Porcine Reproductive and Respiratory Syndrome Surveillance in breeding Herds and Nurseries Using Tongue Tips from Dead Animals
Abstract
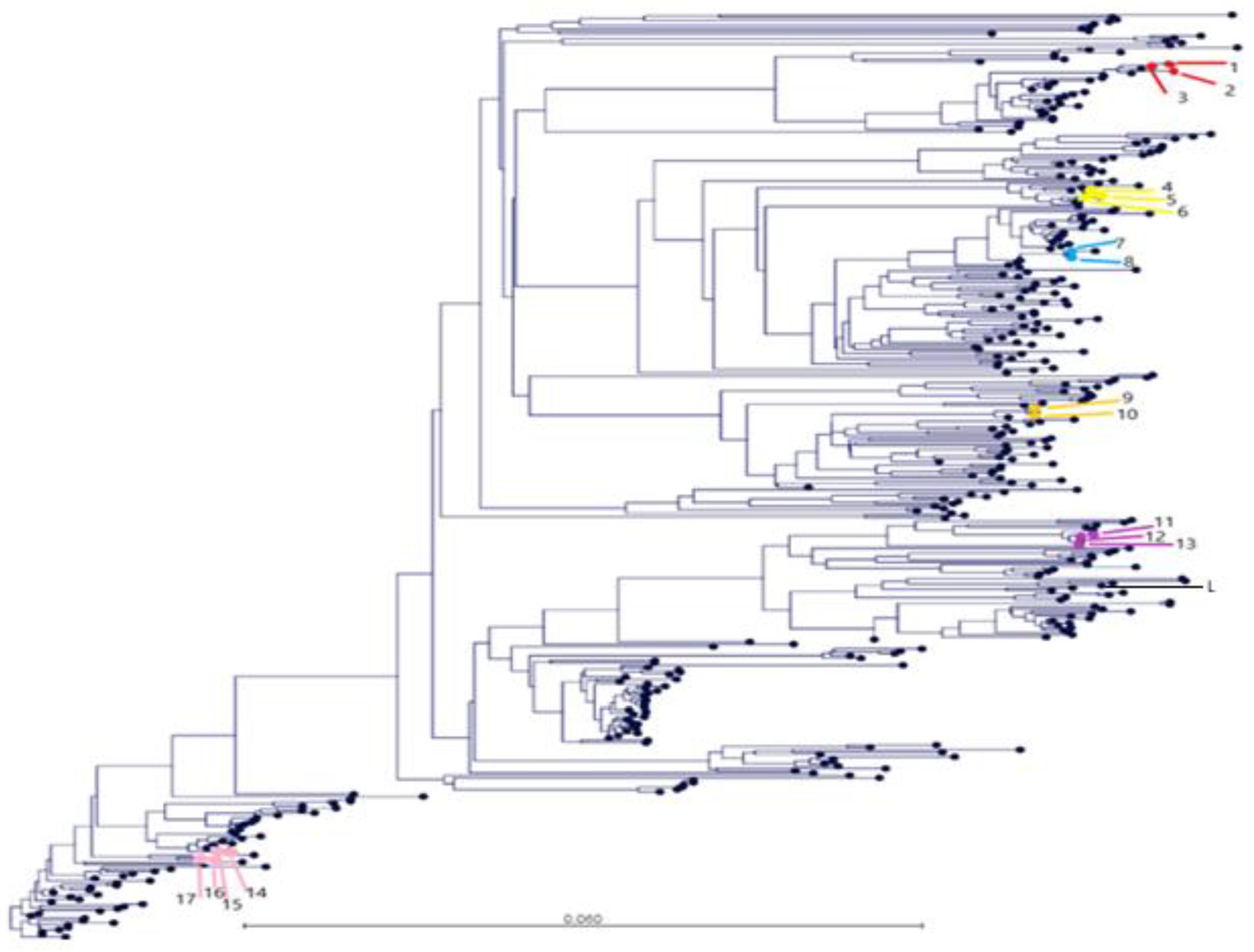
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Sample Collection Protocol
2.3. Diagnostic Testing
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rowland, R.R.; Lunney, J.; Dekkers, J. Control of porcine reproductive and respiratory syndrome (PRRS) through genetic improvements in disease resistance and tolerance. Front. Genet. 2012, 3, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraile, L.; Fernández, N.; Pena, R.N.; Balasch, S.; Castellà, G.; Puig, P.; Estany, J.; Valls, J. A probabilistic Poisson-based model to detect PRRSV recirculation using sow production records. Prev. Vet. Med. 2020, 177, 104948. [Google Scholar] [CrossRef]
- Corzo, C.A.; Mondaca, E.; Wayne, S.; Torremorell, M.; Dee, S.; Davies, P.; Morrison, R.B. Control and elimination of porcine reproductive and respiratory syndrome virus. Virus Res. 2010, 154, 185–192. [Google Scholar] [CrossRef]
- Torremorell, M.; Henry, S.; Christianson, W.T. Eradication Using Herd Closure. In Pork Checkoff; Zimmerman, J., Yoon, K.J., Neumann, E., Eds.; National Pork Board PRRS Compendium: Des Moines, IO, USA, 2003; pp. 157–160. [Google Scholar]
- Nathues, H.; Alarcon, P.; Rushton, J.; Jolie, R.; Fiebig, K.; Jimenez, M.; Geurts, V.; Nathues, C. Modelling the economic efficiency of using different strategies to control Porcine Reproductive & Respiratory Syndrome at herd level. Prev. Vet. Med. 2018, 152, 89–102. [Google Scholar] [CrossRef] [PubMed]
- McCaw, M.B. Effect of reducing crossfostering at birth on piglet mortality and performance during an acute outbreak of porcine reproductive and respiratory syndrome. J. Swine Health Prod. 2000, 8, 15–21. Available online: https://www.aasv.org/shap/issues/v8n1/v8n1p15.pdf (accessed on 1 October 2021).
