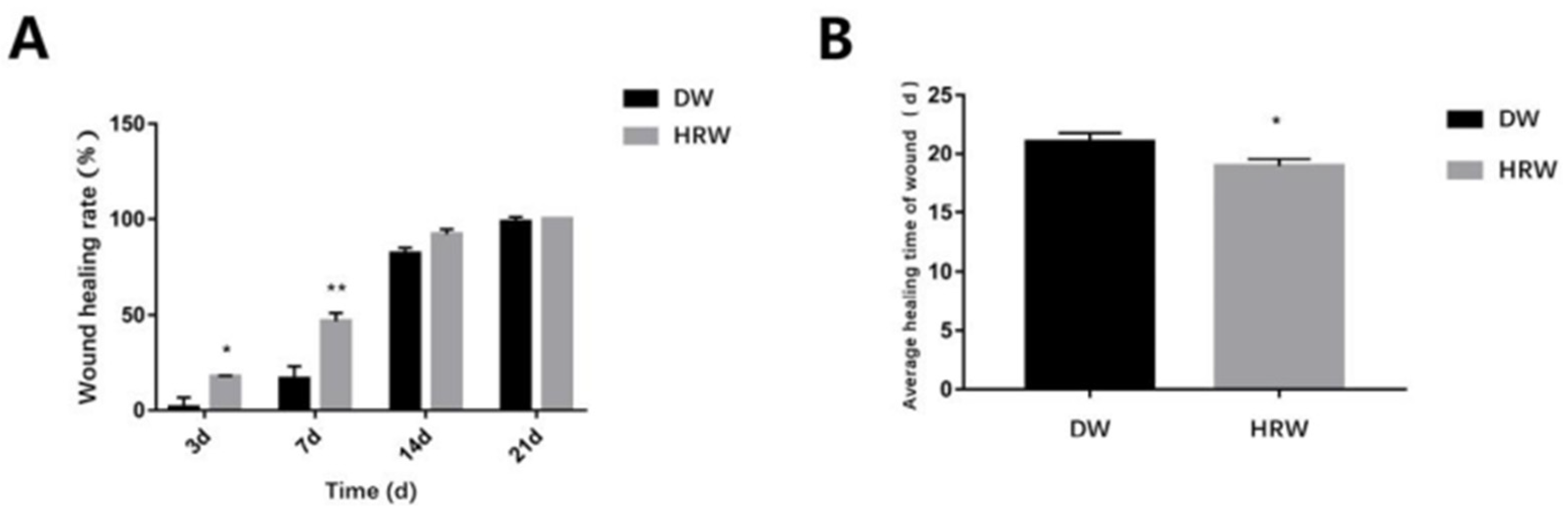
3.1.2. Wound Healing Rate and Average Healing Time
3.2. Histopathology
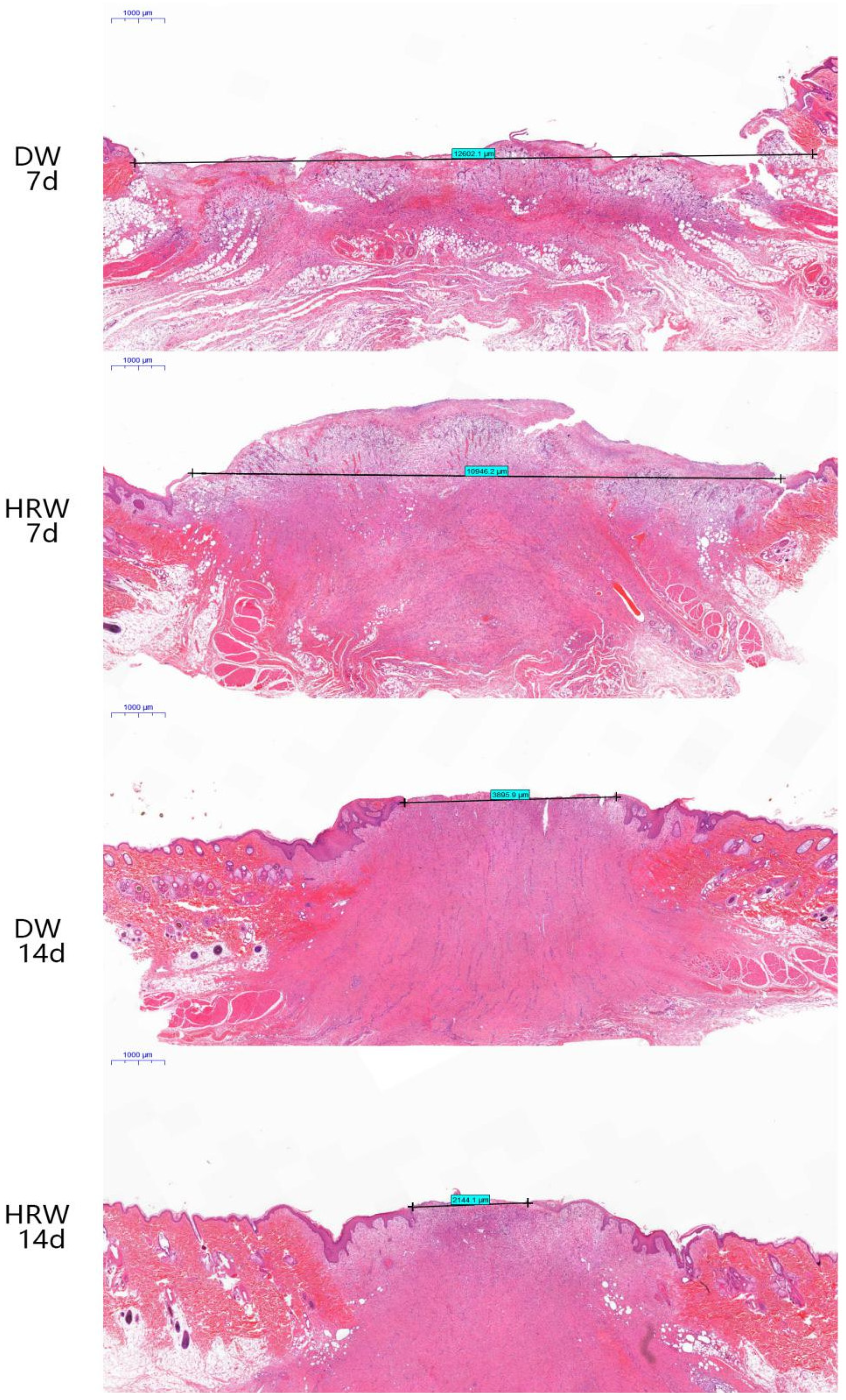
3.2.1. Wound Length
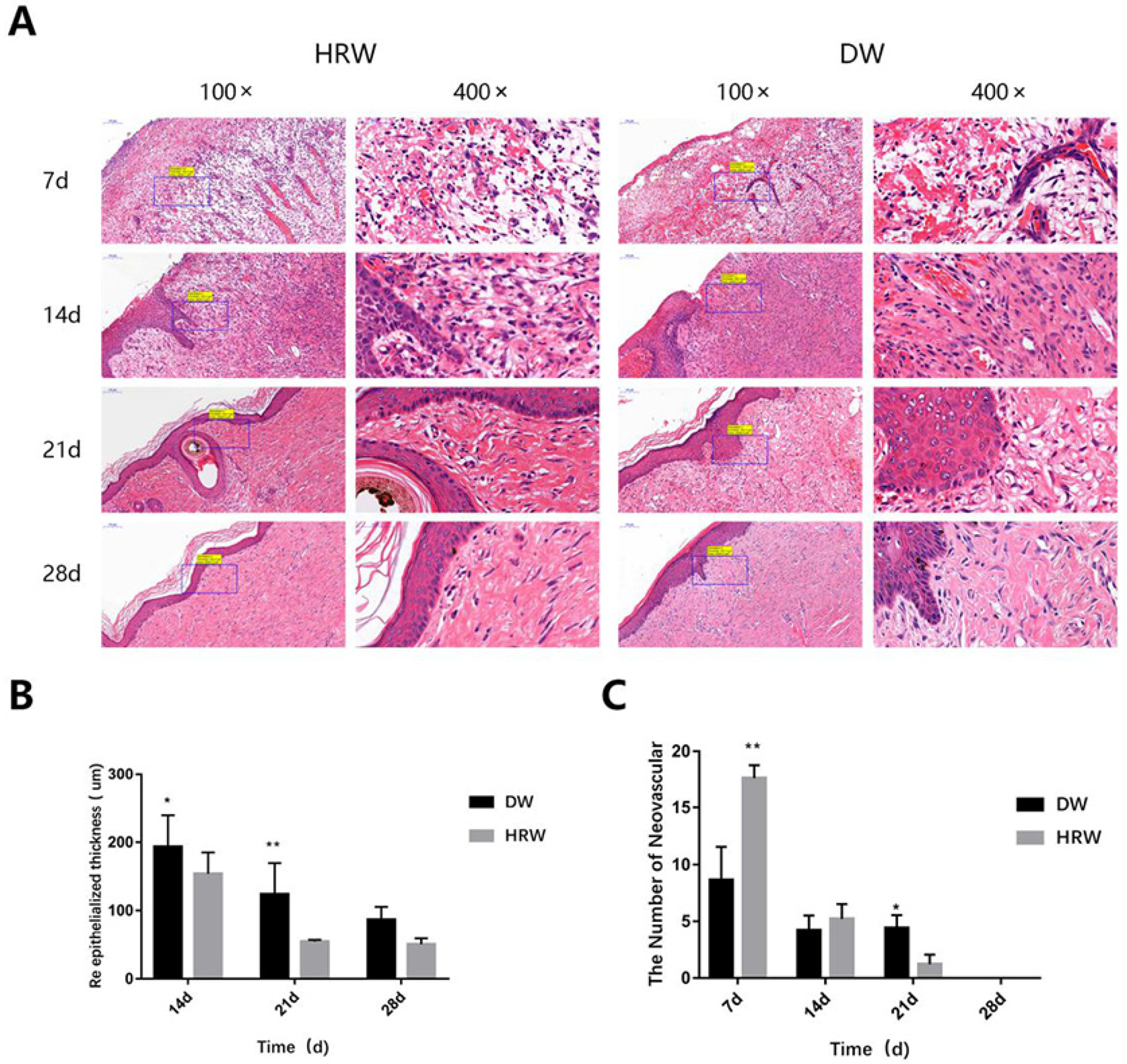
3.2.2. Re-Epithelialization
3.2.3. Neovascularization
3.2.4. Collagen Fiber
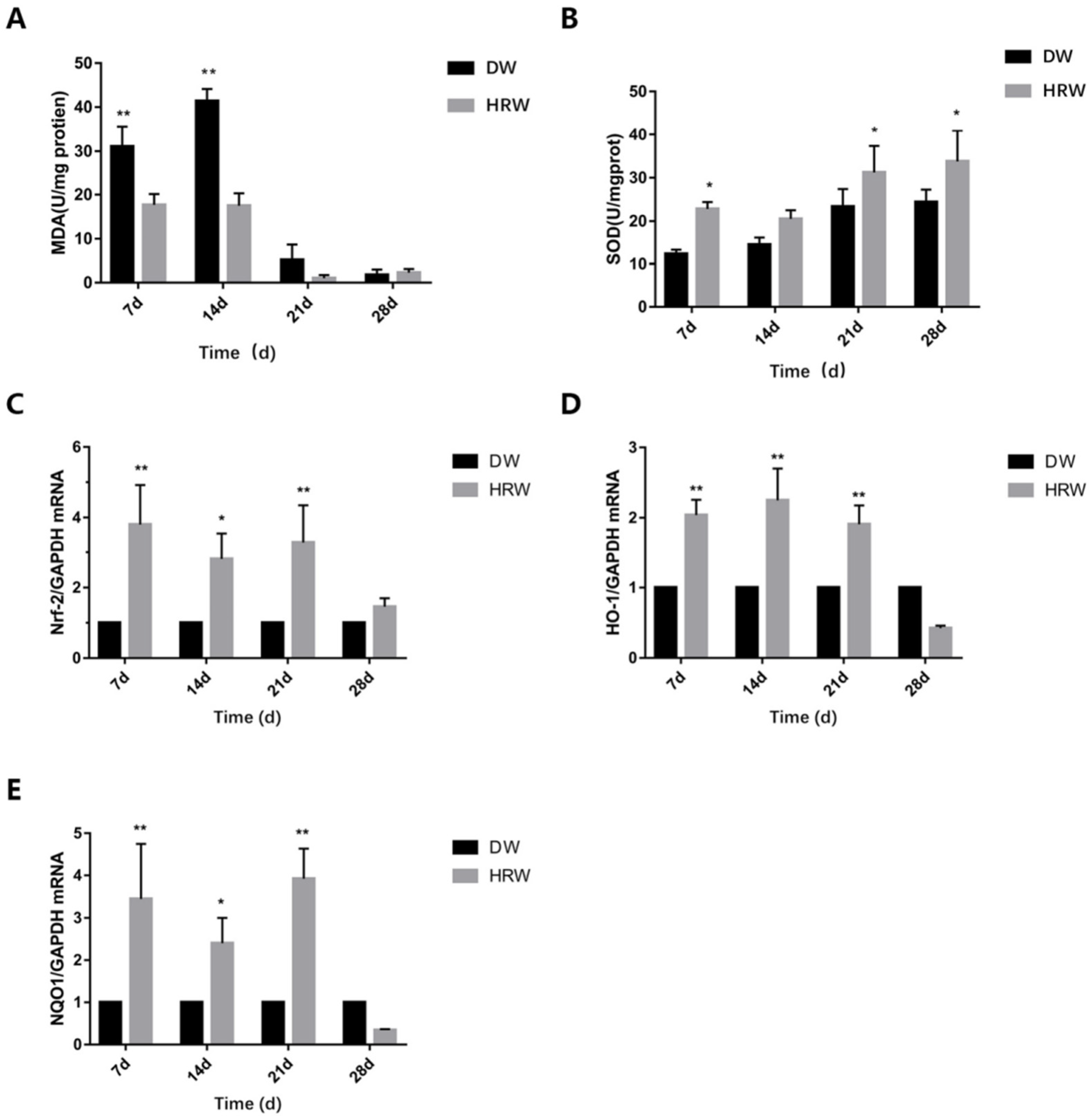
3.3. Oxidative Stress Detection
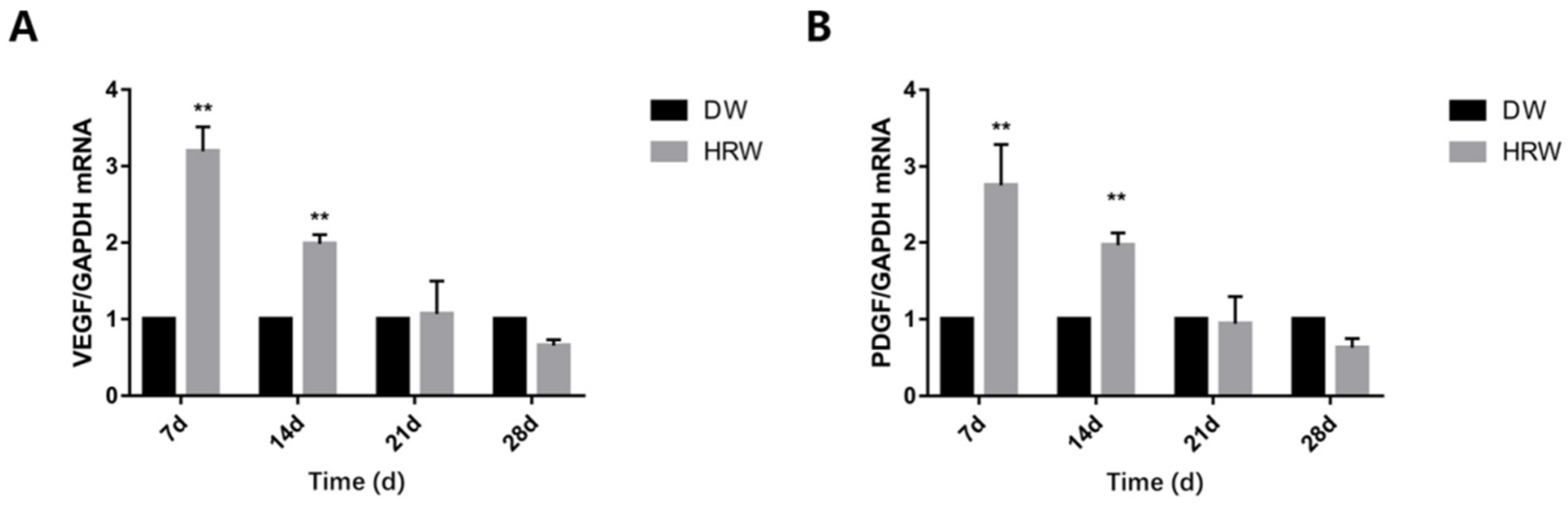
3.4. Growth Factor Detection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joanna, J.K. Novel method for border irregularity assessment in dermoscopic color images. Comput. Math. Methods Med. 2015, 2015, 496202. [Google Scholar]
- Wang, Y.; Jing, L.; Zhao, X.M.; Han, J.J.; Xia, Z.L.; Qin, S.C.; Wu, Y.P.; Sun, X.J. Protective effects of hydrogen-rich saline on monocrotaline-induced pulmonary hypertension in a rat model. Respir. Res. 2011, 12, 26–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Mclennan, S.V.; Lo, L.; Natfaji, A.; Bolton, T.; Liu, Y.; Twigg, S.M.; Yue, D.K. Bacterial load predicts healing rate in neuropathic diabetic foot ulcers. Diabetes Care 2007, 30, 378–380. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.B.; Fang, L.J.; Li, H.H.; Li, X.K.; Cheng, B.; Sheng, Z.Y. Adipose tissue extract enhances skin wound healing. Wound Repair Regener. 2010, 15, 540–548. [Google Scholar] [CrossRef]
- Hage, R.; Plapler, H.; Bitar, R.A. Photodynamic therapy (PDT) to treat a chronic skin wound in a dog. Proc. Soc. Photo. Opt. Instrum. Eng. 2008, 16, 68450X-1–68450X-6. [Google Scholar]
