Screening of Cyanide-Utilizing Bacteria from Rumen and In Vitro Evaluation of Fresh Cassava Root Utilization with Pellet Containing High Sulfur Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1
2.1.1. Screening of Cyanide-Utilizing Bacteria in the Rumen
Animals and Feeding
Sampling and Enrichment Culture for Cyanide-Utilizing Bacteria in Rumen
Media and Culture Conditions
Bacterial Growth
2.2. Experiment 2
2.2.1. In Vitro Study
Pellets Containing High Sulfur (PELFUR) Preparation and Experimental Design
Inoculum
Substrate
Analysis of Samples
2.2.2. Statistical Analysis
3. Results and Discussions
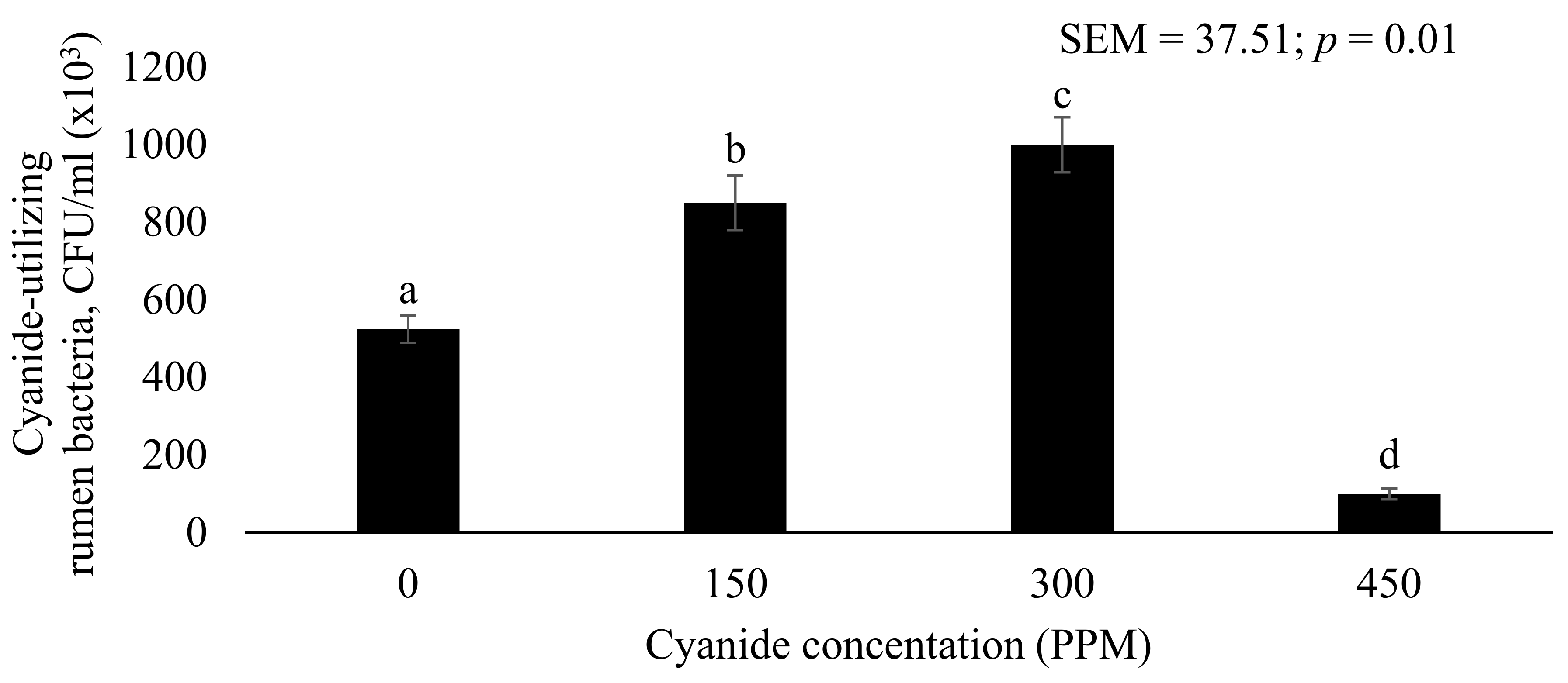
3.1. Screening of Cyanide-Utilizing Bacteria from Rumen
3.2. In Vitro Gas Production Study
3.2.1. Gas Production Parameters and Cumulative Gas Production
3.2.2. In Vitro Fermentation and Ruminal Microbial Population
3.2.3. In Vitro Degradability
3.2.4. Concentration of Volatile Fatty Acids (VFAs)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wanapat, M.; Khampa, S. Effect of levels of supplementation of concentrate containing high levels of cassava chip on rumen ecology, microbial N supply and digestibility of nutrients in beef cattle. Asian Australas. J. Anim. Sci. 2007, 20, 75–81. [Google Scholar] [CrossRef]
- Cherdthong, A.; Khonkhaeng, B.; Seankamsorn, A.; Supapong, C.; Wanapat, M.; Gunun, N.; Gunun, P.; Chanjula, P.; Polyorach, S. Effects of feeding fresh cassava root with high-sulfur feed block on feed utilization, rumen fermentation and blood metabolites in Thai native cattle. Trop. Anim. Health Prod. 2018, 50, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Dagaew, G.; Cherdthong, A.; Wanapat, M.; Chanjula, P. In vitro rumen gas production kinetics, hydrocyanic acid concentration and fermentation characteristics of fresh cassava root and feed block sulfur concentration. Anim. Prod. Sci. 2020, 60, 659–664. [Google Scholar] [CrossRef]
- Promkot, C.; Wanapat, M.; Wachirapakorn, C.; Navanukraw, C. Influence of sulphur on fresh cassava foliage and cassava hay incubate in rumen fluid of beef cattle. Asian Australas. J. Anim. Sci. 2007, 20, 1424–1432. [Google Scholar] [CrossRef]
- Aminlari, M. Distribution of the cyanide metabolizing enzyme rhodanese in different tissues of domestic animals. J. Vet. Pharmacol. Ther. 2006, 29, 128. [Google Scholar] [CrossRef]
