Protective Effect of Hydroxytyrosol on LPS-Induced Inflammation and Oxidative Stress in Bovine Endometrial Epithelial Cell Line
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Treatment
2.3. Cell Viability Assay
2.4. ELISA
2.5. Reactive Oxygen Species (ROS) Evaluation
2.6. RNA Extraction-cDNA Synthesis
2.7. Real-Time PCR
2.8. Statistical Analysis
3. Results
3.1. HT Effect on BEND Cells Viability
3.2. HT Effect on LPS-Induced Inflammatory Response and Oxidative Stress in BEND Cells
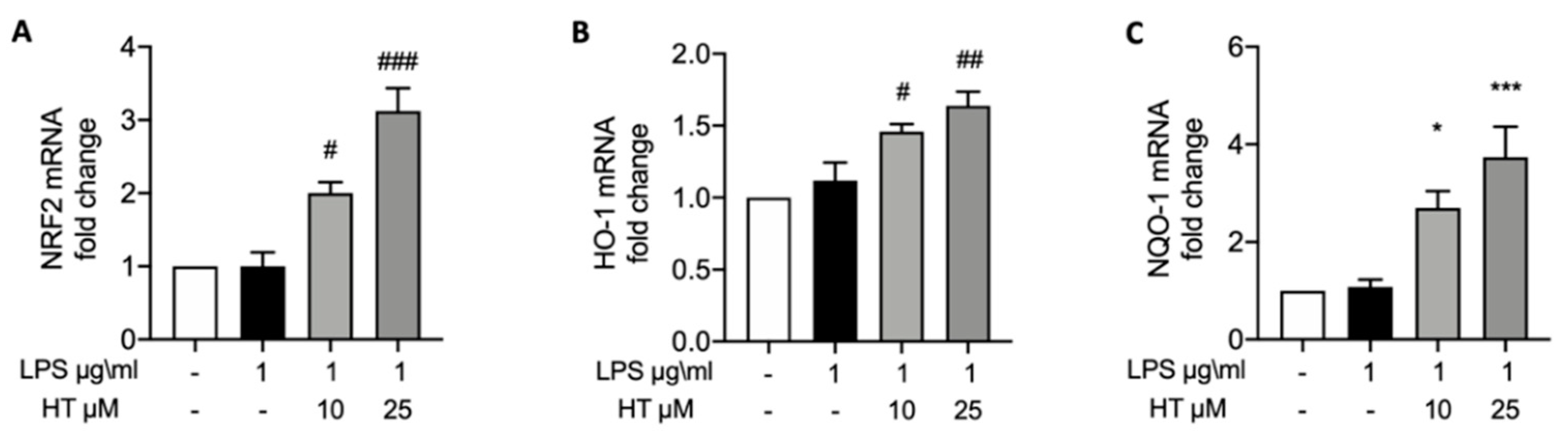
3.3. HT Effect on LPS-Induced Nrf2 Pathway
3.4. HT Effect on and Expressions of Tight Junction Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hansen, P.J.; Soto, P.; Natzke, R.P. Mastitis and fertility in cattle—Possible involvement of inflammation or immune activation in embryonic mortality. Am. J. Reprod. Immunol. 2004, 51, 294–301. [Google Scholar] [CrossRef]
- Siemieniuch, M.J.; Szostek, A.Z.; Gajos, K.; Kozdrowski, R.; Nowak, M.; Okuda, K. Type of Inflammation Differentially Affects Expression of Interleukin 1beta and 6, Tumor Necrosis Factor-alpha and Toll-Like Receptors in Subclinical Endometritis in Mares. PLoS ONE 2016, 11, e0154934. [Google Scholar] [CrossRef]
- Burdet, J.; Rubio, A.P.; Salazar, A.I.; Ribeiro, M.L.; Ibarra, C.; Franchi, A.M. Inflammation, infection and preterm birth. Curr. Pharm. Des. 2014, 20, 4741–4748. [Google Scholar] [CrossRef]
- LeBlanc, S.J.; Osawa, T.; Dubuc, J. Reproductive tract defense and disease in postpartum dairy cows. Theriogenology 2011, 76, 1610–1618. [Google Scholar] [CrossRef]
- Koyama, T.; Omori, R.; Koyama, K.; Matsui, Y.; Sugimoto, M. Optimization of diagnostic methods and criteria of endometritis for various postpartum days to evaluate infertility in dairy cows. Theriogenology 2018, 119, 225–232. [Google Scholar] [CrossRef]
- Roche, J.R.; Burke, C.R.; Crookenden, M.A.; Heiser, A.; Loor, J.L.; Meier, S.; Mitchell, M.D.; Phyn, C.V.C.; Turner, S.A. Fertility and the transition dairy cow. Reprod. Fertil. Dev. 2017, 30, 85–100. [Google Scholar] [CrossRef]
- Raheem, K.A. An insight into maternal recognition of pregnancy in mammalian species. J. Saudi Soc. Agric. Sci. 2017, 16, 1–6. [Google Scholar] [CrossRef]
- Herath, S.; Fischer, D.P.; Werling, D.; Williams, E.J.; Lilly, S.T.; Dobson, H.; Bryant, C.E.; Sheldon, I.M. Expression and function of Toll-like receptor 4 in the endometrial cells of the uterus. Endocrinology 2006, 147, 562–570. [Google Scholar] [CrossRef]
- Amjadi, F.; Salehi, E.; Mehdizadeh, M.; Aflatoonian, R. Role of the innate immunity in female reproductive tract. Adv. Biomed. Res. 2014, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Owens, S.E. Postpartum uterine infection and endometritis in dairy cattle. Anim. Reprod. 2018, 14, 622–629. [Google Scholar] [CrossRef]
