Colistin Resistant mcr Genes Prevalence in Livestock Animals (Swine, Bovine, Poultry) from a Multinational Perspective. A Systematic Review
Abstract
1. Introduction
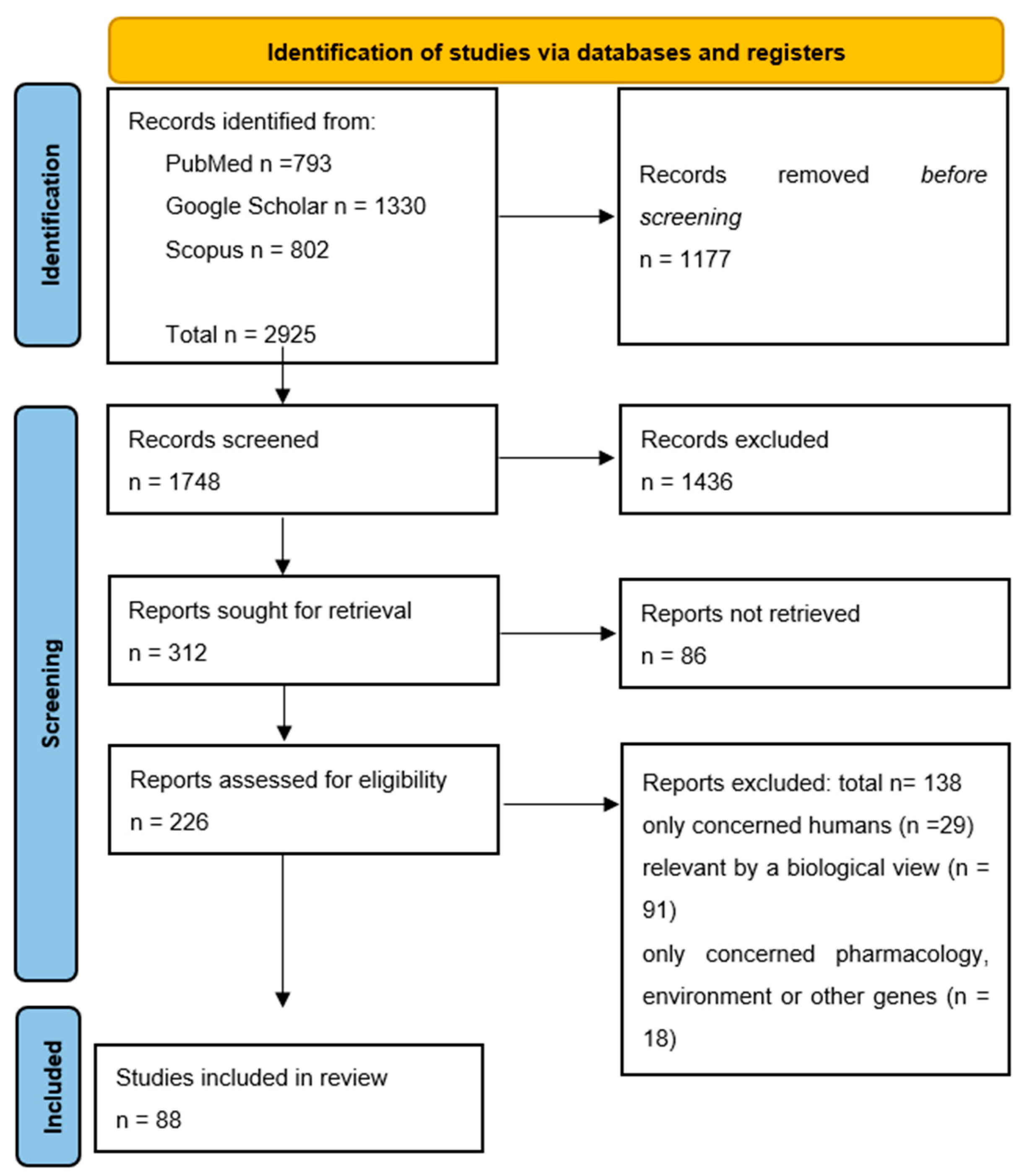
2. Materials and Methods
3. Results
3.1. Swine
3.1.1. China
3.1.2. Thailand
3.1.3. Spain
3.1.4. Germany
3.1.5. Japan
3.1.6. Great Britain
3.1.7. Portugal
3.1.8. Vietnam
3.1.9. France
3.1.10. Italy
3.1.11. Belgium
3.1.12. Brazil
3.1.13. Canada
3.1.14. South Korea
3.1.15. Switzerland
3.1.16. European Studies
3.2. Cattle
3.2.1. The Netherlands
3.2.2. Belgium
3.2.3. China
3.2.4. France
3.2.5. Portugal
3.2.6. Vietnam
3.2.7. Brazil
3.2.8. Spain
3.2.9. S. Korea
3.2.10. Italy
3.2.11. Greece
3.2.12. Europe
3.3. Poultry
3.3.1. China
3.3.2. Belgium
3.3.3. The Netherlands
3.3.4. Brazil
3.3.5. Vietnam
3.3.6. Portugal
3.3.7. Romania
3.3.8. Bangladesh
3.3.9. Iraq
3.3.10. South Korea
3.3.11. Tunisia
3.3.12. Pakistan
3.3.13. Nigeria
3.3.14. Turkey
3.3.15. Paraguay
3.3.16. Lebanon
3.3.17. Algeria
3.3.18. Europe
| Country | Bacteria | mcr Genes Were Searched | Number of Isolated Samples | Colistin Resistant Samples | mcr Number | mcr | % mcr Prevalence | Year | Citation | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Japan | E. coli | mcr-1 to mcr-8 | 90 pre-ban, 511 after-ban | 23 pre-ban, 19 after-ban | 23 pre-ban, 19 after-ban | mcr-1 | 25% pre-ban, 4% after-ban | 2021 | [94] |
| 2 | Belgium | E. coli | mcr-1, mcr-2, mcr-3, mcr-4, mcr-5, mcr-6, mcr-7, mcr-8, mcr-9, mcr-10 | 40 | 40 | 3 | mcr-1 | 9.6 | 2021 | [107] |
| 3 | Switzerland | E. coli | mcr-1, mcr-2, mcr-3, mcr-4, mcr-5, mcr-6, mcr-7, mcr-8, mcr-9 | 81 | 3 | mcr-1 to mcr-9 | 0 | 2021 | [112] | |
| 4 | Thailand | Salmonella | mcr-1, mcr-2, mcr-3 | 300 | 4 | 5 | mcr-3 | 1.3 | 2021 | [83] |
| 5 | Thailand, Cambodia, Lao, Myanmor | E. coli Salmonella | mcr-1, mcr-2, mcr-3, mcr-4, mcr-5, mcr-6, mcr-7, mcr-8, mcr-9, mcr-10 | 809 | 80 | 68 | mcr-1 | 62.5 | 2021 | [82] |
| 31 | Mcr-2 | 6.25 | ||||||||
| 6 | Spain | E. coli | mcr-1, mcr-2, mcr-3, mcr-4, mcr-5 | 70 | 15 | 14 | mcr-1 | 20 | 2020 | [90] |
| 1 | mcr-4 | 1.42 | ||||||||
| 7 | China | E. coli | 115 | 10 | 10 | mcr-1 | 8.70 | 2019 | [78] | |
| 8 | China | E. coli, Klebsiella pneumoniae, Kluyvera ascorbata, Enterobacter cloacae | mcr-1, mcr-2, mcr-3, mcr-4, mcr-5, mcr-6, mcr-7, mcr-8 | 65 | 33 | 28 | mcr-1 | 43.07 | 2019 | [80] |
| 4 | mcr-1 +mcr-3 | 6.15 | ||||||||
| 1 | mcr-3 | 1.54 | ||||||||
| 9 | China | E. coli | mcr-1, mcr-2 | 811 | 440 | 303 | mcr-1 | 37.36 | 2019 | [79] |
| 88 | mcr-1+ mcr-2 | 10.85 | ||||||||
| 206 | mcr-2 | 25.40 | ||||||||
| 10 | China | E. coli | mcr-1 | 600 | 457 | 152 | mcr-1 | 25.33 | 2018 | [72] |
| 11 | China | Enterobacteriaceae, Aeromonas hydrophila, Ε. Coli, A. Veronii, A. Caviae | mcr-1, mcr-2, mcr-3, mcr-4, mcr-5 | 336 | 8 | 1 | mcr-5 | 0.30 | 2018 | [77] |
| 12 | China | E. coli | mcr-4, mcr-5 | 1552 | 1454 | 621 | mcr-4 | 40.01 | 2018 | [76] |
| 478 | mcr-5 | 30.80 | ||||||||
| 266 | mcr-4 +mcr-5 | 17.14 | ||||||||
| 13 | China | Enterobacteriaceae, Moraxella spp., Aeromonas Veronii | mcr-1, mcr-2, mcr-3 | 4895 | 1454 | 1152 | mcr-1 | 23.53 | 2018 | [73] |
| 818 | mcr-2 | 16.71 | ||||||||
| 272 | mcr-3 | 5.55 | ||||||||
| 14 | China | E. coli | mcr-1, mcr-2, mcr-3, mcr-4, mcr-5 | 417 | 71 | mcr-1 | 17.02 | 2018 | [75] | |
| 5 | mcr-3 | 1.20 | ||||||||
| 15 | China | E. coli | mcr-1 | 204 | 81 | 78 | mcr-1 | 38.23 | 2018 | [71] |
| 16 | Japan | E. coli | mcr-1, mcr-2, mcr-3, mcr-4, mcr-5 | 676 | 120 | 36 | mcr-1 | 5.32 | 2018 | [96] |
| 10 | mcr-3 | 1.15 | ||||||||
| 34 | mcr-5 | 5.02 | ||||||||
| 17 | Great Britain | Moraxella spp., E. coli | mcr-1, mcr-2 | 657 | 0 | mcr-1 | 0 | 2017 | [32] | |
| 18 | Italy, Spain, Belgium | Salmonella enterica serovar, Typhimurium, Salmonella enterica, E. coli | mcr-1, mcr-2 mcr-3 | 125 | 50 | 32 | mcr-1 | 25.6 | 2017 | [40] |
| 3 | mcr-2 | 2.40 | ||||||||
| 11 | mcr-4 | 8.80 | ||||||||
| 19 | Germany | E. coli | mcr-1 | 216 | 26 | 12 | mcr-1 | 5.55 | 2018 | [93] |
| 20 | China | E. coli | mcr-1 | 1026 | 302 | 302 | mcr-1 | 30 | 2016 | [69] |
| 21 | Germany | Enterobacteriaceae | mcr-1, mcr-2 | 436 | 43 | 15 | mcr-1 | 3.44 | 2017 | [92] |
| 22 | China | Salmonella spp. | mcr-1 | 279 | 20 | 7 | mcr-1 | 2.50 | 2017 | [70] |
| 23 | Great Britain | E. coli | mcr-1 | 556 | 163 | 8 | mcr-1 | 1.44 | 2017 | [97] |
| 24 | China | E. coli | mcr-1 | 93 | 10 | 2 | mcr-1 | 2.15 | 2016 | [67] |
| 25 | China | E. coli | mcr-1 | 16 | 6 | mcr-1 | 37.5 | 2016 | [68] | |
| 26 | Vietnam | E. coli | mcr-2 | 250 | 180 | 17 | mcr-1 | 6.80 | 2016 | [101] |
| 27 | Belgium | E. coli | mcr-1 | 105 | 52 | 7 | mcr-1 | 6.67 | 2016 | [108] |
| 28 | Germany | E. coli | mcr-1 | 557 | 4 | 3 | 3mcr-1 | 2016 | [91] | |
| 29 | South Korea | E. coli | mcr-1, mcr-2, mcr-3, mcr4, mcr-5, mcr-6, mcr-7, mcr-8 | 59 | 4 | 4 | mcr-1 | 6.78 | 2020 | [111] |
| 30 | Canada | Salmonella spp. E. coli | mcr-1 | 10 | 6 | 1 | mcr-1 | 0.10 | 2019 | [110] |
| 31 | Portugal | E. coli, Salmonella | mcr-1 | 398 | 42 | 40 | mcr-1 | 10.05 | 2019 | [47] |
| 32 | Spain | Citrobacter freundii, E. coli, Klebsiella pneumoniae, Elisabethkingia spp. | mcr-1, mcr-2, mcr-3, mcr-4 | 76 | 72 | 63 | mcr-1 | 82.90 | 2019 | [87] |
| 3 | mcr-4 | 3.95 | ||||||||
| 33 | France | E. coli | mcr-1 | 339 | 86 | 19 | mcr-1 | 5.60 | 2019 | [105] |
| 34 | Italy | E. coli, Salmonella | mcr-1, mcr2, mcr-3, mcr-4, mcr-5 | 304 | 14 | 13 | mcr-1 | 0.33 | 2018 | [106] |
| 35 | Vietnam | E. coli | mcr-1, mcr-2, mcr-3, mcr-4, mcr-5 | 261 | 62 | 60 | mcr-1 | 97 | 2018 | [104] |
| 2 | mcr-3 | 0.77 | ||||||||
| 36 | China | E. coli | mcr-1 | 15193 | 1416 | 274 | mcr-1 | 1.80 | 2020 | [81] |
| 37 | Japan | E. coli | mcr-1 | 684 | 309 | 90 | mcr-1 | 13.16 | 2016 | [95] |
| 38 | Brazil | Enterobacteriaceae | mcr-1 | 113 | 79 | 2 | mcr-1 | 1.77 | 2016 | [109] |
| 39 | Europe | E. coli, Salmonella spp. | mcr-1, mcr-2 | 3510 | 78 | 25 | mcr-1 | 0.81 | 2018 | [113] |
| 40 | China | E. coli | mcr-3 | 6497 | 49 | 4 | mcr-3 | 0.06 | 2018 | [74] |