- Toyokawa, H.; Matsui, Y.Y.; Uhara, J.; Tsuchiya, H.; Teshima, S.; Nakanishi, H.; Kwon, A.H.; Azuma, Y.; Nagaoka, T.; Ogawa, T.; et al. Promotive effects of far-infrared ray on full-thickness skin wound healing in rats. Exp. Biol. Med. 2003, 228, 724–729. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, G.; Huang, H.; Wu, J. Advances and impact of arginine-based materials in wound healing. J. Mater. Chem. B 2021, 9, 6738–6750. [Google Scholar] [CrossRef]
- Xu, Z.; Liang, B.; Tian, J.; Wu, J. Anti-inflammation biomaterial platforms for chronic wound healing. Biomater. Sci. 2021, 9, 4388–4409. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, D.C.; Chen, S.Y. Hydrogen water reduces NSE, IL-6, and TNF-α levels in hypoxic-ischemic encephalopathy. Open Med. 2016, 11, 399–406. [Google Scholar] [CrossRef]
- Dole, M.; Wilson, F.; Fife, W. Hyperbaric hydrogen therapy: A possible treatment for cancer. Science 1975, 190, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Kajiyama, S.; Hasegawa, G.; Asano, M.; Hosoda, H.; Fukui, M.; Nakamura, N.; Kitawaki, J.; Imai, S.; Nakano, K.; Ohta, M.; et al. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr. Res. 2008, 28, 137–143. [Google Scholar] [CrossRef]
- Toru, I. Molecular hydrogen: New antioxidant and anti-inflammatory therapy for rheumatoid arthritis and related diseases. Curr. Pharm. Des. 2013, 19, 6375–6381. [Google Scholar]
- Kawamura, T.; Wakabayashi, N.; Shigemura, N.; Huang, C.S.; Masutani, K.; Tanaka, Y.; Noda, K.; Peng, X.; Takahashi, T.; Billiar, T.R.; et al. Hydrogen gas reduces hyperoxic lung injury via the Nrf2 pathway in vivo. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 304, 646–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, S.; Fujita, M.; Ishihara, M.; Tachibana, S.; Yamamoto, Y.; Kaji, T.; Kawauchi, T.; Kanatani, Y. Protective effect of inhalation of hydrogen gas on radiation-induced dermatitis and