- Dohmen, M.; Joop, K.; Sturk, A.; Bols, P.; Lohuis, J. Relationship between intra-uterine bacterial contamination, endotoxin levels and the development of endometritis in postpartum cows with dystocia or retained placenta. Theriogenology 2000, 54, 1019–1032. [Google Scholar] [CrossRef]
- Rosenfeld, Y.; Shai, Y. Lipopolysaccharide (Endotoxin)-host defense antibacterial peptides interactions: Role in bacterial resistance and prevention of sepsis. Biochim. Biophys. Acta 2006, 1758, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Harju, K.; Ojaniemi, M.; Rounioja, S.; Glumoff, V.; Paananen, R.; Vuolteenaho, R.; Hallman, M. Expression of toll-like receptor 4 and endotoxin responsiveness in mice during perinatal period. Pediatr. Res. 2005, 57, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Healy, L.L.; Cronin, J.G.; Sheldon, I.M. Endometrial cells sense and react to tissue damage during infection of the bovine endometrium via interleukin 1. Sci. Rep. 2014, 4, 7060. [Google Scholar] [CrossRef]
- Splichal, I.; Trebichavsky, I. Cytokines and other important inflammatory mediators in gestation and bacterial intraamniotic infections. Folia Microbiol. 2001, 46, 345–351. [Google Scholar] [CrossRef]
- Willi, J.; Kupfer, P.; Evequoz, D.; Fernandez, G.; Katz, A.; Leumann, C.; Polacek, N. Oxidative stress damages rRNA inside the ribosome and differentially affects the catalytic center. Nucleic Acids Res. 2018, 46, 1945–1957. [Google Scholar] [CrossRef]
- Blekas, G.; Vassilakis, C.; Harizanis, C.; Tsimidou, M.; Boskou, D.G. Biophenols in table olives. J. Agric. Food Chem. 2002, 50, 3688–3692. [Google Scholar] [CrossRef]
- Vilaplana-Perez, C.; Aunon, D.; Garcia-Flores, L.A.; Gil-Izquierdo, A. Hydroxytyrosol and potential uses in cardiovascular diseases, cancer, and AIDS. Front. Nutr. 2014, 1, 18. [Google Scholar] [CrossRef]
- Parkinson, L.; Cicerale, S. The Health Benefiting Mechanisms of Virgin Olive Oil Phenolic Compounds. Molecules 2016, 21, 1734. [Google Scholar] [CrossRef]
- Fuccelli, R.; Fabiani, R.; Rosignoli, P. Hydroxytyrosol Exerts Anti-Inflammatory and Anti-Oxidant Activities in a Mouse Model of Systemic Inflammation. Molecules 2018, 23, 3212. [Google Scholar] [CrossRef]
- Larussa, T.; Imeneo, M.; Luzza, F. Olive Tree Biophenols in Inflammatory Bowel Disease: When Bitter is Better. Int. J. Mol. Sci. 2019, 20, 1390. [Google Scholar] [CrossRef]
- Amini, A.; Liu, M.; Ahmad, Z. Understanding the link between antimicrobial properties of dietary olive phenolics and bacterial ATP synthase. Int. J. Biol. Macromol. 2017, 101, 153–164. [Google Scholar] [CrossRef]
- Furneri, P.M.; Piperno, A.; Sajia, A.; Bisignano, G. Antimycoplasmal activity of hydroxytyrosol. Antimicrob. Agents Chemother. 2004, 48, 4892–4894. [Google Scholar] [CrossRef] [PubMed]