- Promkot, C.; Wanapat, M. Effect of elemental sulfur supplementation on rumen environment parameters and utilization efficiency of fresh cassava foliage and cassava hay in dairy cattle. Asian Australas. J. Anim. Sci. 2009, 22, 1366–1376. [Google Scholar] [CrossRef]
- Jones, R.J.; Megarrity, R.G. Successful transfer of DHP-degrading bacteria from Hawaiian goats to Australian ruminants to overcome the toxicity of Leucaena. Aust. Vet. J. 1986, 63, 259–262. [Google Scholar] [CrossRef]
- Supapong, C.; Cherdthong, A. Effect of sulfur concentrations in fermented total mixed rations containing fresh cassava root on rumen fermentation. Anim. Prod. Sci. 2020, 60, 1429–1434. [Google Scholar] [CrossRef]
- Tele, F.F. Chronic poisoning by hydrogen cyanide in cassava and its prevention in Africa and Latin America. Food. Nutr. Bull. 2002, 23, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Supapong, C.; Cherdthong, A.; Wanapat, M.; Chanjula, P.; Uriyapongson, S. Effects of sulfur levels in fermented total mixed ration containing fresh cassava root on feed utilization, rumen characteristics, microbial protein synthesis, and blood metabolites in Thai native beef cattle. Animals 2019, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Südekum, K.H.; Schröder, A.; Fiebelkorn, S.; Schwer, R.; Thalmann, A. Quality characteristics of pelleted compound feeds under varying storage conditions as influenced by purity and concentration of glycerol from biodiesel production. J. Anim. Feed Sci. 2008, 17, 120–136. [Google Scholar] [CrossRef]
- Seankamsorn, A.; Cherdthong, A.; Wanapat, M.; Supapong, C.; Khonkhaeng, B.; Uriyapongson, S.; Gunun, N.; Gunun, P.; Chanjula, P. Effect of dried rumen digesta pellet levels on feed use, rumen ecology, and blood metabolite in swamp buffalo. Trop. Anim. Health Prod. 2017, 48, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Cherdthong, A.; Prachumchai, R.; Wanapat, M. In vitro evaluations of pellets containing Delonix regia seed meal for ruminants. Trop. Anim. Health Prod. 2019, 51, 2003–2010. [Google Scholar] [CrossRef]
- Kandasamy, S.; Dananjeyan, B.; Krishnamurthy, K.; Benckise, G. Aerobic cyanide degradation by bacterial isolates from cassava factory wastewater. Braz. J. Microbiol. 2015, 3, 659–666. [Google Scholar] [CrossRef]
- Moradkhani, M.; Yaghmaei, S.; Ghobadi Nejad, Z. Biodegradation of cyanide under alkaline conditions by a strain of pseudomonas putida isolated from gold mine soil and optimization of process variables through response surface methodology (RSM). Period. Polytech. Chem. 2018, 62, 265–273. [Google Scholar] [CrossRef]