| 41 | Portugal | E. coli Klebsiella pneumoniae Enterobacter Aerogenes Enterobacteriaceae | mcr-1 | 93 | 62 | 12 | mcr-1 | 12.90 | 2020 | [100] |
| 42 | Spain | E. coli | mcr-1, mcr-2, mcr-3, mcr-4, mcr-5 | 200 | 43 | 14 | mcr-1 | 7 | 2020 | [88] |
| 26 | mcr-4 | 13 | ||||||||
| 6 | mcr-5 | 3 |
| Country | Bacteria | mcr Genes Were Searched | Number of Isolated Samples | Colistin Resistant Samples | mcr Number | mcr | % mcr Prevalence | Year | Authors | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Belgium | E. coli | mcr-1, mcr-2, mcr-3, mcr-4, mcr-5, mcr-6, mcr-7, mcr-8, mcr-9, mcr-10 | 40 | 40 | 27 | mcr-1 | 87.1 | 2021 | [107] |
| 2 | China | E. coli | mcr-1, mcr-2 | 156 | 42 | 30 | mcr-1 | 71.43 | 2019 | [79] |
| 8 | mcr-2 | 19.05 | ||||||||
| 4 | mcr-1 + mcr-2 | 9.52 | ||||||||
| 3 | Europe | E. coli, Salmonella spp. | mcr-1, mcr-2 | 2553 | 32 | 0 | mcr-1 | 0 | 2018 | [113] |
| 4 | Belgium | E. coli | mcr-1 | 105 | 52 | 13 | mcr-1 | 12.38 | 2016 | [108] |
| 5 | S. Korea | E. coli | mcr-1, mcr-2, mcr-3, mcr-4, mcr-5, mcr-6, mcr-7, mcr-8, mcr-9 | 57 | 0 | mcr-1 | mcr-1 | 0 | 2020 | [111] |
| 6 | Greece | E. coli | 400 | 89 | 6 | mcr-1 | 1.50 | 2020 | [118] | |
| 7 | France | E. coli | mcr-1 | 14 | 9 | 8 | mcr-1 | 57.14 | 2019 | [117] |
| 8 | Portugal | Salmonella, E. coli | mcr-1 | 350 | 0 | 0 | 0 | 2019 | [47] | |
| 9 | Belgium | E. coli | mcr-1, mcr-2, mcr-3, mcr-4, mcr-5 | 94 | 45 | 9 | mcr-1 | 9.57 | 2019 | [115] |
| 10 | China | E. coli | mcr-1, mcr-2, mcr-3 | 120 | 7 | mcr-2 | 7.45 | 2018 | [67] | |
| 1 | mcr-1 | 0.83 | ||||||||
| 11 | Italy | E. coli | mcr-1, mcr-2, mcr-3, mcr-4, mcr-5 | 678 | 8 | 5 | mcr-1 | 2.24 | 2018 | [106] |
| 12 | Vietnam | E. coli | mcr-1, mcr-2, mcr-3, mcr-4, mcr-5 | 29 | 0 | 1 | mcr-4 | 0.45 | 2018 | [104] |
| 0 | mcr-1 | 0 | ||||||||
| 13 | Netherlands | E. coli | 15 | 15 | 11 | mcr-1 | 73.33 | 2016 | [114] | |
| 14 | France | E. coli | mcr-1 | 517 | 106 | 75 | mcr-1 | 14.50 | 2016 | [116] |
| 15 | Brazil | Enterobacteriaceae | mcr-1 | 158 | 22 | 0 | mcr-1 | 0 | 2016 | [109] |
| 16 | Spain | E. coli | mcr-1, mcr-2, mcr-3 | 152 | 6 | 5 1 | mcr-1 mcr-3 | 3.29 0.66 | 2017 | [53] |
| Country | Bacteria | mcr Genes Were Searched | Number of Isolates | Colistin Resistant Isolates | mcr Number | mcr | % mcr Prevalence | Year | Authors | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bangladesh | E. coli | mcr-1 | 40 | 24 | 8 | mcr-1 | 33.3 | 2021 | [124] |
| 1 | mcr-2 | 4.16 | ||||||||
| 4 | mcr-3 | 16.6 | ||||||||
| 2 | Bangladesh | E. coli | mcr-1 | 159 | 59 | 2 | mcr-1 | 34 | 2021 | [125] |
| 3 | Algeria | E. coli Klebsiella | mcr-1 | 181 | 17 | 11 | mcr-1 | 64.7 | 2021 | [136] |
| pneumoniae | mcr-1 | 5 | 0 | |||||||
| 4 | Bangladesh | Salmonella | mcr-1 | 82 | 10 | 5 | mcr-1 | 50 | 2021 | [126] |
| 5 | Pakistan | E. coli | mcr-1 | 100 | 59 | 5 | mcr-1 | 15 | 2021 | [130] |
| 6 | Libanon | E. coli | mcr-1 | 84 | 32 | 27 | mcr-1 | 84.3 | 2021 | [135] |
| 7 | Libanon | E. coli | mcr-1 | 93 | 19 | 9 | mcr-1 | 47.4 | 2021 | [134] |
| 8 | China | Enterobacteriae | mcr-1, mcr-2, mcr-3, mcr-4, mcr-5, mcr-6, mcr-7, mcr-8, mcr-9, mcr-10 | 910 (gut microbiomes) | 293 | mcr-1 | 32.2 | 2021 | [120] | |
| 14 | mcr-10 | 1.5 | ||||||||
| 9 | China | E. coli | mcr-1 | 72 | 3 | 3 | mcr-1 | 100 | 2021 | [119] |
| 10 | Paraguay | E. coli | mcr-5 | 62 | 29 | 6 | mcr-1 | 20.6 | 2021 | [133] |
| 11 | Belgium | E. coli | mcr-1 to mcr-10 | 40 | 40 | 1 | mcr-1 | 100 | 2021 | [107] |
| 12 | Turkey | E. coli | mcr-1 | 200 | 15 | 0 | mcr-1 | 0 | 2021 | [132] |
| 13 | Nigeria | E. coli | mcr-1, mcr-2, mcr-3, mcr-4, mcr-5, mcr-6, mcr-7, mcr-8, mcr-9, mcr-10 | 785 | 45 | 23 | mcr-1 | 62.5 | 2021 | [131] |
| mcr-1.22 | 6.25 | |||||||||
| 14 | China | E. coli | mcr-1, mcr-2 | 1232 | 443 | 388 | mcr-1 | 31.50 | 2019 | [79] |
| 66 | mcr-2 | 5.36 | ||||||||
| 32 | mcr-1 +mcr-2 | 2.60 | ||||||||
| 15 | Netherlands | E. coli, Salmonella | mcr-1 | 10 | 5 | 2 | mcr-1 | 20 | 2016 | [114] |
| 16 | Brazil | Enterobacteriaceae | mcr-1 | 280 | 113 | 14 | mcr-1 | 12.40 | 2016 | [109] |
| 17 | Netherlands | E. coli | mcr-1-mcr2 | 214 | 53 | 34 | mcr-1 | 15.89 | 2017 | [121] |
| 18 | Brazil | E. coli | mcr-1 | 41 | 8 | 5 | mcr-1 | 12.19 | 2017 | [122] |
| 19 | China | E. colli | mcr-4, mcr5 | 1836 | 1498 | 257 | mcr-4 | 14 | 2018 | [76] |
| 20 | China | E. coli | mcr-1, mcr-2, mcr-3 | 1836 | 1498 | 477 | mcr-1 | 25.98 | 2018 | [73] |
| 82 | mcr-2 | 4.47 | ||||||||
| 78 | mcr-3 | 4.24 | ||||||||
| 21 | Europe | E. coli, Salmonella spp. | mcr-1, mcr-2 | 2973 | 114 | 40 | mcr-1 | 1.80 | 2018 | [113] |
| 22 | China | E. coli, Salmonella spp. | mcr-1, mcr-3 | 450 | 17 | 2 | mcr-1 | 0.44 | 2017 | [70] |
| 23 | Vietnam | E. coli | mcr-2 | 180 | 20 | 1 | mcr-1 | 7.78 | 2016 | [101] |
| 24 | Iraq | A. Baumannii | mcr-1 | 424 | 80 | 2 | mcr-1 | 0.47 | 2020 | [128] |
| 25 | S. Korea | Enterobacteriaceae | mcr-1, mcr-2, mcr-3, mcr-4, mcr-5, mcr-6, mcr-7, mcr-8 | 34 | 2 | 2 | mcr-1 | 5.88 | 2020 | [111] |
| 26 | Romania | E. coli | mcr-1, mcr-2 | 92 | 11 | 17 | mcr-1 | 18.47 | 2019 | [123] |
| 27 | Bangladesh | E. coli | mcr-1 | 60 | 37 | 18 | mcr-1 | 0.30 | 2019 | [127] |
| 28 | Portugal | E. coli, Salmonella spp. | mcr-1 | 202 | 6 | 4 | mcr-1 | 1.98 | 2019 | [47] |
| 29 | Vietnam | E. coli | mcr-1, mcr-2, mcr-3, mcr-4, mcr-5 | 144 | 143 | 56 | mcr-1 | 39.16 | 2018 | [104] |
| 30 | Tunisia | E. coli | mcr-1 | 50 | 12 | 7 | mcr-1 | 0.14 | 2020 | [129] |
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poirel, L.; Jayol, A.; Nordmann, P. Polymyxins: Antibacterial Activity, Susceptibility Testing, and Resistance Mechanisms Encoded by Plasmids or Chromosomes. Clin. Microbiol. Rev. 2017, 30, 557–596. [Google Scholar] [CrossRef]
- Dijkmans, A.C.; Wilms, E.B.; Kamerling, I.M.; Birkhoff, W.; Ortiz-Zacarias, N.V.; van Nieuwkoop, C.; Verbrugh, H.A.; Touw, D.J. Colistin: Revival of an Old Polymyxin Antibiotic. Ther. Drug Monit. 2015, 37, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Son, S.J.; Huang, R.; Squire, C.J.; Leung, I.K.H. Mcr-1: A Promising Target for Structure-Based Design of Inhibitors to Tackle Polymyxin Resistance. Drug Discov. Today 2016, 24, 206–216. [Google Scholar] [CrossRef]
