Abstract
Sarcocystosis is considered one of the major parasitic diseases with a worldwide distribution. It is caused by the obligatory intracellular protozoan parasites of the genus Sarcocystis. Besides its public health issues, sarcocystosis results in significant economic losses due to its impact on productivity and milk yield. A wide range of final and intermediate hosts have been identified, including mammals, birds, and reptiles; however, few studies have investigated the contribution of camels to maintaining the epidemiological foci of the disease in countries such as Egypt. The present study was conducted to grossly and histopathologically identify the prevalence rate of Sarcocystis spp. in camels (N = 100) from the Aswan Governorate, Egypt. Furthermore, the major risk factors related to the development of sarcocystosis in camels were investigated. Samples from the diaphragm, cardiac muscle, esophagus, and testes of the slaughtered camels were collected. Interestingly, Sarcocystis was detected in 75% of the examined camels. Following the studied variable factors, camels aged 5 years or more were found to be at higher risk, with an infection rate of 87.7% (57 of 65) than those younger than 5 years. The infection rate was 81.4% (57 of 70) in males and 60% (18 of 30) in females. The esophagus was the most affected organ (49%), followed by the diaphragm (26%) and cardiac muscle (17%), whereas none of the testes samples were affected. Taken together, the present study demonstrates the high prevalence of Sarcocystis in the examined camels and suggests the importance of these animals in preserving the epidemiological foci of sarcocystosis in Egypt. Future research should map the circulating strains in Egypt and aim to raise public health awareness about the importance of sarcocystosis and other related zoonotic diseases.
1. Introduction
Sarcocystis spp. are intracellular protozoan parasites of the phylum Apicomplexa. They are considered one of the most common parasites with global distribution in humans and various animal species [,,]. The parasite can infect a variety of intermediate hosts, including mammals, birds, and reptiles, whereas carnivores act as the final hosts [,,,,,]. The final hosts contract the infection by ingestion of muscle cysts containing bradyzoites, whereas schizonts and merozoites are not infective for the final hosts []. Camels may serve as intermediate hosts and can develop an infection following the ingestion of sporulated oocysts excreted in feces from infected carnivores. In the camel gut, sporozoites excyst, divide rapidly in the gut wall, and then migrate to the skeletal and cardiac muscles to produce the distinctive sarcocyst. The life cycle then terminates when infective sarcocysts are ingested by the final host, which is typically a member of the Canidae family in the case of Sarcocystis cameli [,,]. Sporulated oocysts undergo asexual and sexual reproduction and are passed in the feces of final hosts []. Sarcocysts are located mainly in the skeletal and cardiac muscles, occasionally in the brain. The size of these cysts varies by species and ranges from a few millimeters to centimeters in length [].
Note that Sarcocystis spp. include macroscopic and microscopic species; however, macroscopic species are not important pathogenic agents when compared with microscopic ones, which comprise some zoonotic species. The microscopic species cannot be identified through routine meat inspection and therefore do not lead to carcass condemnation. However, the high prevalence of these microscopic forms has a high economic impact on animal production [,,]. The first study of Sarcocystis infection in camels (Camelus dromedarius) from Egypt was reported in 1910; the observed sarcocysts were less than 12 mm in length, 1 mm in width, and appeared to be white lines with thin or thick cyst walls []. Clearly, at least two morphologically different Sarcocystis were affecting the camels (thin- and thick-walled) [,,].
Humans acquire the infection through the ingestion of undercooked meat of animals contaminated with certain species of Sarcocystis []. Humans are the final hosts of Sarcocystis hominis and Sarcocystis suihominis, with cattle and pigs as intermediate hosts, respectively []. Given the predator–prey relationship of the parasite, sarcocystosis does not represent a serious health threat to humans who might serve as the dead-end hosts [,]. Hence, the disease is often asymptomatic in definitive hosts [,,]. In humans, the infection may lead to two different scenarios. In the first one, intestinal infection is usually caused by two species of coccidian parasites, namely, S. hominis and S. suihominis, developed through the consumption of raw infected beef and pork, respectively. The resulting symptoms may include nausea, vomiting, stomachache, diarrhea, and dyspnea. The second scenario is muscular involvement, which happens when humans serve as intermediate hosts. This presentation may be associated with muscle pain, transitory edema, and fever [,,]. However, no reports of the transmission of S. cameli to humans through the consumption of camel meat are available. Clinical manifestations of the acute form of sarcocystosis in animal intermediate hosts may include encephalitis, bleeding diathesis, and inflammation of the brain and spinal cord [,]. The disease may also result in death, premature delivery, or abortion in pregnant animals [,]. Meanwhile, mild and chronic sarcocystosis leads to a drop in body weight and fur count, as well as significant changes in animal behavior []. Collectively, the parasite can affect animal growth and weight gain; reduce meat quality and milk yield; and cause anorexia, fever, anemia, abortion, muscle weakness, and even death of the intermediate hosts [].