- Kozaki, M.; Uchimura, T.; Okada, S. Experimental Manual for Lactic Acid Bacteria; Asakurasyoten: Tokyo, Japan, 1992. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1998; Volume 2. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Bradbury, J.H.; Egan, S.M.; Lynch, M.J. Analysis of cyanide in cassava using acid hydrolysis of cyanogenic glucosides. J. Sci. Food. Agric. 1991, 55, 277–290. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and gas production using rumen fluid. Anim. Res. Dev. 1998, 28, 7–55. [Google Scholar]
- Schofield, P. Gas production methods. Chapter 10. In Farm Animal Metabolism and Nutrition; D’Mello, J.P.F., Ed.; CABI Publishing: Ithaca, NY, USA, 2000; pp. 209–232. [Google Scholar]
- Samuel, M.; Sagathewan, S.; Thomas, J.; Mathen, G. An HPLC method for estimation of volatile fatty acids of ruminal fluid. Indian J. Anim. Sci. 1997, 67, 805–811. [Google Scholar]
- Galyean, M. Laboratory Procedure in Animal Nutrition Research; Department of Animal and Range Sciences, New Mexico State University: Las Cruces, NM, USA, 1989. [Google Scholar]
- Lambert, J.L.; Ramasamy, J.; Paukstelis, J.F. Stable reagents for the colorimetric determination of cyanide by modified Konig reactions. Anal. Chem. 1975, 47, 916–918. [Google Scholar] [CrossRef]
- Tilley, J.M.A.; Terry, R.A. A two-stage technique for the digestion of forage crops. J. Br. Grassl. Soc. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Statistical Analysis Systems. SAS/STAT User’s Guide: Version 6.12, 4th ed.; SAS Inc.: Cary, NC, USA, 1996. [Google Scholar]
- Razanamahandry, L.C.; Karoui, H.; Andrianisa, H.A.; Yacouba, H. Bioremediation of soil and water polluted by cyanide: A review. Afr. J. Environ. Sci. Technol. 2017, 11, 272–291. [Google Scholar]
- McMahon, J.M.; White, W.L.B.; Sayre, R.T. Cyanogenesis in cassava (Manihot esculanta Crantz). J. Exp. Bot. 1995, 46, 731–741. [Google Scholar] [CrossRef]
- Kang, S.M.; Kim, D.J. Degradation of cyanide by a bacterial mixture composed of new types of cyanide-degrading bacteria. Biotechnol. Lett. 1993, 15, 201–206. [Google Scholar] [CrossRef]
- Intanoo, M.; Kongkeitkajorn, M.B.; Pattarajinda, V.; Bernard, J.K.; Callaway, T.R.; Suriyasathaporn, W.; Phasuk, Y. Isolation and screening of aflatoxin-detoxifying yeast and bacteria from ruminal fluids to reduce aflatoxin B1contamination in dairy cattle feed. J. Appl. Microbiol. 2018, 125, 1603–1613. [Google Scholar] [CrossRef] [PubMed]