skin injury in rats. J. Radiat. Res. 2014, 55, 1107–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christian, R.L.; Cassie, N.L.; Sarah, R.; Marti, G.D.; Cheryl, B.; Mee, M.S. Effects of hyperbaric oxygen therapy on uncomplicated incisional and open wound healing in dogs. Vet. Surg. 2018, 47, 827–836. [Google Scholar]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant byselectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.H.; Nie, Q.R.; Dong, L.P.; Zhang, J.H.; Song, D.M. Hydrogen attenuates allergic inflammation by reversing energy metabolic pathway switch. Sci. Rep. 2020, 10, 1962–1974. [Google Scholar] [CrossRef]
- Masanori, K.; Michio, H.; Yoko, T.; Osamu, S. Hydrogen-rich water inhibits glucose and α, β-dicarbonyl compound-induced reactive oxygen species production in the SHR.Cg-Leprcp/NDmcr rat kidney. Med. Gas Res. 2012, 2, 18–24. [Google Scholar]
- Shin, E.M.; Zhou, H.Y.; Guo, L.Y.; Kim, J.A.; Lee, S.H.; Merfort, I.; Kang, S.S.; Kim, H.S.; Kim, S.; Kim, Y.S. Anti-inflammatory effects of glycyrol isolated from Glycyrrhiza uralensis in LPS-stimulated RAW264.7 macrophages. Int. Immunopharmacol. 2008, 8, 1524–1532. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, J.M.; Cai, J.M.; Zhu, Z.; Sun, X.J.; Jiang, C.L. Anti-inflammation effects of hydrogen saline in LPS activated macrophages and carrageenan induced paw oedema. BioMed Cent. 2012, 9, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Botek, M.; Krejčí, J.; McKune, A.J.; Sládečková, B. Hydrogen-rich water supplementation and Up-Hill running performance: Effect of athlete performance level. Int. J. Sports Physiol. Perform. 