- Belaqziz, M.; Tan, S.P.; El-Abbassi, A.; Kiai, H.; Hafidi, A.; O’Donovan, O.; McLoughlin, P. Assessment of the antioxidant and antibacterial activities of different olive processing wastewaters. PLoS ONE 2017, 12, e0182622. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Huelamo, M.; Rodriguez-Morato, J.; Boronat, A.; de la Torre, R. Modulation of Nrf2 by Olive Oil and Wine Polyphenols and Neuroprotection. Antioxidants 2017, 6, 73. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [PubMed]
- Swanson, A.M.; David, A.L. Animal models of fetal growth restriction: Considerations for translational medicine. Placenta 2015, 36, 623–630. [Google Scholar] [CrossRef]
- Bahr, A.; Wolf, E. Domestic animal models for biomedical research. Reprod. Domest. Anim. 2012, 47 (Suppl. 4), 59–71. [Google Scholar] [CrossRef]
- Cibelli, J.; Emborg, M.E.; Prockop, D.J.; Roberts, M.; Schatten, G.; Rao, M.; Harding, J.; Mirochnitchenko, O. Strategies for improving animal models for regenerative medicine. Cell Stem Cell 2013, 12, 271–274. [Google Scholar] [CrossRef]
- Bonney, E.A. Demystifying animal models of adverse pregnancy outcomes: Touching bench and bedside. Am. J. Reprod. Immunol. 2013, 69, 567–584. [Google Scholar] [CrossRef]
- Sharifi, T.; Ghayeb, Y. A computational study to identify the key residues of peroxisome proliferator-activated receptor gamma in the interactions with its antagonists. J. Biomol. Struct. Dyn. 2018, 36, 1822–1833. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.-C.; Zhang, C.; Jin, Q.; Wei, C.; Zhao, H.-B.; Zhang, X.-L.; You, W.; Liu, X.-M.; Liu, G.-F.; Liu, Y.-F. Protective effects of astaxanthin on lipopolysaccharide-induced inflammation in bovine endometrial epithelial cells. Biol. Reprod. 2020, 102, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.I.; Kim, T.G.; Lim, D.H.; Kim, S.B.; Park, S.M.; Hur, T.Y.; Ki, K.S.; Kwon, E.G.; Vijayakumar, M.; Kim, Y.J. Preparation of Nanoemulsions of Vitamin A and C by Microfluidization: Efficacy on the Expression Pattern of Milk-Specific Proteins in MAC-T Cells. Molecules 2019, 24, 2566. [Google Scholar] [CrossRef] [PubMed]
- Pallio, G.; Bitto, A.; Pizzino, G.; Galfo, F.; Irrera, N.; Squadrito, F.; Squadrito, G.; Pallio, S.; Anastasi, G.P.; Cutroneo, G.; et al. Adenosine Receptor Stimulation by Polydeoxyribonucleotide Improves Tissue Repair and Symptomology in Experimental Colitis. Front. Pharmacol. 2016, 7, 273. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, F.; Micali, A.; Rinaldi, M.; Irrera, N.; Marini, H.; Puzzolo, D.; Pisani, A.; Lorenzini, C.; Valenti, A.; Laura, R.; et al. Polydeoxyribonucleotide, an Adenosine-A2A Receptor Agonist, Preserves Blood Testis Barrier from Cadmium-Induced Injury. Front Pharmacol 2016, 7, 537. [Google Scholar] [CrossRef]