- Cherdthong, A.; Prachumchai, R.; Wanapat, M.; Foiklang, S.; Chanjula, P. Effects of supplementation with royal poinciana seed meal (Delonix regia) on ruminal fermentation pattern, microbial protein synthesis, blood metabolites and mitigation of methane emissions in native Thai beef cattle. Animals 2019, 9, 625. [Google Scholar] [CrossRef] [PubMed]

| Item | PELFUR 0 | PELFUR 15 | PELFUR 30 | FCR | Rice Straw |
|---|---|---|---|---|---|
| Ingredients, g/kg DM | |||||
| Cassava chips | 556 | 556 | 556 | - | |
| Soybean meal | 110 | 110 | 110 | - | |
| Rice bran | 110 | 100 | 105 | - | |
| Coconut meal | 89 | 89 | 70 | - | |
| Palm kernel meal | 78 | 73 | 72 | - | |
| Urea | 10 | 10 | 10 | - | |
| Salt | 10 | 10 | 10 | - | |
| sulfur powder | 0 | 15 | 30 | - | |
| Mineral premix | 10 | 10 | 10 | - | |
| Molasses, liquid | 27 | 27 | 27 | - | |
| Chemical composition | |||||
| DM, g/kg as basis | 946 | 943 | 941 | 351 | 925 |
| Organic matter, g/kg DM | 925 | 921 | 932 | 924 | 895 |
| Ash, g/kg DM | 75 | 79 | 68 | 76 | 105 |
| Crude protein, g/kg DM | 132 | 130 | 130 | 21 | 23 |
| Neutral detergent fiber, g/kg DM | 228 | 231 | 226 | 156 | 712 |
| Acid detergent fiber, g/kg DM | 104 | 106 | 104 | 89 | 442 |
| Cyanide, ppm | - | - | - | 106 | - |
| Item | FCR (g/kg) | PELFUR (g/kg) | Gas Production Parameters | Cumulative Gas (mL) | ||
|---|---|---|---|---|---|---|
| Vf | k | L | ||||
| 0 | 0 | 91.85 | 0.02 | 1.60 | 75.43 | |
| 0 | 15 | 115.80 | 0.02 | 1.45 | 94.16 | |
| 0 | 30 | 153.95 | 0.02 | 0.50 | 111.40 | |
| 260 | 0 | 139.30 | 0.02 | 1.95 | 114.82 | |
| 260 | 15 | 159.75 | 0.02 | 1.90 | 131.04 | |
| 260 | 30 | 156.75 | 0.02 | 1.60 | 129.49 | |
| 350 | 0 | 173.65 | 0.02 | 2.10 | 139.68 | |
| 350 | 15 | 169.50 | 0.02 | 1.70 | 142.94 | |
| 350 | 30 | 161.40 | 0.02 | 1.75 | 133.71 | |
| 440 | 0 | 134.45 | 0.03 | 1.70 | 124.40 | |
| 440 | 15 | 173.65 | 0.02 | 2.10 | 143.45 | |
| 440 | 30 | 183.50 | 0.02 | 1.75 | 150.62 | |
| 530 | 0 | 139.75 | 0.02 | 1.55 | 116.05 | |
| 530 | 15 | 193.70 | 0.02 | 1.80 | 161.09 | |
| 530 | 30 | 166.05 | 0.02 | 2.00 | 146.27 | |
| SEM | 13.19 | 0.01 | 0.95 | 9.08 | ||
| Main effects | ||||||
| FCR (g/kg) | 0 | 120.53 a | 0.02 | 1.18 a | 93.66 a | |
| 260 | 151.93 b | 0.02 | 1.82 b | 125.12 b | ||
| 350 | 168.18 b | 0.02 | 1.85 b | 138.78 bc | ||