2020, 10, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Song, D.D.; Liu, X.L.; Diao, Y.G.; Sun, Y.J.; Gao, G.J.; Zhang, T.Z.; Chen, K.Y.; Pei, L. Hydrogen-rich solution against myocardial injury and aquaporin expression via the PI3K/Akt signaling pathway during cardiopulmonary bypass in rats. Mol. Med. Rep. 2018, 18, 1925–1938. [Google Scholar] [CrossRef] [Green Version]
- Madigan, M.C.; McEnaney, R.M.; Shukla, A.J.; Hong, G.; Kelley, E.E.; Tarpey, M.M.; Gladwin, M.; Zuckerbraun, B.S.; Tzeng, E. Xanthine oxidoreductase function contributes to normal wound healing. Mol. Med. 2015, 21, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Naofumi, T.; Cristina, O.R.; Makoto, F.; Hiro, O.I. Hydrogen-rich water intake accelerates oral palatal wound healing via activation of the Nrf2/antioxidant defense pathways in a rat model. Oxid. Med. Cell Longev. 2016, 2016, 5679040. [Google Scholar]
- Ping, Z.; Bing, L.; Peng, W.; Tao, P.; Shun, W.; Wei, S.C.; Shao, W.C.; Sha, L. The healing effect of hydrogen-rich water on acute radiation-induced skin injury in rats. J. Radiat. Res. 2019, 60, 17–22. [Google Scholar]
- Jayachandran, M.; Rodriguez, S.; Solis, E.; Lei, J.L.; Godavarty, A. Critical review of noninvasive optical technologies for wound imaging. Adv. Wound Care. 2016, 5, 349–359. [Google Scholar] [CrossRef] [Green Version]
- Ashcroft, G.S.; Greenwell, W.T.; Horan, M.A.; Wahl, S.M.; Ferguson, M.J. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. AM J. Pathol. 1999, 155, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Balgobin, S.; Montoya, T.I.; Shi, H.L.; Acevedo, J.F.; Keller, P.W.; Riegel, M.; Wai, C.Y.; Word, R.A. Estrogen alters remodeling of the vaginal wall after surgical injury in guinea pigs. Biol. Reprod. 