- Kim, Y.C.; Kim, B.G.; Lee, J.H. Thymosin beta10 expression driven by the human TERT promoter induces ovarian cancer-specific apoptosis through ROS production. PLoS ONE 2012, 7, e35399. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Bitto, A.; Pallio, G.; Mannino, F.; Arcoraci, V.; Aliquo, F.; Minutoli, L.; De Ponte, C.; D’Andrea, P.; et al. Cadmium-Induced Oxidative Stress Impairs Glycemic Control in Adolescents. Oxid. Med. Cell. Longev. 2017, 2017, 6341671. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.G.; Bromfield, J.J. Tolerance and Innate Immunity Shape the Development of Postpartum Uterine Disease and the Impact of Endometritis in Dairy Cattle. Annu. Rev. Anim. Biosci. 2019, 7, 361–384. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.J. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef]
- Swangchan-Uthai, T.; Lavender, C.R.; Cheng, Z.; Fouladi-Nashta, A.A.; Wathes, D.C. Time course of defense mechanisms in bovine endometrium in response to lipopolysaccharide. Biol. Reprod. 2012, 87, 135. [Google Scholar] [CrossRef]
- Castillo, C.; Hernandez, J.; Bravo, A.; Lopez-Alonso, M.; Pereira, V.; Benedito, J.L. Oxidative status during late pregnancy and early lactation in dairy cows. Vet. J. 2005, 169, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, L.; Zhang, C.; Cheng, X.; Zhang, Y.; Guo, Y.; Long, M.; Yang, S.; He, J. Palmitic Acid and beta-Hydroxybutyrate Induce Inflammatory Responses in Bovine Endometrial Cells by Activating Oxidative Stress-Mediated NF-kappaB Signaling. Molecules 2019, 24, 2421. [Google Scholar] [CrossRef]
- Mandhwani, R.; Bhardwaz, A.; Kumar, S.; Shivhare, M.; Aich, R. Insights into bovine endometritis with special reference to phytotherapy. Vet. World 2017, 10, 1529–1532. [Google Scholar] [CrossRef][Green Version]
- Sharma, P.; Srivastava, S.; Kumar, R.; Singh, V. Phytotherapy: An Alternative Low Cost Therapeutic Management of Endometritis in Dairy Animals: A Review. Int. J. Curr. Microbiol. App. Sci 2018, 7, 4581–4591. [Google Scholar]
- Bhardwaz, A.; Nema, S.; Mahour, S.; Chabbra, D.; Rajput, N.; Sudarshan, K. Effect of Garlic (Allium sativum) Extract on Recovery and Conception Rate in Infectious Repeat Breeder Crossbred Cows. Indian J. Vet. Sci. Biotechnol. 2018, 14, 60–63. [Google Scholar] [CrossRef]
- Rathaur, P.; Raja, W.; Ramteke, P.; John, S.A. Turmeric: The golden spice of life. Int. J. Pharm. Sci. Res. 2012, 3, 1987. [Google Scholar]
- Karkovic Markovic, A.; Toric, J.; Barbaric, M.; Jakobusic Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Fusco, R.; Cordaro, M.; Siracusa, R.; Peritore, A.F.; D’Amico, R.; Licata, P.; Crupi, R.; Gugliandolo, E. Effects of Hydroxytyrosol against Lipopolysaccharide-Induced Inflammation and Oxidative Stress in Bovine Mammary Epithelial Cells: A Natural Therapeutic Tool for Bovine Mastitis. Antioxidants 2020, 9, 693. [Google Scholar] [CrossRef]