| 440 | 163.87 b | 0.02 | 1.85 b | 139.49 bc | ||
| 530 | 166.50 b | 0.02 | 1.78 b | 141.14 c | ||
| PELFUR (g/kg) | 0 | 135.80 a | 0.02 | 1.78 | 114.07 a | |
| 15 | 162.48 b | 0.02 | 1.79 | 134.53 b | ||
| 30 | 164.33 b | 0.02 | 1.52 | 134.30 b | ||
| Significance of main effect and interaction | ||||||
| FCR | 0.01 | 0.64 | 0.01 | 0.01 | ||
| PELFUR | 0.01 | 0.82 | 0.16 | 0.01 | ||
| FCR × PELFUR | 0.15 | 0.99 | 0.15 | 0.29 | ||
| Item | FCR (g/kg) | PELFUR (g/kg) | pH | NH3-N (mg/dL) | Protozoa (×106 cell/mL) | Bacteria (×108 cell/mL) | Cyanide (ppm) |
|---|---|---|---|---|---|---|---|
| 0 | 0 | 6.91 | 16.86 | 7.00 | 9.50 | 0.00 a | |
| 0 | 15 | 6.89 | 14.96 | 6.00 | 11.00 | 0.00 a | |
| 0 | 30 | 6.90 | 15.21 | 6.00 | 11.50 | 0.00 a | |
| 260 | 0 | 6.89 | 15.71 | 6.00 | 10.50 | 0.26 f | |
| 260 | 15 | 6.86 | 11.51 | 6.00 | 11.50 | 0.18 c | |
| 260 | 30 | 6.87 | 12.91 | 5.00 | 12.00 | 0.15 b | |
| 350 | 0 | 6.87 | 14.31 | 7.00 | 11.00 | 0.34 k | |
| 350 | 15 | 6.86 | 12.21 | 7.00 | 12.00 | 0.24 e | |
| 350 | 30 | 6.85 | 12.21 | 7.00 | 12.50 | 0.21 d | |
| 440 | 0 | 6.85 | 15.71 | 6.00 | 11.50 | 0.43 l | |
| 440 | 15 | 6.83 | 12.96 | 9.00 | 12.50 | 0.29 h | |
| 440 | 30 | 6.83 | 12.41 | 8.00 | 12.50 | 0.27 g | |
| 530 | 0 | 6.82 | 17.26 | 8.50 | 12.00 | 0.51 m | |
| 530 | 15 | 6.83 | 16.01 | 8.00 | 13.00 | 0.32 j | |
| 530 | 30 | 6.83 | 15.01 | 9.00 | 13.00 | 0.31 i | |
| SEM | 0.01 | 0.53 | 1.85 | 0.73 | 0.003 | ||
| Main effects | |||||||
| FCR (g/kg) | 0 | 6.90 a | 15.68 a | 6.30 | 10.70 | 0.00 a | |
| 260 | 6.87 b | 13.38 b | 5.70 | 11.30 | 0.20 b | ||
| 350 | 6.86 c | 12.91 b | 7.00 | 11.80 | 0.26 c | ||
| 440 | 6.84 d | 13.69 b | 7.70 | 12.20 | 0.33 d | ||
| 530 | 6.83 d | 16.09 a | 8.50 | 12.70 | 0.38 e | ||
| PELFUR (g/kg) | 0 | 6.87 a | 15.97 a | 6.90 | 10.90 | 0.31 a | |
| 15 | 6.85 b | 13.53 b | 7.20 | 12.00 | 0.21 b | ||
| 30 | 6.86 b | 13.55 b | 7.00 | 12.30 | 0.19 c | ||
| Significance of main effect and interaction | |||||||
| FCR | 0.01 | 0.01 | 0.40 | 0.22 | 0.01 | ||
| PELFUR | 0.04 | 0.01 | 0.97 | 0.65 | 0.01 | ||
| FCR × PELFUR | 0.28 | 0.23 | 0.98 | 0.90 | 0.01 | ||
| Item | FCR (g/kg) | PELFUR (g/kg) | IVDMD (g/kg DM) | IVOMD (g/kg DM) | ||||
|---|---|---|---|---|---|---|---|---|
| 12 h | 24 h | Mean | 12 h | 24 h | Mean | |||
| 0 | 0 | 562.6 a | 598.8 a | 580.7 a | 600.8 a | 639.5 a | 620.2 a | |
| 0 | 15 | 569.5 a | 603.7 ab | 586.6 a | 612.7 ab | 647.3 ab | 630.0 ab | |