- Hémonic, A.; Chauvin, C.; Corrégé, I. Utilisations d’antibiotiques en élevage de porcs: Motifs et stratégies thérapeutiques associées. Journ. Rech. Porc. 2014, 46, 135–140. [Google Scholar]
- Rhouma, M.; Beaudry, F.; Letellier, A. Resistance to Colistin: What Is the Fate for This Antibiotic in Pig Production? Int. J. Antimicrob. Agents 2016, 48, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Kasiakou, S.K. Colistin: The Revival of Polymyxins for the Management of Multidrug-Resistant Gram-Negative Bacterial Infections. Clin. Infect. Dis. 2005, 40, 1333–1341. [Google Scholar] [CrossRef]
- Srinivas, P.; Rivard, K. Polymyxin Resistance in Gram-Negative Pathogens. Curr. Infect. Dis. Rep. 2017, 19, 38. [Google Scholar] [CrossRef]
- Jeannot, K.; Bolard, A.; Plesiat, P. Resistance to Polymyxins in Gram-Negative Organisms. Int. J. Antimicrob. Agents 2017, 49, 526–535. [Google Scholar] [CrossRef]
- John, E.B.; Bennett, R.D.; Blaser, M.J. Polymyxins (polymyxin B and colistin). In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier/Saunders: Philadelphia, PA, USA, 2015; pp. 549–555. [Google Scholar]
- Azzopardi, E.A.; Boyce, D.E.; Thomas, D.W.; Dickson, W.A. Colistin in Burn Intensive Care: Back to the Future? Burns 2013, 39, 7–15. [Google Scholar] [CrossRef]
- Velkov, T.; Philip, E.T.; Roger, L.N.; Li, J. Structure--Activity Relationships of Polymyxin Antibiotics. J. Med. Chem. 2010, 53, 1898–1916. [Google Scholar] [CrossRef]
- Biswas, S.; Brunel, J.M.; Dubus, J.C.; Reynaud-Gaubert, M.; Rolain, J.M. Colistin: An Update on the Antibiotic of the 21st Century. Expert Rev. Anti. Infect. Ther. 2012, 10, 917–934. [Google Scholar] [CrossRef]
- Falagas, M.E.; Rafailidis, P.I. Re-Emergence of Colistin in Today’s World of Multidrug-Resistant Organisms: Personal Perspectives. Expert Opin. Investig. Drugs 2008, 17, 973–981. [Google Scholar] [CrossRef]
- Lim, L.M.; Ly, N.; Anderson, D.; Yang, J.C.; Macander, L.; Jarkowski, A., 3rd; Forrest, A.; Bulitta, J.B.; Tsuji, B.T. Resurgence of Colistin: A Review of Resistance, Toxicity, Pharmacodynamics, and Dosing. Pharmacotherapy 2010, 30, 1279–1291. [Google Scholar] [CrossRef]
- Bialvaei, A.Z.; Kafil, H.S. Colistin, Mechanisms and Prevalence of Resistance. Curr. Med. Res. Opin. 2015, 31, 707–721. [Google Scholar] [CrossRef]
- Koch-Weser, J.; Sidel, V.W.; Federman, E.B.; Kanarek, P.; Finer, D.C.; Eaton, A.E. Adverse Effects of Sodium Colistimethate. Manifestations and Specific Reaction Rates During 317 Courses of Therapy. Ann. Intern. Med. 1970, 72, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, H.; Liu, Y.H.; Feng, Y. Towards Understanding Mcr-Like Colistin Resistance. Trends Microbiol. 2018, 26, 794–808. [Google Scholar] [CrossRef]
- McClure, N.S.; Day, T. A Theoretical Examination of the Relative Importance of Evolution Management and Drug Development for Managing Resistance. Proc. Biol. Sci. 2014, 281, 20141861. [Google Scholar] [CrossRef] [PubMed]
- Kempf, I.; Jouy, E.; Chauvin, C. Colistin Use and Colistin Resistance in Bacteria from Animals. Int. J. Antimicrob. Agents 2016, 48, 598–606. [Google Scholar] [CrossRef]
- Kempf, I.; Fleury, M.A.; Drider, D.; Bruneau, M.; Sanders, P.; Chauvin, C.; Madec, J.Y.; Jouy, E. What Do We Know About Resistance to Colistin in Enterobacteriaceae in Avian and Pig Production in Europe? Int. J. Antimicrob. Agents 2013, 42, 379–383. [Google Scholar] [CrossRef]
- Katsunuma, Y.; Hanazumi, M.; Fujisaki, H.; Minato, H.; Hashimoto, Y.; Yonemochi, C. Associations between the Use of Antimicrobial Agents for Growth Promotion and the Occurrence of Antimicrobial-Resistant Escherichia coli and Enterococci in the Feces of Livestock and Livestock Farmers in Japan. J. Gen. Appl. Microbiol. 2007, 53, 273–279. [Google Scholar] [CrossRef]
- Catry, B.; Cavaleri, M.; Baptiste, K.; Grave, K.; Grein, K.; Holm, A.; Jukes, H.; Liebana, E.; Navas, A.L.; Mackay, D.; et al. Use of Colistin-Containing Products within the European Union and European Economic Area (Eu/Eea): Development of Resistance in Animals and Possible Impact on Human and Animal Health. Int J. Antimicrob. Agents 2015, 46, 297–306. [Google Scholar] [CrossRef]
- Hung, C.C.; Chen, C.Y.; Chen, B.J. Colistin and Tylosin Enhances Disaccharidase Activities, and Improves Morphology and Permeability of the Intestine of Broilers. Br. Poult. Sci. 2020, 61, 465–470. [Google Scholar] [CrossRef]
- Walsh, T.R.; Wu, Y. China Bans Colistin as a Feed Additive for Animals. Lancet Infect. Dis. 2016, 16, 1102–1103. [Google Scholar] [CrossRef]
- Looft, T.; Johnson, T.A.; Allen, H.K.; Bayles, D.O.; Alt, D.P.; Stedtfeld, R.D.; Sul, W.J.; Stedtfeld, T.M.; Chai, B.; Cole, J.R.; et al. In-Feed Antibiotic Effects on the Swine Intestinal Microbiome. Proc. Natl. Acad. Sci. USA 2012, 109, 1691–1696. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, Y.; Sun, Y.; Ma, L.; Zeng, Q.; Jiang, X.; Li, A.; Zeng, Z.; Zhang, T. Antibiotic-Mediated Changes in the Fecal Microbiome of Broiler Chickens Define the Incidence of Antibiotic Resistance Genes. Microbiome 2018, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Su, J.Q.; An, X.L.; Huang, F.Y.; Rensing, C.; Brandt, K.K.; Zhu, Y.G. Feed Additives Shift Gut Microbiota and Enrich Antibiotic Resistance in Swine Gut. Sci. Total Environ. 2018, 621, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of Plasmid-Mediated Colistin Resistance Mechanism mcr-1 in Animals and Human Beings in China: A Microbiological and Molecular Biological Study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- World Health Organization. Joint Fao/Oie/Who Expert Workshop on Non-Human Antimicrobial Usage and Antimicrobial Resistance: Scientific Assessment; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- American Veterinary Medical Association (AVMA). One Health: A New Professional Imperative. One Health Initiative Task Force: Final Report; American Veterinary Medical Association: Schaumburg, IL, USA, 2008. [Google Scholar]