- Martelli, P.; Gozio, S.; Ferrari, L.; Rosina, S.; Angelis, E.D.; Quintavalla, C.; Bottarelli, E.; Borghetti, P. Efficacy of a modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine in pigs naturally exposed to a heterologous European (Italian cluster) field strain: Clinical protection and cell-mediated immunity. Vaccine 2009, 27, 3788–3799. [Google Scholar] [CrossRef] [PubMed]
- Lunney, J.K.; Chen, H. Genetic control of host resistance to porcine reproductive and respiratory syndrome virus (PRRSV) infection. Virus Res. 2010, 154, 161–169. [Google Scholar] [CrossRef]
- Tousignant, S.J.P.; Perez, A.M.; Lowe, J.F.; Yeske, P.E.; Morrison, R.B. Temporal and spatial dynamics of porcine reproductive and respiratory syndrome virus infection in the United States. Am. J. Vet. Res. 2015, 76, 70–76. [Google Scholar] [CrossRef]
- Vilalta, C.; Arruda, A.G.; Tousignant, S.J.P.; Valdes-Donoso, P.; Muellner, P.; Muellner, U.; Alkhamis, M.A.; Morrison, R.B.; Perez, A.M. A Review of Quantitative Tools Used to Assess the Epidemiology of Porcine Reproductive and Respiratory Syndrome in U.S. Swine Farms Using Dr. Morrison’s Swine Health Monitoring Program Data. Front Vet Sci. 2017, 4, 94. Available online: https://www.frontiersin.org/articles/10.3389/fvets.2017.00094/full (accessed on 1 October 2021). [CrossRef]
- Holtkamp, D.J.; Polson, D.D.; Torremorell, M.; Morrison, B.; Rowland, R.R.; Snelson, H. Terminology for classifying swine herds by porcine reproductive and respiratory syndrome virus status. J. Swine Health Prod. 2011, 19, 13. Available online: https://www.aasv.org/shap/issues/v19n1/v19n1p44.pdf (accessed on 1 October 2021).