Importantly, mapping the epidemiology of these parasites is one of the key strategies for controlling this disease, and epidemiological data can inform efforts to intercept the life cycle. It is often very difficult to diagnose the acute stage of the disease in intermediate hosts. Various diagnostic methods are available for the detection of sarcocystosis, which include muscle squashing, pepsin digestion, and histological techniques []. Histopathological examination of samples offers many advantages in the detection of Sarcocystis in the major host groups [,,]. Furthermore, the exploration of various epidemiological variables and risk factors through field surveys appears to be critical to the implementation of effective intervention strategies besides raising public health awareness of the disease. Given the scarcity of information on sarcocystosis in camels in countries such as Egypt, the present study was undertaken to identify the prevalence of Sarcocystis infection in camels in the Aswan Governorate (Egypt) by examining histopathological changes and estimating the major variable risk factors potentially associated with the infection.
2. Materials and Methods
2.1. Ethics Statement
The study protocol was carefully reviewed and approved by the local guidance of Research, Publication, and Ethics of the Faculty of Veterinary Medicine, Mansoura University, Egypt, which complies with all the relevant Egyptian laws.
2.2. Sampling and Study Area
The present study included 100 camels. Animals were slaughtered in the abattoir of Daraw, Aswan Governorate (Egypt), from March to November 2019. The Aswan Governorate (Egypt) is the southernmost part of Upper Egypt (latitude, 24°5′20.1768″ N and longitude 32°53′59.3880″ E) and is located near the borders of Sudan. This province has a very strategic location for the importation of animals from other African countries.
2.3. Macroscopic Examination
Camels were admitted to Daraw abattoir for slaughter. The skeletal muscles of the fore and hind limbs, diaphragm, intercostal muscles, heart, tongue, esophagus, and masseter muscles were carefully inspected by the naked eye for the presence of Sarcocystis, as described elsewhere [].
2.4. Microscopic Examination
Microscopic examination was conducted as follows:
- (A)
- Direct examination: Small tissue samples (2 mm × 8 mm) from the cardiac muscle, esophagus, diaphragm, and testes were squashed between two glass slides and examined by light microscopy (×100). Later on, two preparations were made from each muscle sample and examined for the positivity of Sarcocystis, as described elsewhere [].
- (B)
- Digestion method: Tissue digestion was used for enabling the observation of bradyzoites in the tested organ samples. Seventy grams of each tissue sample was ground and digested in 1.5% HCL acid and 0.5% pepsin at 29 °C overnight. The digested samples were then filtered through a mesh and centrifuged at 1500 rpm for 10 min. Next, the supernatant fluid was discarded. The sediment was stained with Giemsa and examined microscopically for the detection of bradyzoites [].
- (C)
- Histopathological examination: Tissue specimens from positive cases were fixed in 10% neutral buffered formalin, dehydrated in graded alcohol series, cleared in xylene, embedded in paraffin, sliced into 5 μm thick sections, and mounted on slides. The slides were then stained with hematoxylin and eosin and were examined microscopically [].
2.5. Statistical Analysis
Statistical analysis was performed using the statistical software SPSS (Version 22, SPSS Inc., Chicago, MI, USA) for Windows, and chi-square (χ2) tests were used to evaluate the correlation between the occurrence of Sarcocystis spp. and major variable risk factors for infection (age, sex, and organ involved). A probability (p) value of <0.05 was considered to indicate statistical significance.
3. Results
3.1. Prevalence of Sarcocystis spp. by Macroscopic and Direct Examination Methods
Note that Sarcocystis could not be detected macroscopically during the postmortem examination of the slaughtered camels. Table 1 shows the prevalence of infection with Sarcocystis spp. and associated risk factors. The prevalence of microscopic Sarcocystis by direct examination in examined camels was 75% (75 of 100).

Table 1.
Prevalence of Sarcocystis infection in camels slaughtered at Aswan Governorate.
3.2. Prevalence of Sarcocystis spp. by the Tissue Digestion Method
The tissue digestion method was used to verify the results of the prevalence obtained by direct examination. Similarly to direct examination, Sarcocystis could be detected in 75% of examined camels using the tissue digestion method.