- Michael, J. One Health: The Importance of Companion Animal Vector-Borne Diseases. Parasites Vectors 2011, 4, 49. [Google Scholar]
- AbuOun, M.; Stubberfield, E.J.; Duggett, N.A.; Kirchner, M.; Dormer, L.; Nunez-Garcia, J.; Randall, L.P.; Lemma, F.; Crook, D.W.; Teale, C.; et al. mcr-1 and mcr-2 Variant Genes Identified in Moraxella Species Isolated from Pigs in Great Britain from 2014 to 2015. J. Antimicrob. Chemother. 2017, 72, 2745–2749. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Zhou, Y.; Li, J.; Yin, W.; Wang, S. Emergence of a Novel Mobile Colistin Resistance Gene, mcr-8, in NDM-producing Klebsiella Pneumoniae. Emerg. Microbes Infect. 2018, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Li, Y.X.; Lei, C.W.; Zhang, A.Y.; Wang, H.N. Novel Plasmid-Mediated Colistin Resistance Gene mcr-7.1 in Klebsiella pneumoniae. J. Antimicrob. Chemother 2018, 73, 1791–1795. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.M.; Gaballa, A.; Guldimann, C.; Sullivan, G.; Henderson, L.O.; Wiedmann, M. Identification of Novel Mobilized Colistin Resistance Gene mcr-9 in a Multidrug-Resistant, Colistin-Susceptible Salmonella enterica Serotype Typhimurium Isolate. mBio 2019, 10, e00853-19. [Google Scholar] [CrossRef]
- Wang, C.; Feng, Y.; Liu, L.; Wei, L.; Kang, M.; Zong, Z. Identification of Novel Mobile Colistin Resistance Gene mcr-10. Emerg. Microbes Infect. 2020, 9, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Hussein, N.H.; Al-Kadmy, I.M.S.; Taha, B.M.; Hussein, J.D. Mobilized Colistin Resistance (mcr) Genes from 1 to 10: A Comprehensive Review. Mol. Biol. Rep. 2021, 48, 2897–2907. [Google Scholar] [CrossRef]
- Xavier, B.B.; Lammens, C.; Ruhal, R.; Kumar-Singh, S.; Butaye, P.; Goossens, H.; Malhotra-Kumar, S. Identification of a Novel Plasmid-Mediated Colistin-Resistance Gene, Mcr-2, in Escherichia Coli, Belgium, June 2016. Euro Surveill. 2016, 21, 30280. [Google Scholar] [CrossRef]
- Borowiak, M.; Fischer, J.; Hammerl, J.A.; Hendriksen, R.S.; Szabo, I.; Malorny, B. Identification of a Novel Transposon-Associated Phosphoethanolamine Transferase Gene, mcr-5, Conferring Colistin Resistance in D-Tartrate Fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J. Antimicrob. Chemother 2017, 72, 3317–3324. [Google Scholar] [CrossRef]
- Carattoli, A.; Villa, L.; Feudi, C.; Curcio, L.; Orsini, S.; Luppi, A.; Pezzotti, G.; Magistrali, C.F. Novel Plasmid-Mediated Colistin Resistance Mcr-4 Gene in Salmonella and Escherichia Coli, Italy 2013, Spain and Belgium, 2015 to 2016. Euro Surveill 2017, 22, 30589. [Google Scholar] [CrossRef]
- Yin, W.; Li, H.; Shen, Y.; Liu, Z.; Wang, S.; Shen, Z.; Zhang, R.; Walsh, T.R.; Shen, J.; Wang, Y. Novel Plasmid-Mediated Colistin Resistance Gene mcr-3 in Escherichia Coli. mBio 2017, 8, e00543-17. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, C.; Sun, Q.; Schwarz, S.; Ou, Y.; Yang, L.; Huang, Z.; Eichhorn, I.; Walsh, T.R.; Wang, Y.; et al. Prevalence and Genetic Analysis of mcr-3-Positive Aeromonas Species from Humans, Retail Meat, and Environmental Water Samples. Antimicrob. Agents Chemother 2018, 62, e00404-18. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Graells, C.; de Keersmaecker, S.C.J.; Vanneste, K.; Pochet, B.; Vermeersch, K.; Roosens, N.; Dierick, K.; Botteldoorn, N. Detection of Plasmid-Mediated Colistin Resistance, Mcr-1 and Mcr-2 Genes, in Salmonella Spp. Isolated from Food at Retail in Belgium from 2012 to 2015. Foodborne Pathog. Dis. 2018, 15, 114–117. [Google Scholar] [CrossRef]
- Quiroga, C.; Nastro, M.; di Conza, J. Current Scenario of Plasmid-Mediated Colistin Resistance in Latin America. Rev. Argent Microbiol. 2019, 51, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Feng, Y.; Lü, X.; McNally, A.; Zong, Z. Remarkable Diversity of Escherichia coli Carrying mcr-1 from Hospital Sewage with the Identification of Two New mcr-1 Variants. Front. Microbiol. 2017, 8, 2094. [Google Scholar] [CrossRef]
- Wang, R.; van Dorp, L.; Shaw, L.P.; Bradley, P.; Wang, Q.; Wang, X.; Jin, L.; Zhang, Q.; Liu, Y.; Rieux, A.; et al. The Global Distribution and Spread of the Mobilized Colistin Resistance Gene mcr-1. Nat. Commun. 2018, 9, 1179. [Google Scholar] [CrossRef] [PubMed]
- Clemente, L.; Manageiro, V.; Correia, I.; Amaro, A.; Albuquerque, T.; Themudo, P.; Ferreira, E.; Caniça, M. Revealing mcr-1-Positive Esbl-Producing Escherichia coli Strains among Enterobacteriaceae from Food-Producing Animals (Bovine, Swine and Poultry) and Meat (Bovine and Swine), Portugal, 2010–2015. Int. J. Food Microbiol. 2019, 296, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Tyson, G.H.; Li, C.; Hsu, C.H.; Ayers, S.; Borenstein, S.; Mukherjee, S.; Tran, T.T.; McDermott, P.F.; Zhao, S. The mcr-9 Gene of Salmonella and Escherichia coli Is Not Associated with Colistin Resistance in the United States. Antimicrob. Agents Chemother 2020, 64, e00573-20. [Google Scholar] [CrossRef]
- Partridge, S.R.; di Pilato, V.; Doi, Y.; Feldgarden, M.; Haft, D.H.; Klimke, W.; Kumar-Singh, S.; Liu, J.H.; Malhotra-Kumar, S.; Prasad, A.; et al. Proposal for Assignment of Allele Numbers for Mobile Colistin Resistance (mcr) Genes. J. Antimicrob. Chemother 2018, 73, 2625–2630. [Google Scholar] [CrossRef]
- Schwarz, S.; Johnson, A.P. Transferable Resistance to Colistin: A New but Old Threat. J. Antimicrob Chemother 2016, 71, 2066–2070. [Google Scholar] [CrossRef]
- Chen, K.; Chan, E.W.; Xie, M.; Ye, L.; Dong, N.; Chen, S. Widespread Distribution of mcr-1-bearing Bacteria in the Ecosystem, 2015 to 2016. Euro Surveill. 2017, 22, 17–00206. [Google Scholar] [CrossRef]
- Feng, Y. Transferability of mcr-1/2 Polymyxin Resistance: Complex Dissemination and Genetic Mechanism. ACS Infect. Dis. 2018, 4, 291–300. [Google Scholar] [CrossRef]
- Hernández, M.; Iglesias, M.R.; Rodríguez-Lázaro, D.; Gallardo, A.; Quijada, N.; Miguela-Villoldo, P.; Campos, M.J.; Píriz, S.; López-Orozco, G.; de Frutos, C.; et al. Co-Occurrence of Colistin-Resistance Genes mcr-1 and mcr-3 among Multidrug-Resistant Escherichia coli Isolated from Cattle, Spain, September 2015. Euro Surveill 2017, 22, 30586. [Google Scholar] [CrossRef]