- Dee, S.A.; Molitor, T.W.; Rossow, K.D. Epidemiological and diagnostic observations following the elimination of porcine reproductive and respiratory syndrome virus from a breeding herd of pigs by the test and removal protocol. Vet. Rec. 2000, 146, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Bierk, M.D.; Dee, S.A.; Rossow, K.D.; Collins, J.E.; Guedes, M.I.; Pijoan, C.; Molitor, T.W. Diagnostic investigation of chronic porcine reproductive and respiratory syndrome virus in a breeding herd of pigs. Vet. Rec. 2001, 148, 687–690. [Google Scholar] [CrossRef]
- Linhares, D.C.L.; Cano, J.P.; Torremorell, M.; Morrison, R.B. Comparison of time to PRRSV-stability and production losses between two exposure programs to control PRRSV in sow herds. Prev. Vet. Med. 2014, 116, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, D.; Torremorell, M.; Corzo, C.A.; Linhares, D.C.L.; Almeida, M.N.; Yeske, P.; Polson, D.D.; Becton, L.; Snelson, H.; Donovan, T.; et al. Proposed modifications to porcine reproductive and respiratory syndrome virus herd classification. J. Swine Health Prod. 2021, 29, 261–270. Available online: https://www.aasv.org/shap/issues/v29n5/v29n5p261.html (accessed on 1 October 2021).
- Kittawornrat, A.; Rickett, J.; Chittick, W.; Wang, C.; Engle, M.; Johnson, J. Porcine reproductive and respiratory syndrome virus (PRRSV) in serum and oral fluid samples from individual boars: Will oral fluid replace serum for PRRSV surveillance? Virus Res. 2010, 154, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Rotolo, M.L.; Sun, Y.; Wang, C.; Giménez-Lirola, L.; Baum, D.H.; Gauger, P.C.; Harmon, K.M.; Hoogland, M.; Main, R.; Zimmerman, J.J. Sampling guidelines for oral fluid-based surveys of group-housed animals. Vet. Microbiol. 2017, 209, 20–29. [Google Scholar] [CrossRef]
- Lopez, W.A.; Angulo, J.; Zimmerman, J.J.; Linhares, D.C.L. Porcine reproductive and respiratory syndrome monitoring in breeding herds using processing fluids. J. Swine Health Prod. 2018, 26, 5. Available online: https://www.aasv.org/jshap/issues/v26n3/v26n3p146.pdf (accessed on 1 October 2021).
- Vilalta, C.; Sanhueza, J.; Alvarez, J.; Murray, D.; Torremorell, M.; Corzo, C.; Morrison, R.B. Use of processing fluids and serum samples to characterize porcine reproductive and respiratory syndrome virus dynamics in 3 day-old pigs. Vet. Microbiol. 2018, 225, 149–156. [Google Scholar] [CrossRef]
- Vilalta, C.; Sanhueza, J.M.; Schwartz, M.; Kikuti, M.; Torremorell, M.; Corzo, C.A. Assessing the litter level agreement of RT-PCR results for porcine reproductive and respiratory syndrome virus in testicles, tails and udder wipes diagnostic samples relative to serum from piglets. Prev. Vet. Med. 2021, 186, 105211. [Google Scholar] [CrossRef]
- Guzmán, M.; Meléndez, R.; Jiménez, R.; Piche, M.; Jiménez, E.; León, B.; Cordero, J.M.; Ramirez-Carvajal, L.; Uribe, A.; Van Neas, A.; et al. Analysis of ORF5 sequences of Porcine Reproductive and Respiratory Syndrome virus (PRRSV) circulating within swine farms in Costa Rica. BMC Vet. Res. 2021, 17, 217. [Google Scholar] [CrossRef]
- Thrusfield, M. Veterinary Epidemiology, 3rd ed.; Blackwell Science: Oxford, UK, 2005; pp. 110–126. [Google Scholar]
- Cannon, R.M.; Roe, R.T. A Livestock Disease Surveys: A Field Manual for Veterinarians. Australian Government Publishing Service. 1982. Available online: https://www.aphis.usda.gov/animal_health/surveillance_toolbox/docs/epi_surv/cannon_roe_1982_livestock_disease_surveys.pdf (accessed on 1 October 2021).