2013, 89, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Horng, H.C.; Chang, W.H.; Yeh, C.C.; Huang, B.S.; Chang, C.P.; Chen, Y.J.; Tsui, K.H.; Wang, P.H. Estrogen effects on wound healing. Int. J. Mol. Sci. 2017, 18, 2325–2338. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Jiao, J.; Yan, H. Hydrogen-rich saline attenuates steroid-associated femoral head necrosis through inhibition of oxidative stress in a rabbit model. Exp. Ther. Med. 2016, 11, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soundia, A.; Hadaya, D.; Esfandi, N.; Gkouveris, I.; Christensen, R.; Dry, S.M.; Bezouglaia, O.; Pirih, F.; Nikitakis, N.; Aghaloo, T.; et al. Zoledronate impairs socket healing after extraction of teeth with experimental periodontitis. J. Dent. Res. 2018, 97, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Park, U.; Kim, K. Multiple growth factor delivery for skin tissue engineering application. Biotechnol. Bioprocess Eng. 2017, 22, 659–670. [Google Scholar] [CrossRef]
- Clark, R.A. Biology of dermal wound repair. Dermatol. Clin. 1993, 11, 647–666. [Google Scholar] [CrossRef]
- Blache, U.; Ehrbar, M. Ins pired by nature: Hydrogels as versatile tools for vascular engineering. Adv. Wound Care 2018, 7, 232–246. [Google Scholar] [CrossRef]
- Mazzon, E.; Esposito, E.; Paola, R.D.; Muià, C.; Crisafulli, C.; Genovese, T.; Caminiti, R.; Meli, R.; Bramanti, P.; Cuzzocrea, S. Effect of tumour necrosis factor-α receptor 1 genetic deletion on carrageenan-induced acute inflammation: A comparison with etanercept. Clin. Exp. Immunol. 2008, 153, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P.; Lecouter, J. The biology of VEGF and its receptors. J. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzicannella, J.; Gugliandolo, A.; Orsini, T.; Fontana, A.; Trubiani, O. Engineered extracellular vesicles from human periodontal-ligament stem cells increase VEGF/VEGFR2 expression during bone regeneration image_1.TIF image_2.TIF. Front. Physiol. 2019, 10, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.J.; Krieg, S.; Kuo, C.J.; Wiegand, S.J.; Rabinovitch, M.; Druzin, M.L.; Brenner, R.M.; Giudice, L.C.; Nayak, N.R. VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. FASEB J. 2008, 22, 3571–3580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, T.; Lei, P.X.; Zhou, H.; Liang, R.P.; Sun, Y.L. Sigmareceptor protects against ferroptosis in hepatocellular carcinoma cells. Cell Mol. Med. 2019, 23, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.H.; Si, Z.H.; Li, X.L.; Lu, L.; Pan, Y.L.; Liu, J.Z. FK506 Attenuated pilocarpine-induced epilepsy by reducing inflammation in rats. Front. Neurol. 2019, 10, 971–979. [Google Scholar] [CrossRef]
- Xiao, W.G.; Jia, Z.H.; Zhang, Q.Y.; Wei, C.; Wang, H.T.; Wu, Y.L. Inflammation and oxidative stress, rather than hypoxia, are predominant factors promoting angiogenesis in the initial phases of atherosclerosis. Mol. Med. Rep. 2015, 12, 3315–3322. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.S.; Chen, H.P.; Yu, H.H.; Yan, Y.F.; Liao, Z.P.; Huang, Q.R. Nrf2-dependent upregulation of antioxidative enzymes: A novel pathway for hypoxic preconditioning-mediated delayed cardioprotection. Mol. Cell Biochem. 2014, 385, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Singh, R.L.; Raghubir, R. Antioxidant status during cutaneous wound healing in immunocompromised rats. Recent Res. Dev. Mol. Cell Biochem. 2002, 241, 1–7. [Google Scholar]