- Roer, L.; Frank, H.; Marc, S.; Sönksen, U.W.; Hasman, H.; Hammerum, A.M. Novel mcr-3 Variant, Encoding Mobile Colistin Resistance, in an St131 Escherichia coli Isolate from Bloodstream Infection, Denmark, 2014. Eurosurveillance 2017, 22, 30584. [Google Scholar] [CrossRef]
- Hinchliffe, P.; Yang, Q.E.; Portal, E.; Young, T.; Li, H.; Tooke, C.L.; Carvalho, M.J.; Paterson, N.G.; Brem, J.; Niumsup, P.R.; et al. Insights into the Mechanistic Basis of Plasmid-Mediated Colistin Resistance from Crystal Structures of the Catalytic Domain of mcr-1. Sci. Rep. 2017, 7, 39392. [Google Scholar] [CrossRef]
- Venter, H.; Henningsen, M.L.; Begg, S.L. Antimicrobial Resistance in Healthcare, Agriculture and the Environment: The Biochemistry Behind the Headlines. Essays Biochem. 2017, 61, 1–10. [Google Scholar] [CrossRef]
- Gurjar, M. Colistin for Lung Infection: An Update. J. Intensive Care 2015, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Godoy, A.; Muldoon, C.; Becker, B.; Elliott, A.G.; Lash, L.H.; Huang, J.X.; Butler, M.S.; Pelingon, R.; Kavanagh, A.M.; Ramu, S.; et al. Activity and Predicted Nephrotoxicity of Synthetic Antibiotics Based on Polymyxin B. J. Med. Chem. 2016, 59, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Olaitan, A.O.; Morand, S.; Rolain, J.M. Mechanisms of Polymyxin Resistance: Acquired and Intrinsic Resistance in Bacteria. Front. Microbiol. 2014, 5, 643. [Google Scholar] [CrossRef]
- Lima, W.G.; Alves, M.C.; Cruz, W.S.; Paiva, M.C. Chromosomally Encoded and Plasmid-Mediated Polymyxins Resistance in Acinetobacter baumannii: A Huge Public Health Threat. Eur J. Clin. Microbiol. Infect. Dis. 2018, 37, 1009–1019. [Google Scholar] [CrossRef]
- Needham, B.D.; Trent, M.S. Fortifying the Barrier: The Impact of Lipid a Remodelling on Bacterial Pathogenesis. Nat. Rev. Microbiol. 2013, 11, 467–481. [Google Scholar] [CrossRef]
- Hancock, R.E. Peptide Antibiotics. Lancet 1997, 349, 418–422. [Google Scholar] [CrossRef]
- Martis, N.; Leroy, S.; Blanc, V. Colistin in Multi-Drug Resistant Pseudomonas aeruginosa Blood-Stream Infections: A Narrative Review for the Clinician. J. Infect 2014, 69, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Landman, D.; Georgescu, C.; Martin, D.A.; Quale, J. Polymyxins revisited. Clin. Microbiol. Rev. 2008, 21, 449–465. [Google Scholar] [CrossRef]
- Apostolakos, I.; Piccirillo, A. A Review on the Current Situation and Challenges of Colistin Resistance in Poultry Production. Avian Pathol. 2018, 47, 546–558. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.H. Monitoring Colistin Resistance in Food Animals, an Urgent Threat. Expert Rev. Anti. Infect. Ther. 2018, 16, 443–446. [Google Scholar] [CrossRef]
- Bai, L.; Hurley, D.; Li, J.; Meng, Q.; Wang, J.; Fanning, S.; Xiong, Y. Characterisation of Multidrug-Resistant Shiga Toxin-Producing Escherichia coli Cultured from Pigs in China: Co-Occurrence of Extended-Spectrum Β-Lactamase- and mcr-1-Encoding Genes on Plasmids. Int. J. Antimicrob. Agents 2016, 48, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tan, C.; Lin, J.; Feng, Y. Diversified Variants of the mcr-1-Carrying Plasmid Reservoir in the Swine Lung Microbiota. Sci. China Life Sci. 2016, 59, 971–973. [Google Scholar] [CrossRef][Green Version]
- Wang, Q.; Li, Z.; Lin, J.; Wang, X.; Deng, X.; Feng, Y. Complex Dissemination of the Diversified Mcr-1-Harbouring Plasmids in Escherichia Coli of Different Sequence Types. Oncotarget 2016, 7, 82112–82122. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.S.; Chen, Y.T.; Wang, Y.W.; Liu, Y.Y.; Kuo, H.C.; Tu, Y.H.; Lin, A.C.; Liao, Y.S.; Hong, Y.P. Dissemination of mcr-1-Carrying Plasmids among Colistin-Resistant Salmonella Strains from Humans and Food-Producing Animals in Taiwan. Antimicrob Agents Chemother 2017, 61, e00338-17. [Google Scholar] [CrossRef]
- Li, X.S.; Liu, B.G.; Dong, P.; Li, F.L.; Yuan, L.; Hu, G.Z. The Prevalence of mcr-1 and Resistance Characteristics of Escherichia coli Isolates from Diseased and Healthy Pigs. Diagn. Microbiol. Infect. Dis. 2018, 91, 63–65. [Google Scholar] [CrossRef]
- Tong, H.; Liu, J.; Yao, X.; Jia, H.; Wei, J.; Shao, D.; Liu, K.; Qiu, Y.; Ma, Z.; Li, B. High Carriage Rate of mcr-1 and Antimicrobial Resistance Profiles of mcr-1-Positive Escherichia coli Isolates in Swine Faecal Samples Collected from Eighteen Provinces in China. Vet. Microbiol. 2018, 225, 53–57. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Wang, J.; Yassin, A.K.; Butaye, P.; Kelly, P.; Gong, J.; Guo, W.; Li, J.; Li, M.; et al. Molecular Detection of Colistin Resistance Genes (mcr-1, mcr-2 and mcr-3) in Nasal/Oropharyngeal and Anal/Cloacal Swabs from Pigs and Poultry. Sci. Rep. 2018, 8, 3705. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhong, L.L.; Srinivas, S.; Sun, J.; Huang, M.; Paterson, D.L.; Lei, S.; Lin, J.; Li, X.; Tang, Z.; et al. Spread of mcr-3 Colistin Resistance in China: An Epidemiological, Genomic and Mechanistic Study. EBioMedicine 2018, 34, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hulth, A.; Nilsson, L.E.; Borjesson, S.; Chen, B.; Bi, Z.; Wang, Y.; Schwarz, S.; Wu, C. Occurrence of the Mobile Colistin Resistance Gene mcr-3 in Escherichia coli from Household Pigs in Rural Areas. J. Antimicrob. Chemother 2018, 73, 1721–1723. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Wang, J.; Butaye, P.; Kelly, P.; Li, M.; Yang, F.; Gong, J.; Yassin, A.K.; Guo, W.; et al. Newly Identified Colistin Resistance Genes, mcr-4 and mcr-5, from Upper and Lower Alimentary Tract of Pigs and Poultry in China. PLoS ONE 2018, 13, e0193957. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Sun, C.; Hulth, A.; Li, J.; Nilsson, L.E.; Zhou, Y.; Borjesson, S.; Bi, Z.; Bi, Z.; Sun, Q.; et al. Mobile Colistin Resistance Gene mcr-5 in Porcine Aeromonas Hydrophila. J. Antimicrob. Chemother 2018, 73, 1777–1780. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Wang, Z.; Fu, Y.; Du, X.D.; Gao, B.; Zhou, Y.; He, J.; Wang, Y.; Shen, J.; Jiang, H.; et al. Association of Colistin Residues and Manure Treatment with the Abundance of mcr-1 Gene in Swine Feedlots. Environ. Int. 2019, 127, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, B.; Guo, Y.; Wang, J.; Zhao, P.; Liu, J.; He, K. Colistin Resistance Prevalence in Escherichia coli from Domestic Animals in Intensive Breeding Farms of Jiangsu Province. Int. J. Food Microbiol. 2019, 291, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fu, Y.; Schwarz, S.; Yin, W.; Walsh, T.R.; Zhou, Y.; He, J.; Jiang, H.; Wang, Y.; Wang, S. Genetic Environment of Colistin Resistance Genes mcr-1 and mcr-3 in Escherichia coli from One Pig Farm in China. Vet. Microbiol. 2019, 230, 56–61. [Google Scholar] [CrossRef]