- Almeida, M.N.; Zhang, M.; Lopez, W.A.L.; Vilalta, C.; Sanhueza, J.; Corzo, C.; Zimmerman, J.J.; Linhares, D.C.L. A comparison of three surveillance approaches for detecting PRRSV in suckling piglets. Prev. Vet. Med. 2021, 194, 105427. [Google Scholar] [CrossRef] [PubMed]
- Prickett, J.R.; Cutler, S.; Kinyon, J.M.; Naberhaus, N.; Stensland, W.R.; Yoon, K.J. Stability of porcine reproductive and respiratory syndrome virus and antibody in swine oral fluid. J. Swine Health Prod. 2010, 18, 9. Available online: https://www.aasv.org/shap/issues/v18n4/v18n4p187.pdf (accessed on 1 October 2021).
- Kleiboeker, S.B. Applications of Competitor RNA in Diagnostic Reverse Transcription-PCR. J. Clin. Micol. 2003, 41, 2055–2061. [Google Scholar] [CrossRef] [Green Version]
- Makau, D.N.; Alkhamis, M.A.; Paploski, I.A.D.; Corzo, C.A.; Lycett, S.; VanderWaal, K. Integrating animal movements with phylogeography to model the spread of PRRSV in the USA. Virul. Evol. 2021, 7, veab060. [Google Scholar] [CrossRef] [PubMed]


| Farm (Pair) | Farm Size 1 | Type of Farm 2 | PRRSV History 3 | Age 4 | Sample Date | Timing from Diagnosis to Sampling | PRRSV Batch Results Serum/Tongues | Agreement (Y/N) 5 |
|---|---|---|---|---|---|---|---|---|
| 4 (1) | 2500 | FTF | Positive | 3 weeks | 5 February 2019 | 371 | −/+ | N |
| 4 (2) | 2500 | FTF | Positive | 1 day | 5 February 2019 | 371 | −/+ | N |
| 4 (3) | 2500 | FTF | Positive | 1 day | 12 March 2019 | 406 | +/+ | Y |
| 4 (4) | 2500 | FTF | Positive | 3 weeks | 12 March 2019 | 406 | −/+ | N |
| 4 (5) | 2500 | FTF | Positive | 1 day | 9 May 2019 | 464 | −/+ | N |
| 4 (6) | 2500 | FTF | Positive | 1 day | 28 July 2020 | 910 | +/+ | Y |
| 5 (1) | 2300 | FTF | Positive | 1 day | 12 March 2019 | 464 | +/+ | Y |
| 5 (2) | 2300 | FTF | Positive | 1 day | 28 July 2020 | 206 | +/+ | Y |
| 6 (1) | 1700 | FTF | Positive | 1 day | 6 March 2019 | 15 | +/+ | Y |
| 6 (2) | 1700 | FTF | Positive | 3 weeks | 6 March 2019 | 15 | +/+ | Y |
| 8 (1) | 2400 | FTF | Positive | 3 weeks | 19 February 2019 | 120 | −/+ | N |
| 8 (2) | 2400 | FTF | Positive | 1 day | 19 February 2019 | 120 | +/+ | Y |
| 9 (1) | 2350 | FTF | Positive | 1 day | 11 March 2020 | 109 | +/+ | Y |
| 9 (2) | 2350 | FTF | Positive | 3 weeks | 11 March 2020 | 109 | +/+ | Y |
| 24 (1) | 3000 | FTF | Positive | 1 day | 16 July 2020 | 87 | −/+ | N |
| 24 (2) | 3000 | FTF | Positive | 1 day | 18 June 2020 | 59 | +/+ | Y |
| 36 (1) | 2200 | FTW | Positive | 1 day | 28 July 2019 | 203 | +/+ | Y |