- Imene, A.; Sana, B.; Massara, M.; Sahnoun, Z.; Rebaii, T.; Attia, H.; Ennouri, M. Antioxidant, antibacterial and in vivo dermal wound healing effects of Opuntia flower extracts. Int. J. Biol. Macromol. 2015, 81, 483–490. [Google Scholar]
- Li, X.T.; Zhang, Y.K.; Kuang, H.X.; Jin, F.X.; Liu, D.W.; Gao, M.B.; Liu, Z.; Xin, X.J. Mitochondrial protection and anti-aging activity of Astragalus polysaccharides and their potential mechanism. Int. J. Mol. Sci. 2012, 13, 1747–1761. [Google Scholar] [CrossRef] [Green Version]
- Dhall, S.; Hoffman, T.; Sathyamoorthy, M.; Lerch, A.; Danilkovitch, A. A viable lyopreserved amniotic membrane modulates diabetic wound microenvironment and accelerates wound closure. Adv. Wound Care. 2019, 8, 355–367. [Google Scholar] [CrossRef] [Green Version]
- Eisner, M.D.; Thompson, T.; Hudson, L.D.; Luce, J.M.; Matthay, M.A. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2001, 164, 231–236. [Google Scholar] [CrossRef]


| Gene | Gene Sequence Numbers | Primer Sequence (5’ to 3’) | Size (bp) |
|---|---|---|---|
| GAPDH | NM_008084.2 | Forward: TCATGAGGCCCTCCACGAT Reverse: GATGGGCGTGAACCATGAG | 157 |
| NRF-2 | NM_009925.4 | Forward: CCCATCGGAAACCAGTGCAT Reverse: CATCTACGAACGGGAATGTCTCTG | 150 |
| HO-1 | GI: 678541 | Forward: GCCAAGACTGCCTTCCTGCTG Reverse: ACCCGTTGTCGTAGCCCTGAG | 113 |
| NQO1 | NM_008607.2 | Forward: ACCTGTACGCCATGAACTTCAACC Reverse: CTTCTGCTCGGCCACGATGTC | 149 |
| VEGF | NM_008607.2 | Forward: GCCTTGCCTTGCTGCTCTACC Reverse: CTTCGTGGGGTTTGTGCTCTCC | 84 |
| PDGF | NM_008607.2 | Forward: CCTGGCGTGCAAGTGTGAGAC Reverse: CCGAATGGTCACCCGAGTTTGG | 112 |
| Time | DW | HRW |
|---|---|---|
| 3 d | 1.59 ± 5.04 | 17.57 ± 0.55 * |
| 7 d | 16.44 ± 6.43 | 46.82 ± 4.29 ** |
| 14 d | 82.03 ± 3.09 | 92.07 ± 2.58 |