- Shen, C.; Zhong, L.; Yang, Y.; Doi, Y.; Paterson, D.L.; Stoesser, N.; Ma, F.; Ahmed, M.A.E.E.; Feng, S.; Huang, S.; et al. Dynamics of mcr-1 Prevalence and Mcr-1-Positive Escherichia coli after the Cessation of Colistin Use as a Feed Additive for Animals in China: A Prospective Cross-Sectional and Whole Genome Sequencing-Based Molecular Epidemiological Study. Lancet Microbe 2020, 1, e34–e43. [Google Scholar] [CrossRef]
- Lay, K.K.; Jeamsripong, S.; Sunn, K.P.; Angkititrakul, S.; Prathan, R.; Srisanga, S.; Chuanchuen, R. Colistin Resistance and Esbl Production in Salmonella and Escherichia Coli from Pigs and Pork in the Thailand, Cambodia, Lao Pdr, and Myanmar Border Area. Antibiotics 2021, 10, 657. [Google Scholar] [CrossRef]
- Wongsrichai, S.; Phuektes, P.; Jittimanee, P. Multidrug-resistance and mobile colistin resistance (mcr) genes of Salmonella isolates from pork in Thailand during 2014–2017: Comparison between two different types of slaughterhouses and retails. Vet. Integr. Sci. 2021, 19, 333–348. [Google Scholar]
- Quesada, A.; Ugarte-Ruiz, M.; Iglesias, M.R.; Porrero, M.C.; Martinez, R.; Florez-Cuadrado, D.; Campos, M.J.; Garcia, M.; Piriz, S.; Saez, J.L.; et al. Detection of Plasmid Mediated Colistin Resistance (Mcr-1) in Escherichia Coli and Salmonella Enterica Isolated from Poultry and Swine in Spain. Res. Vet. Sci. 2016, 105, 134–135. [Google Scholar] [CrossRef] [PubMed]
- AEMPSPlan. Nacional Resistencia Antibióticos. Informe JIACRA España. Primer análisis integrado del consumo de antibióticos y su relación con la aparición de Resistencia; Spanish Agency for Medicines and Health Products (AEMPS): Madrid, Spain, 2018; pp. 1–165. [Google Scholar]
- National Plan against Antibiotic Resistance (PRAN); Spanish Agency for Medicines and Health Products (AEMPS): Madrid, Spain, 2018.
- Miguela-Villoldo, P.; Hernandez, M.; Moreno, M.A.; Rodriguez-Lazaro, D.; Quesada, A.; Dominguez, L.; Ugarte-Ruiz, M. National Colistin Sales Versus Colistin Resistance in Spanish Pig Production. Res. Vet. Sci. 2019, 123, 141–143. [Google Scholar] [CrossRef]
- Aguirre, L.; Vidal, A.; Seminati, C.; Tello, M.; Redondo, N.; Darwich, L.; Martín, M. Antimicrobial Resistance Profile and Prevalence of Extended-Spectrum Beta-Lactamases (Esbl), Ampc Beta-Lactamases and Colistin Resistance (Mcr) Genes in Escherichia coli from Swine between 1999 and 2018. Porc. Health Manag. 2020, 6, 8. [Google Scholar] [CrossRef]
- Rebelo, A.R.; Bortolaia, V.; Kjeldgaard, J.S.; Pedersen, S.K.; Leekitcharoenphon, P.; Hansen, I.M.; Guerra, B.; Malorny, B.; Borowiak, M.; Hammerl, J.A.; et al. Multiplex Pcr for Detection of Plasmid-Mediated Colistin Resistance Determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for Surveillance Purposes. Euro Surveill. 2018, 23, 17–00672. [Google Scholar] [CrossRef] [PubMed]
- Migura-Garcia, L.; González-López, J.J.; Martinez-Urtaza, J.; Sánchez, J.R.A.; Moreno-Mingorance, A.; de Rozas, A.P.; Höfle, U.; Ramiro, Y.; Gonzalez-Escalona, N. mcr-Colistin Resistance Genes Mobilized by Incx4, Inchi2, and Inci2 Plasmids in Escherichia coli of Pigs and White Stork in Spain. Front. Microbiol. 2019, 10, 3072. [Google Scholar] [CrossRef]
- Falgenhauer, L.; Waezsada, S.E.; Yao, Y.; Imirzalioglu, C.; Käsbohrer, A.; Roesler, U.; Michael, G.B.; Schwarz, S.; Werner, G.; Kreienbrock, L.; et al. Colistin Resistance Gene mcr-1 in Extended-Spectrum Β-Lactamase-Producing and Carbapenemase-Producing Gram-Negative Bacteria in Germany. Lancet Infect. Dis. 2016, 16, 282–283. [Google Scholar] [CrossRef]
- Roschanski, N.; Falgenhauer, L.; Grobbel, M.; Guenther, S.; Kreienbrock, L.; Imirzalioglu, C.; Roesler, U. Retrospective Survey of mcr-1 and mcr-2 in German Pig-Fattening Farms, 2011–2012. Int. J. Antimicrob. Agents 2017, 50, 266–271. [Google Scholar] [CrossRef]
- Hille, K.; Roschanski, N.; Ruddat, I.; Woydt, J.; Hartmann, M.; Rösler, U.; Kreienbrock, L. Investigation of Potential Risk Factors for the Occurrence of Escherichia coli Isolates from German Fattening Pig Farms Harbouring the mcr-1 Colistin-Resistance Gene. Int. J. Antimicrob. Agents 2018, 51, 177–180. [Google Scholar] [CrossRef]
- Usui, M.; Nozawa, Y.; Fukuda, A.; Sato, T.; Yamada, M.; Makita, K.; Tamura, Y. Decreased Colistin Resistance and mcr-1 Prevalence in Pig-Derived Escherichia coli in Japan after Banning Colistin as a Feed Additive. J. Glob. Antimicrob. Resist. 2021, 24, 383–386. [Google Scholar] [CrossRef]
- Kusumoto, M.; Ogura, Y.; Gotoh, Y.; Iwata, T.; Hayashi, T.; Akiba, M. Colistin-Resistant Mcr-1-Positive Pathogenic Escherichia Coli in Swine, Japan, 2007–2014. Emerg Infect. Dis. 2016, 22, 1315–1317. [Google Scholar] [CrossRef]
- Fukuda, A.; Sato, T.; Shinagawa, M.; Takahashi, S.; Asai, T.; Yokota, S.I.; Usui, M.; Tamura, Y. High Prevalence of mcr-1, mcr-3 and mcr-5 in Escherichia coli Derived from Diseased Pigs in Japan. Int. J. Antimicrob. Agents 2018, 51, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Duggett, N.A.; Sayers, E.; AbuOun, M.; Ellis, R.J.; Nunez-Garcia, J.; Randall, L.; Horton, R.; Rogers, J.; Martelli, F.; Smith, R.P.; et al. Occurrence and Characterization of mcr-1-Harbouring Escherichia coli Isolated from Pigs in Great Britain from 2013 to 2015. J. Antimicrob. Chemother 2017, 72, 691–695. [Google Scholar] [PubMed]
- European Centre for Disease, Prevention, Control, Authority European Food Safety, and Agency European Medicines. Ecdc/Efsa/Ema Second Joint Report on the Integrated Analysis of the Consumption of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Humans and Food-Producing Animals. EFSA J. 2017, 15, e04872. [Google Scholar]