| 0 | 30 | 559.6 a | 613.9 bc | 586.7 a | 601.6 a | 652.2 bc | 626.9 ab | |
| 260 | 0 | 582.9 c | 611.7 abc | 597.3 b | 623.1 bc | 652.6 bc | 637.8 bc | |
| 260 | 15 | 601.7 dh | 621.7 c | 611.7 c | 644.6 de | 663.5 c | 654.1 de | |
| 260 | 30 | 597.2 d | 637.2 de | 617.2 cd | 637.9 d | 679.0 d | 658.5 ef | |
| 350 | 0 | 603.9 bdh | 616.2 bc | 610.1 c | 630.5 cd | 663.1 c | 646.8 cd | |
| 350 | 15 | 616.4 e | 649.3 ef | 632.9 e | 653.2 e | 689.6 de | 671.4 g | |
| 350 | 30 | 608.6 beh | 654.7 fg | 631.7 e | 644.0 de | 693.2 e | 668.6 fg | |
| 440 | 0 | 612.3 be | 635.7 d | 624.0 de | 637.8 d | 681.2 de | 659.5 ef | |
| 440 | 15 | 647.8 g | 668.5 h | 658.1 gh | 684.3 gh | 720.7 fg | 702.5 ij | |
| 440 | 30 | 629.0 f | 664.2 gh | 646.6 f | 668.4 f | 713.0 f | 690.7 h | |
| 530 | 0 | 617.5 e | 643.5 def | 630.5 e | 642.2 de | 689.3 de | 665.7 fg | |
| 530 | 15 | 651.7 g | 672.1 h | 661.9 h | 690.0 h | 725.9 g | 708.0 j | |
| 530 | 30 | 633.7 f | 665.9 gh | 649.8 fg | 672.4 fg | 720.4 fg | 696.4 hi | |
| SEM | 3.4 | 4.3 | 3.1 | 4.9 | 4.1 | 3.7 | ||
| Main effects | ||||||||
| FCR (g/kg) | 0 | 563.9 a | 605.4 a | 584.7 a | 605.0 a | 646.3 a | 625.7 a | |
| 260 | 593.9 b | 623.5 b | 608.7 b | 635.2 b | 665.0 b | 650.1 b | ||
| 350 | 609.6 c | 640.1 c | 624.9 c | 642.6 c | 682.0 c | 662.3 c | ||
| 440 | 629.7 d | 656.1 d | 642.9 d | 663.5 d | 705.0 d | 684.2 d | ||
| 530 | 634.3 d | 660.5 d | 647.4 d | 668.2 d | 711.9 d | 690.0 d | ||
| PELFUR (g/kg) | 0 | 605.6 a | 621.2 a | 608.5 a | 626.9 a | 665.1 a | 646.0 a | |
| 15 | 617.4 b | 643.0 b | 630.2 b | 657.0 b | 689.4 b | 673.2 b | ||
| 30 | 595.8 c | 647.2 b | 626.4 b | 644.9 c | 691.6 b | 668.2 b | ||
| Significance of main effect and interaction | ||||||||
| FCR | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | ||
| PELFUR | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | ||
| FCR × PELFUR | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | ||
| Item | FCR (g/kg) | PELFUR (g/kg) | Total VFAs (mmol/L) | Acetate C2 | Propionate C3 | Butyrate C4 | C2:C3 |
|---|---|---|---|---|---|---|---|
| (mol/100 mol) | |||||||
| 0 | 0 | 91.40 | 67.26 | 23.44 | 9.30 | 2.87 | |
| 0 | 15 | 91.60 | 67.41 | 23.37 | 9.22 | 2.89 | |
| 0 | 30 | 92.24 | 66.91 | 23.61 | 9.49 | 2.83 | |
| 260 | 0 | 93.07 | 66.19 | 24.82 | 8.98 | 2.67 | |
| 260 | 15 | 93.54 | 65.41 | 25.47 | 9.12 | 2.57 | |
| 260 | 30 | 94.83 | 65.06 | 25.49 | 9.45 | 2.55 | |