- Basirico, L.; Morera, P.; Dipasquale, D.; Bernini, R.; Santi, L.; Romani, A.; Lacetera, N.; Bernabucci, U. (-)-Epigallocatechin-3-gallate and hydroxytyrosol improved antioxidative and anti-inflammatory responses in bovine mammary epithelial cells. Animal 2019, 13, 2847–2856. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Bond, D.R.; Jankowski, H.; Weidenhofer, J.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. The Olive Biophenols Oleuropein and Hydroxytyrosol Selectively Reduce Proliferation, Influence the Cell Cycle, and Induce Apoptosis in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1937. [Google Scholar] [CrossRef]
- Chase, C.; Kaushik, R.S. Mucosal Immune System of Cattle: All Immune Responses Begin Here. Vet Clin. N. Am. Food Anim. Pract. 2019, 35, 431–451. [Google Scholar] [CrossRef] [PubMed]

| LPS -/HT - | |
|---|---|
| Ctrl | 100 ± 0 |
| HT 10 μM | 98.6 ± 0.50 |
| HT 25 μM | 98.4± 0.67 |
| HT 50 μM | 97.8 ± 0.86 |
| HT 100 μM | 94.6 ± 1.20# |
| HT 250 μM | 93.6 ± 1.56## |
| LPS -/HT - | LPS +/HT- | LPS +/HT 10 μM | LPS +/HT 25 μM | |
|---|---|---|---|---|
| ROS | 16.6 ± 0.02 | 33.8 ± 1.68 *** | 22.4 ± 1.20 ## | 15.6 ± 1.07 ### |
| TNF-α | 252.8 ± 10.8 | 304.8 ± 7.41 ** | 241.8 ± 5.2 # | 237 ± 7.36 ### |
| IL-6 | 1.70 ± 0.1 | 4.30 ± 120.8 ** | 1.50 ± 0.11 ## | 1.32 ± 0.16 ### |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gugliandolo, E.; Fusco, R.; Licata, P.; Peritore, A.F.; D’amico, R.; Cordaro, M.; Siracusa, R.; Cuzzocrea, S.; Crupi, R. Protective Effect of Hydroxytyrosol on LPS-Induced Inflammation and Oxidative Stress in Bovine Endometrial Epithelial Cell Line. Vet. Sci. 2020, 7, 161. https://doi.org/10.3390/vetsci7040161
Gugliandolo E, Fusco R, Licata P, Peritore AF, D’amico R, Cordaro M, Siracusa R, Cuzzocrea S, Crupi R. Protective Effect of Hydroxytyrosol on LPS-Induced Inflammation and Oxidative Stress in Bovine Endometrial Epithelial Cell Line. Veterinary Sciences. 2020; 7(4):161. https://doi.org/10.3390/vetsci7040161
Chicago/Turabian StyleGugliandolo, Enrico, Roberta Fusco, Patrizia Licata, Alessio Filippo Peritore, Ramona D’amico, Marika Cordaro, Rosalba Siracusa, Salvatore Cuzzocrea, and Rosalia Crupi. 2020. "Protective Effect of Hydroxytyrosol on LPS-Induced Inflammation and Oxidative Stress in Bovine Endometrial Epithelial Cell Line" Veterinary Sciences 7, no. 4: 161. https://doi.org/10.3390/vetsci7040161
APA StyleGugliandolo, E., Fusco, R., Licata, P., Peritore, A. F., D’amico, R., Cordaro, M., Siracusa, R., Cuzzocrea, S., & Crupi, R. (2020). Protective Effect of Hydroxytyrosol on LPS-Induced Inflammation and Oxidative Stress in Bovine Endometrial Epithelial Cell Line. Veterinary Sciences, 7(4), 161. https://doi.org/10.3390/vetsci7040161