- European Medicines Agency, European Surveillance of Veterinary Antimicrobial Consumption. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2017; European Medicines Agency: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Fournier, C.; Aires-de-Sousa, M.; Nordmann, P.; Poirel, L. Occurrence of Ctx-M-15- and Mcr-1-Producing Enterobacterales in Pigs in Portugal: Evidence of Direct Links with Antibiotic Selective Pressure. Int. J. Antimicrob. Agents 2020, 55, 105802. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Nguyen, H.M.; Nguyen, C.V.; Nguyen, T.V.; Nguyen, M.T.; Thai, H.Q.; Ho, M.H.; Thwaites, G.; Ngo, H.T.; Baker, S.; et al. Use of Colisti.in and Other Critical Antimicrobials on Pig and Chicken Farms in Southern Vietnam and Its Association with Resistance in Commensal Escherichia Coli Bacteria. Appl. Environ. Microbiol. 2016, 82, 3727–3735. [Google Scholar] [CrossRef]
- Nakayama, T.; Ueda, S.; Huong, B.T.; Le, D.T.; Komalamisra, C.; Kusolsuk, T.; Hirai, I.; Yamamoto, Y. Wide Dissemination of Extended-Spectrum Β-Lactamase-Producing Escherichia Coli in Community Residents in the Indochinese Peninsula. Infect. Drug Resist. 2015, 8, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Jinnai, M.; Kawahara, R.; Diep, K.T.; Thang, N.N.; Hoa, T.T.; Hanh, L.K.; Khai, P.N.; Sumimura, Y.; Yamamoto, Y. Frequent Use of Colistin-Based Drug Treatment to Eliminate Extended-Spectrum Beta-Lactamase-Producing Escherichia coli in Backyard Chicken Farms in Thai Binh Province, Vietnam. Trop Anim. Health Prod. 2017, 49, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Kawahara, R.; Harada, K.; Teruya, S.; Nakayama, T.; Motooka, D.; Nakamura, S.; Nguyen, P.D.; Kumeda, Y.; van Dang, C.; et al. The Presence of Colistin Resistance Gene mcr-1 and -3 in Esbl Producing Escherichia coli Isolated from Food in Ho Chi Minh City, Vietnam. FEMS Microbiol. Lett. 2018, 365, fny100. [Google Scholar] [CrossRef]
- Mourand, G.; Andraud, M.; Jouy, E.; Chauvin, C.; le Devendec, L.; Paboeuf, F.; Kempf, I. Impact of Colistin Administered before or after Inoculation on the Transmission of a mcr-1 Colistin-Resistant Escherichia coli Strain between Pigs. Vet. Microbiol. 2019, 230, 164–170. [Google Scholar] [CrossRef]
- Alba, P.; Leekitcharoenphon, P.; Franco, A.; Feltrin, F.; Ianzano, A.; Caprioli, A.; Stravino, F.; Hendriksen, R.S.; Bortolaia, V.; Battisti, A. Molecular Epidemiology of mcr-Encoded Colistin Resistance in Enterobacteriaceae from Food-Producing Animals in Italy Revealed through the Eu Harmonized Antimicrobial Resistance Monitoring. Front. Microbiol. 2018, 9, 1217. [Google Scholar] [CrossRef]
- Timmermans, M.; Wattiau, P.; Denis, O.; Boland, C. Colistin Resistance Genes mcr-1 to mcr-5, Including a Case of Triple Occurrence (mcr-1, -3 and -5), in Escherichia coli Isolates from Faeces of Healthy Pigs, Cattle and Poultry in Belgium, 2012–2016. Int. J. Antimicrob. Agents 2021, 57, 106350. [Google Scholar] [CrossRef]
- Malhotra-Kumar, S.; Xavier, B.B.; Das, A.J.; Lammens, C.; Butaye, P.; Goossens, H. Colistin Resistance Gene mcr-1 Harboured on a Multidrug Resistant Plasmid. Lancet Infect. Dis. 2016, 16, 283–284. [Google Scholar] [CrossRef]
- Fernandes, M.R.; Moura, Q.; Sartori, L.; Silva, K.C.; Cunha, M.P.; Esposito, F.; Lopes, R.; Otutumi, L.K.; Gonçalves, D.D.; Dropa, M.; et al. Silent Dissemination of Colistin-Resistant Escherichia coli in South America Could Contribute to the Global Spread of the mcr-1 Gene. Euro Surveill. 2016, 21, 30214. [Google Scholar] [CrossRef]
- Pilote, J.; Létourneau, V.; Girard, M.; Duchaine, C. Quantification of Airborne Dust, Endotoxins, Human Pathogens and Antibiotic and Metal Resistance Genes in Eastern Canadian Swine Confinement Buildings. Aerobiologia 2019, 35, 283–296. [Google Scholar] [CrossRef]
- Oh, S.S.; Song, J.; Kim, J.; Shin, J. Increasing Prevalence of Multidrug-Resistant mcr-1-Positive Escherichia coli Isolates from Fresh Vegetables and Healthy Food Animals in South Korea. Int. J. Infect. Dis. 2020, 92, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Fournier, C.; Nordmann, P.; Pittet, O.; Poirel, L. Does an Antibiotic Stewardship Applied in a Pig Farm Lead to Low Esbl Prevalence? Antibiotics 2021, 10, 574. [Google Scholar] [CrossRef] [PubMed]
- El Garch, F.; de Jong, A.; Bertrand, X.; Hocquet, D.; Sauget, M. mcr-1-Like Detection in Commensal Escherichia coli and Salmonella Spp. From Food-Producing Animals at Slaughter in Europe. Vet. Microbiol. 2018, 213, 42–46. [Google Scholar] [CrossRef]
- Veldman, K.; van Essen-Zandbergen, A.; Rapallini, M.; Wit, B.; Heymans, R.; van Pelt, W.; Mevius, D. Location of Colistin Resistance Gene mcr-1 in Enterobacteriaceae from Livestock and Meat. J. Antimicrob Chemother 2016, 71, 2340–2342. [Google Scholar] [CrossRef] [PubMed]
- Thiry, D.; Berrah, A.; Evrard, J.; Duprez, J.N.; Mainil, J.G.; Saulmont, M. Assessment of Two Selective Agar Media to Isolate Colistin-Resistant Bovine Escherichia coli: Correlation with Minimal Inhibitory Concentration and Presence of mcr Genes. J. Microbiol. Methods 2019, 159, 174–178. [Google Scholar] [CrossRef]
- Haenni, M.; Poirel, L.; Kieffer, N.; Châtre, P.; Saras, E.; Métayer, V.; Dumoulin, R.; Nordmann, P.; Madec, J.Y. Co-Occurrence of Extended Spectrum Β Lactamase and Mcr-1 Encoding Genes on Plasmids. Lancet Infect. Dis. 2016, 16, 281–282. [Google Scholar] [CrossRef]
- Davin-Regli, A.; Guerin-Faublee, V.; Pages, J.M. Modification of Outer Membrane Permeability and Alteration of LPS in Veterinary Enterotoxigenic Escherichia coli. Res. Vet. Sci. 2019, 124, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Filioussis, G.; Kachrimanidou, M.; Christodoulopoulos, G.; Kyritsi, M.; Hadjichristodoulou, C.; Adamopoulou, M.; Tzivara, A.; Kritas, S.K.; Grinberg, A. Short Communication: Bovine Mastitis Caused by a Multidrug-Resistant, Mcr-1-Positive (Colistin-Resistant), Extended-Spectrum Β-Lactamase-Producing Escherichia coli Clone on a Greek Dairy Farm. J. Dairy Sci. 2020, 103, 852–857. [Google Scholar] [CrossRef]