3.3. Occurrence of Sarcocystis spp. in Examined Camels and Major Risk Factors Associated with Infection
The age, sex, and infected organ of the animals were all significantly associated with Sarcocystis infection. In this regard, camels aged 5 years or older were found to be at higher risk of the infection (infection rate, 87.7% (57 of 65)) than those younger than 5 years (infection rate, 51.4% (18 of 35)). Male animals were more affected than female animals (81.4% (57of 70) versus 60% (18 of 30)). Our data showed that the esophagus was the most affected organ with an infection rate of 49%; the diaphragm and cardiac muscle were infected by 26% and 17%, respectively, whereas no sarcocysts were detected in testes samples (Table 1).
3.4. Histopathological Findings
Irregularly shaped Sarcocystis were observed within the muscle fibers of the oesophagus, diaphragm and heart (Figure 1A–C and Figures S1 and S2). Cysts contained several basophilic bradyzoites. Generally, there were no degenerative or inflammatory changes in the infected tissues; however, we observed the infiltration of inflammatory cells around a single sarcocyst in an esophageal muscle sample (Figure 1D).
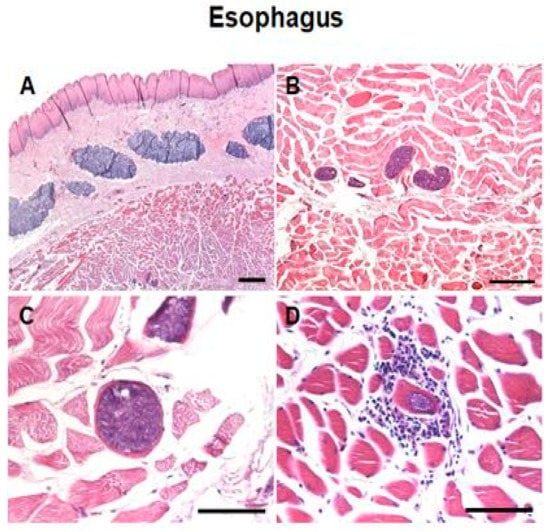
Figure 1.
Photomicrograph of Sarcocystis in the esophagus. Hematoxylin and eosin stain. Encysted parasites in the muscle fibers of the esophagus without (A–C) or with (D) inflammatory cells infiltration. Bar (A) = 500 µM. Bar (B) = 100 µM. Bars (C,D) = 50 µM.
4. Discussion
As previously mentioned, Sarcocystis spp. are a group of coccidian parasites that infect warm-blooded animals, including humans who might act as final or intermediate hosts [,]. The disease is mostly self-limiting in humans; however, several outbreaks of intestinal and muscular sarcocystosis were reported. A review of the available literature shows that approximately 150–200 species of Sarcocystis have been identified and described in a wide range of hosts based on parasite morphology; however, the exact number of species remains unidentified [,]. Importantly, Sarcocystis spp. are among the most common and widespread protozoan parasites of veterinary and economic importance [,].
The present study provides interesting data related to the occurrence of Sarcocystis spp. in camels from the Aswan Governorate, Egypt. To this end, our study revealed the major risk factors associated with the infection in these camels. We were unable to identify any cases of macroscopic Sarcocystis-associated pathology during the postmortem examination of slaughtered camels, which is in agreement with previous reports from Iran and Egypt [,,,]. Prevalence data on Sarcocystis species in camels have been reported in various countries, including Sudan, Jordan, Kazakhstan, Afghanistan, Morocco, the former Union of Soviet Socialist Republics [,], Egypt [], Somalia [], Saudi Arabia [], Iraq [], Southern Ethiopia [], and Mongolia [].
As shown in the present study, the prevalence of Sarcocystis infection in slaughtered camels in the Aswan Governorate using microscopic examination was 75%. The observed prevalence is higher than that reported in Egypt, Southern Ethiopia, and the Yazd Province (Iran), where the reported prevalence rates were 42.3–60.0%, 45.45%, and 51.5%, respectively [,,,]. Moreover, a couple of previous reports on Sarcocystis spp. in camels from Saudi Arabia, Afghanistan, and Morocco reported prevalence rates of 56.7%, 47.3–66.3%, and 60%, respectively [,,]. In a previous study in Jordan, the reported prevalence was 6.6%, which is markedly lower than that identified in the present study []. In stark contrast, higher infection rates of 100% and 91.6% were reported in Mongolia and Iraq, respectively [,]. Furthermore, in a previous study in Egypt, a prevalence of 81% was reported [], whereas a prevalence of 83.6% was reported in the eastern Provinces of Iran []. The variation between our present results and those previously mentioned could be attributed to various factors including the degree of contact between camels and dogs since some camel pastoralists are not using dogs in camel rearing (camels are reared on a free-range basis in the desert); therefore, differences in the systems used for camel keeping could influence the infection rate [,]. Meanwhile, a high prevalence (78.9%) of Sarcocystis infection was reported among slaughtered buffaloes in Beni-Suef, Egypt, and the authors attributed the high prevalence in this region to the close rearing of buffaloes with dogs, cats, and even wild animals that act as final hosts for these protozoa [].