| 36 (2) | 2200 | FTW | Positive | 1 day | 18 September 2019 | 255 | +/+ | Y |
| 47 (1) | 750 | FTW | Positive | 1 day | 10 September 2019 | 369 | +/+ | Y |
| 50 (1) | 2000 | FTF | Positive | 1 day | 9 December 2020 | 330 | +/+ | Y |
| 50 (2) | 2000 | FTF | Positive | 1 day | 3 November 2020 | 294 | +/+ | Y |
| 50 (3) | 2000 | FTF | Positive | 1 day | 8 September 2020 | 238 | +/+ | Y |
| 52 (1) | 3080 | FTF | Positive | 1 day | 18 August 2020 | 120 | +/+ | Y |
| 54 (1) | 650 | FTF | Negative | 1 day | 25 April 2019 | NA | −/− | Y |
| 55 (1) | 2400 | FTF | Positive | 1 day | 15 January 2020 | 93 | +/+ | Y |
| Farm (Pair) | Farm Size 1 | Type of Farm 2 | PRRSV History 3 | Age 4 | Sample Date | Timing from Diagnosis to Sampling | PRRSV Batch Results Serum/Tongues | Agreement (Y/N) 5 |
|---|---|---|---|---|---|---|---|---|
| 1 (1) | 2500 | WTF | Positive | 8 weeks | 4 April 2019 | 64 | +/− | N |
| 2 (1) | 2520 | WTFe | Naive | 8 weeks | 25 March 2019 | NA | −/− | Y |
| 4 (1) | 2350 | WTFe | Positive | 8 weeks | 5 February 2019 | 369 | +/+ | Y |
| 4 (2) | 2350 | WTFe | Positive | 8 weeks | 12 March 2019 | 406 | +/+ | Y |
| 4 (3) | 2350 | WTFe | Positive | 8 weeks | 9 May 2019 | 464 | +/+ | Y |
| 5 (1) | 3050 | WTFe | Positive | 8 weeks | 4 February 2019 | 428 | −/+ | N |
| 8 (1) | 6000 | WTFe | Positive | 8 weeks | 19 February 2019 | 120 | +/+ | Y |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baliellas, J.; Novell, E.; Enric-Tarancón, V.; Vilalta, C.; Fraile, L. Porcine Reproductive and Respiratory Syndrome Surveillance in breeding Herds and Nurseries Using Tongue Tips from Dead Animals. Vet. Sci. 2021, 8, 259. https://doi.org/10.3390/vetsci8110259
Baliellas J, Novell E, Enric-Tarancón V, Vilalta C, Fraile L. Porcine Reproductive and Respiratory Syndrome Surveillance in breeding Herds and Nurseries Using Tongue Tips from Dead Animals. Veterinary Sciences. 2021; 8(11):259. https://doi.org/10.3390/vetsci8110259
Chicago/Turabian StyleBaliellas, Jordi, Elena Novell, Vicens Enric-Tarancón, Carles Vilalta, and Lorenzo Fraile. 2021. "Porcine Reproductive and Respiratory Syndrome Surveillance in breeding Herds and Nurseries Using Tongue Tips from Dead Animals" Veterinary Sciences 8, no. 11: 259. https://doi.org/10.3390/vetsci8110259
APA StyleBaliellas, J., Novell, E., Enric-Tarancón, V., Vilalta, C., & Fraile, L. (2021). Porcine Reproductive and Respiratory Syndrome Surveillance in breeding Herds and Nurseries Using Tongue Tips from Dead Animals. Veterinary Sciences, 8(11), 259. https://doi.org/10.3390/vetsci8110259