- Yin, D.; Cheng, B.; Yang, K.; Xue, M.; Lin, Y.; Li, Z.; Song, X.; Shao, Y.; Tu, J.; Li, P.; et al. Complete Genetic Analysis of Plasmids Carrying mcr-1 and Other Resistance Genes in Avian Pathogenic Escherichia coli Isolates from Diseased Chickens in Anhui Province in China. mSphere 2021, 6, e01135-20. [Google Scholar] [CrossRef]
- Wang, Y.; Lyu, N.; Liu, F.; Liu, W.J.; Bi, Y.; Zhang, Z.; Ma, S.; Cao, J.; Song, X.; Wang, A.; et al. More Diversified Antibiotic Resistance Genes in Chickens and Workers of the Live Poultry Markets. Environ. Int. 2021, 153, 106534. [Google Scholar] [CrossRef]
- Schrauwen, E.J.A.; Huizinga, P.; van Spreuwel, N.; Verhulst, C.; den Bergh, M.F.Q.K.; Kluytmans, J. High Prevalence of the Mcr-1 Gene in Retail Chicken Meat in the Netherlands in 2015. Antimicrob Resist. Infect. Control. 2017, 6, 83. [Google Scholar] [CrossRef]
- Monte, D.F.; Mem, A.; Fernandes, M.R.; Cerdeira, L.; Esposito, F.; Galvão, J.A.; Franco, B.; Lincopan, N. Chicken Meat as a Reservoir of Colistin-Resistant Escherichia coli Strains Carrying mcr-1 Genes in South America. Antimicrob. Agents Chemother 2017, 61, e02718-16. [Google Scholar] [CrossRef] [PubMed]
- Maciuca, I.E.; Cummins, M.L.; Cozma, A.P.; Rimbu, C.M.; Guguianu, E.; Panzaru, C.; Licker, M.; Szekely, E.; Flonta, M.; Djordjevic, S.P.; et al. Genetic Features of Mcr-1 Mediated Colistin Resistance in Cmy-2-Producing Escherichia Coli from Romanian Poultry. Front. Microbiol. 2019, 10, 2267. [Google Scholar] [CrossRef]
- Jalal, M.S.; Dutta, A.; Das, T.; Islam, M.Z. First Detection of Plasmid-Mediated Colistin-Resistance Gene (mcr-1, mcr-2 and mcr-3) in Escherichia coli Isolated from Breeder Poultry of Bangladesh. Int. J. Infect. Dis. 2020, 101, 17. [Google Scholar] [CrossRef]
- Islam, M.; Urmi, U.; Rana, M.; Sultana, F.; Jahan, N.; Hossain, B.; Iqbal, S.; Mosaddek, A.; Nahar, S. Poultry Chicken Gut-Bacteria Carry High Extent of Colistin Resistant mcr-1 Gene in Bangladesh. Int. J. Infect. Dis. 2020, 101, 60. [Google Scholar] [CrossRef]
- Uddin, M.B.; Hossain, S.M.B.; Hasan, M. Multidrug Antimicrobial Resistance and Molecular Detection of mcr-1 Gene in Salmonella Species Isolated from Chicken. Animals 2021, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.S.; Mannan, M.S.; Ali, M.Y.; Bayzid, M.; Ahad, A.; Bupasha, Z.B. Antibiotic Resistance of Escherichia coli Isolated from Broilers Sold at Live Bird Markets in Chattogram, Bangladesh. J. Adv. Vet Anim. Res. 2019, 6, 272–277. [Google Scholar] [CrossRef]
- Ghaffoori Kanaan, M.H.; Al-Shadeedi, S.M.J.; Al-Massody, A.J.; Ghasemian, A. Drug Resistance and Virulence Traits of Acinetobacter baumannii from Turkey and Chicken Raw Meat. Comp. Immunol. Microbiol. Infect Dis. 2020, 70, 101451. [Google Scholar] [CrossRef] [PubMed]
- Dhaouadi, S.; Soufi, L.; Hamza, A.; Fedida, D.; Zied, C.; Awadhi, E.; Mtibaa, M.; Hassen, B.; Cherif, A.; Torres, C.; et al. Co-Occurrence of mcr-1 Mediated Colistin Resistance and Β-Lactamase-Encoding Genes in Multidrug-Resistant Escherichia coli from Broiler Chickens with Colibacillosis in Tunisia. J. Glob. Antimicrob. Resist. 2020, 22, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Zulqarnain, M.; Sarwar, N.; Anjum, A.A.; Firyal, S.; Yaqub, T.; Rabbani, M. Molecular Detection of Colistin Resistance Gene (MCR-1) in E. coli Isolated from Cloacal Swabs of Broilers. Pak. Vet. J. 2021, 41, 284–288. [Google Scholar]
- Anyanwu, M.U.; Marrollo, R.; Lucci, M.P.; Brovarone, F.; Nardini, P.; Chah, K.F.; Shoyinka, S.V.O.; Carretto, E. Isolation and Characterisation of Colistin-Resistant Enterobacterales from Chickens in Southeast Nigeria. J. Glob. Antimicrob. Resist. 2021, 26, 93–100. [Google Scholar] [CrossRef]
- Erzaim, N.; Ikiz, S. Investigation of Phenotypic and mcr-1-Mediated Colistin Resistance in Escherichia coli. Acta Vet. Eurasia 2021, 47, 82–87. [Google Scholar] [CrossRef]
- Nesporova, K.; Valcek, A.; Papagiannitsis, C.; Kutilova, I.; Jamborova, I.; Davidova-Gerzova, L.; Bitar, I.; Hrabak, J.; Literak, I.; Dolejska, M. Multi-Drug Resistant Plasmids with Esbl/Ampc and Mcr-5.1 in Paraguayan Poultry Farms: The Linkage of Antibiotic Resistance and Hatcheries. Microorganisms 2021, 9, 866. [Google Scholar] [CrossRef]
- Kassem, I.I.; Mann, D.; Li, S.; Deng, X. Draft Genome Sequences and Resistome Analysis of Multidrug-Resistant Mcr-1-Harbouring Escherichia Coli Isolated from Pre-Harvest Poultry in Lebanon. J. Glob. Antimicrob. Resist. 2021, 25, 114–116. [Google Scholar] [CrossRef]
- Al-Mir, H.; Osman, M.; Drapeau, A.; Hamze, M.; Madec, J.Y.; Haenni, M. Wgs Analysis of Clonal and Plasmidic Epidemiology of Colistin-Resistance Mediated by Mcr Genes in the Poultry Sector in Lebanon. Front. Microbiol. 2021, 12, 624194. [Google Scholar] [CrossRef]
- Chaalal, N.; Touati, A.; Yahiaoui-Martinez, A.; Aissa, M.A.; Sotto, A.; Lavigne, J.P.; Pantel, A. Colistin-Resistant Enterobacterales Isolated from Chicken Meat in Western Algeria. Microb. Drug Resist. 2021, 27, 991–1002. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valiakos, G.; Kapna, I. Colistin Resistant mcr Genes Prevalence in Livestock Animals (Swine, Bovine, Poultry) from a Multinational Perspective. A Systematic Review. Vet. Sci. 2021, 8, 265. https://doi.org/10.3390/vetsci8110265
Valiakos G, Kapna I. Colistin Resistant mcr Genes Prevalence in Livestock Animals (Swine, Bovine, Poultry) from a Multinational Perspective. A Systematic Review. Veterinary Sciences. 2021; 8(11):265. https://doi.org/10.3390/vetsci8110265
Chicago/Turabian StyleValiakos, George, and Ioanna Kapna. 2021. "Colistin Resistant mcr Genes Prevalence in Livestock Animals (Swine, Bovine, Poultry) from a Multinational Perspective. A Systematic Review" Veterinary Sciences 8, no. 11: 265. https://doi.org/10.3390/vetsci8110265
APA StyleValiakos, G., & Kapna, I. (2021). Colistin Resistant mcr Genes Prevalence in Livestock Animals (Swine, Bovine, Poultry) from a Multinational Perspective. A Systematic Review. Veterinary Sciences, 8(11), 265. https://doi.org/10.3390/vetsci8110265