In the present study, age was found to be a significant variable associated with infection. Older camels appeared to be at greater risk of acquiring infection than younger ones; hence, animals aged 5 years or older were infected to a significantly larger extent (87.7%) than younger animals (51.4%) χ2 = 15.956, p = 0.000). Similar findings were reported in previous studies in the Menofia Governorate (Egypt) [], Yazd Province (Iran) [], and in Riyadh city (Saudi Arabia) []. The higher prevalence of Sarcocystis infection in aged camels may likely reflect the higher rate of slaughtering of aged camels compared with younger animals; moreover, slow development of detectable cysts may explain the lower prevalence in young camels [,,]. Additionally, some owners kept the young camels indoor for breeding, and therefore, the young camels might be less exposed to infection than older ones [,].
In the present investigation, the sex of the animal was another significant risk factor, with males being at higher risk of infection than females (χ2 = 5.143, P = 0.023). As shown in Table 1, prevalence rates of 81.4% and 60% were reported in male and female camels, respectively. Our finding is consistent with several previous studies in Egypt [], southern Ethiopia [], and Iran []. This difference might be attributed to the fact that most female animals are kept indoor for reproduction under good and clean management, whereas most of the males are left for grazing outdoor and used by owners for hard work; they may therefore be more exposed to the infection [].
Sarcocystis spp. infect muscular tissue of the heart, tongue, esophagus, and diaphragm. However, Sarcocystis spp. cysts have been reported in several other types of muscle tissue [,]. Furthermore, some studies reported sarcocystosis in the cremaster muscle of an animal with orchitis, which observation encouraged us to investigate sarcocysts in testicular tissue samples [,]. In the present study, dissemination of Sarcocystis in different organs was observed especially in the esophagus, with a prevalence rate of 49% which is according to several previous reports either in the same species or different species [,,,,]. Meanwhile, some studies found the diaphragm of camels to be the most commonly affected site [,], whereas another study identified the heart as the most commonly infected organ []. However, in other species, such as the water buffalo, the predilection sites for Sarcocystis spp. appear to be the esophagus, tongue, and heart []. This difference could be explained by different S. cameli strains or differences in definite host species [].
Over the last few decades, extraordinary progress was made in developing the criteria for the identification and diagnosis of sarcocystosis in both intermediate and definitive hosts. Several methods were used to evaluate the sarcocyst and sporocyst morphology in their major intermediate hosts using light microscopy to identify the asexual stages in the infected tissues of intermediate hosts or the sexual stages in the gut of the definitive host. In this regard, the digestion method and histopathological analysis have been implemented for the analysis of microscopic sarcocysts in camels. Moreover, ultrastructural analysis, using transmission electron microscopy of the cyst wall appears very useful in the identification of sarcocysts []. Furthermore, several serological techniques, including enzyme-linked immunosorbent assay and an indirect fluorescent antibody test, were used for diagnosing infection. However, these methods are limited by low sensitivity and specificity due to cross-reactivity between various Sarcocystis spp. [,]. Additionally, the use of molecular methods, such as conventional polymerase chain reaction and restriction fragment length polymorphism, represents an essential alternative accurate technique for the identification of endogenous and exogenous stages of Sarcocystis spp. [,,]. However, these methods are typically more expensive compared with microscopic examination, and the lack of financial support remains among the major limitations of using such techniques in the field. Detection of the sporocysts of Sarcocystis spp. in the feces of definitive hosts could play an essential diagnostic role; however, based on morphological identification, the various species are very difficult to distinguish []. Sarcocysts may take years to grow in size to become macroscopically visible. This fact justifies our inability to detect macroscopic sarcocysts in our present study. On the one hand, microscopic evaluation of muscle tissue samples is important in the diagnosis of Sarcocystis infections in camels. On the other hand, detection of the parasite stages (sarcocysts) in tissues using histopathological methods provides a confirmatory tool for the diagnosis []. In the present study, histological examination revealed the presence of two morphologically distinct Sarcocystis embedded within the muscle fibers of the esophagus, diaphragm, and heart. Thin-walled Sarcocystis were found to be most common, which is consistent with some previous reports []. The morphology of Sarcocystis is unique as they are intramyofiber protozoal cysts with two types of cyst walls, a palisade-like thick wall or a smooth thin wall. As shown in Figure 1 and Figures S1 and S2, sarcocysts appear dark blue-colored due to the presence of many crescent-like bradyzoites inside the cysts.