| 21 d | 98.57 ± 2.45 | 100.00 |
| Group | DW | HRW |
|---|---|---|
| Healing time | 21.53 ± 1.68 | 19.25 ± 0.43 * |
| Time | DW | HRW |
|---|---|---|
| 7 d | 8.60 ± 2.97 | 17.60 ± 1.14 ** |
| 14 d | 5.20 ± 1.31 | 4.20 ± 1.41 |
| 21 d | 3.20 ± 0.81 * | 1.20 ± 0.84 * |
| 28 d | 0 | 0 |
| Time | DW | HRW |
|---|---|---|
| 7 d | 31.05 ± 4.51 ** | 17.68 ± 2.48 |
| 14 d | 41.40 ± 2.74 ** | 17.74 ± 2.82 |
| 21 d | 5.20 ± 3.49 | 1.03 ± 0.71 |
| 28 d | 1.78 ± 1.21 | 2.27 ± 0.84 |
| Time | DW | HRW |
|---|---|---|
| 7 d | 31.05 ± 4.51 ** | 17.68 ± 2.48 |
| 14 d | 41.40 ± 2.74 ** | 17.74 ± 2.82 |
| 21 d | 5.20 ± 3.49 | 1.03 ± 0.71 |
| 28 d | 1.78 ± 1.21 | 2.27 ± 0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, D.-D.; Ding, M.-Y.; Wang, T.; Hayat, M.A.; Liu, T.; Zhang, J.-T. The Therapeutic Effects of Oral Intake of Hydrogen Rich Water on Cutaneous Wound Healing in Dogs. Vet. Sci. 2021, 8, 264. https://doi.org/10.3390/vetsci8110264
Qi D-D, Ding M-Y, Wang T, Hayat MA, Liu T, Zhang J-T. The Therapeutic Effects of Oral Intake of Hydrogen Rich Water on Cutaneous Wound Healing in Dogs. Veterinary Sciences. 2021; 8(11):264. https://doi.org/10.3390/vetsci8110264
Chicago/Turabian StyleQi, Dong-Dong, Meng-Yuan Ding, Ting Wang, Muhammad Abid Hayat, Tao Liu, and Jian-Tao Zhang. 2021. "The Therapeutic Effects of Oral Intake of Hydrogen Rich Water on Cutaneous Wound Healing in Dogs" Veterinary Sciences 8, no. 11: 264. https://doi.org/10.3390/vetsci8110264
APA StyleQi, D.-D., Ding, M.-Y., Wang, T., Hayat, M. A., Liu, T., & Zhang, J.-T. (2021). The Therapeutic Effects of Oral Intake of Hydrogen Rich Water on Cutaneous Wound Healing in Dogs. Veterinary Sciences, 8(11), 264. https://doi.org/10.3390/vetsci8110264