| 350 | 0 | 95.13 | 64.99 | 25.18 | 9.84 | 2.58 | |
| 350 | 15 | 98.08 | 64.78 | 25.70 | 9.52 | 2.52 | |
| 350 | 30 | 96.15 | 64.71 | 25.52 | 9.76 | 2.54 | |
| 440 | 0 | 99.42 | 64.56 | 25.64 | 9.81 | 2.52 | |
| 440 | 15 | 100.71 | 63.53 | 27.01 | 9.46 | 2.35 | |
| 440 | 30 | 103.88 | 63.89 | 26.67 | 9.43 | 2.40 | |
| 530 | 0 | 106.45 | 63.86 | 26.93 | 9.21 | 2.37 | |
| 530 | 15 | 108.82 | 63.48 | 28.27 | 8.25 | 2.25 | |
| 530 | 30 | 107.35 | 64.16 | 27.32 | 8.52 | 2.35 | |
| SEM | 2.53 | 0.33 | 0.34 | 0.32 | 0.04 | ||
| Main effects | |||||||
| FCR (g/kg) | 0 | 91.75 a | 67.19 a | 23.47 a | 9.33 b | 2.86 a | |
| 260 | 93.81 ab | 65.55 b | 25.26 b | 9.18 ab | 2.60 b | ||
| 350 | 96.45 b | 64.83 c | 25.47 b | 9.71 b | 2.55 b | ||
| 440 | 101.34 c | 63.99 d | 26.44 c | 9.57 b | 2.42 c | ||
| 530 | 107.54 d | 63.83 d | 27.51 d | 8.66 a | 2.32 d | ||
| PELFUR (g/kg) | 0 | 97.09 | 65.37 | 25.20 a | 9.43 | 2.60 a | |
| 15 | 98.55 | 64.92 | 25.97 b | 9.11 | 2.51 b | ||
| 30 | 98.89 | 64.95 | 25.72 b | 9.33 | 2.53 b | ||
| Significance of main effect and interaction | |||||||
| FCR | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | ||
| PELFUR | 0.51 | 0.09 | 0.01 | 0.32 | 0.01 | ||
| FCR × PELFUR | 0.98 | 0.41 | 0.48 | 0.74 | 0.45 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prachumchai, R.; Cherdthong, A.; Wanapat, M. Screening of Cyanide-Utilizing Bacteria from Rumen and In Vitro Evaluation of Fresh Cassava Root Utilization with Pellet Containing High Sulfur Diet. Vet. Sci. 2021, 8, 10. https://doi.org/10.3390/vetsci8010010
Prachumchai R, Cherdthong A, Wanapat M. Screening of Cyanide-Utilizing Bacteria from Rumen and In Vitro Evaluation of Fresh Cassava Root Utilization with Pellet Containing High Sulfur Diet. Veterinary Sciences. 2021; 8(1):10. https://doi.org/10.3390/vetsci8010010
Chicago/Turabian StylePrachumchai, Rittikeard, Anusorn Cherdthong, and Metha Wanapat. 2021. "Screening of Cyanide-Utilizing Bacteria from Rumen and In Vitro Evaluation of Fresh Cassava Root Utilization with Pellet Containing High Sulfur Diet" Veterinary Sciences 8, no. 1: 10. https://doi.org/10.3390/vetsci8010010
APA StylePrachumchai, R., Cherdthong, A., & Wanapat, M. (2021). Screening of Cyanide-Utilizing Bacteria from Rumen and In Vitro Evaluation of Fresh Cassava Root Utilization with Pellet Containing High Sulfur Diet. Veterinary Sciences, 8(1), 10. https://doi.org/10.3390/vetsci8010010