No inflammatory reaction was observed in the tissue surrounding the cysts. The apparent lack of inflammatory response might be attributed to the fact that protozoa are located in cysts within the muscle fibers, which offers protection from host immunity—a hypothesis that has been confirmed for various parasites [,,,]. Our results are in line with the fact that inflammatory cells are not often reported in Sarcocystis-infected tissue [,,].
5. Conclusions
Current epidemiological and histopathological findings suggest a high occurrence of Sarcocystis in camels in this region of Egypt and indicate that camels may be critical to preserving the epidemiological foci of the disease. The present study also disclosed various major risk factors associated with infection, including animal age, sex, and anatomical predilection site. Further future molecular and epidemiological studies should focus on identifying the major circulating strains in Egyptian ecological niches. Strict hygienic measures may be critical to controlling the disease.
Supplementary Materials
The following are available online at https://www.mdpi.com/2306-7381/7/4/162/s1, Figure S1: Photomicrograph of Sarcocystis in the diaphragm. Encysted parasites in the muscle fibers of the diaphragm without inflammatory cells infiltration (A–D). Hematoxylin and eosin stain. Bar A = 100 µM. Bars B–D = 50 µM; Figure S2: Photomicrograph of Sarcocystis in the heart. Encysted parasite in the muscle fibers of the heart without inflammatory cells infiltration (A and B). Hematoxylin and eosin stain. Bar A = 100 µM. Bar B = 50 µM.
Author Contributions
A.G., M.S., A.A.S., F.A.E.-G., H.M.M.E.-S., R.H.M. and E.K.E. involved in the conception of the idea, methodology design, laboratory work, performed data analysis and interpretation. A.G., M.S. and E.K.E. contributed their scientific advice, prepared the manuscript for publication and revision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors also thank the veterinarians for their support and help in providing data and samples collection throughout the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fayer, R. Sarcocystis spp. in human infections. Clin. Microbiol. Rev. 2004, 17, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Fayer, R.; Esposito, D.H.; Dubey, J.P. Human infections with Sarcocystis species. Clin. Microbiol. Rev. 2015, 28, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.; Calero-Bernal, R.; Rosenthal, B.M.; Speer, C.A.; Fayer, R. Sarcocystosis of Animals and Humans; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Valinezhad, A.; Oryan, A.; Ahmadi, N. Sarcocystis and its complications in camels (Camelus dromedarius) of eastern provinces of Iran. Korean J. Parasitol. 2008, 46, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.A.; Rashid, M.H.; Vaughan, J.; Jabbar, A. Sarcocystosis in South American camelids: The state of play revisited. Parasit. Vectors 2018, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Speer, C.; Fayer, R. Sarcocystosis of Animals and Man; CRC Press, Inc.: Boca Raton, FL, USA, 1989. [Google Scholar]
- Dubey, J.; Hilali, M.; Van Wilpe, E.; Calero-Bernal, R.; Verma, S.K.; Abbas, I. A review of sarcocystosis in camels and redescription of Sarcocystis cameli and Sarcocystis ippeni sarcocysts from the one-humped camel (Camelus dromedarius). Parasitology 2015, 142. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Frenkel, J.K. Recognition of Cyclic Transmission of Sarcocystis muris by Cats. J. Infect. Dis. 1976, 133, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Hilali, M.; Mohamed, A. The dog (Canis familiaris) as the final host of Sarcocystis cameli (Mason, 1910). Tropenmedizin Parasit. 1980, 31, 213–214. [Google Scholar]
- Kuraev, G.T. Sarcocystosis of camel, Kzakhstan Sr. Vet. Moscow 1981, 7, 41. [Google Scholar]
- Niilo, L. Helminths, Arthropods and Protozoa of Domesticated Animals (Sixth Edition of Mönnig’s Veterinary Helminthology and Entomology). Can. Vet. J. 1969, 10, 223. [Google Scholar]
- Oryan, A.; Moghaddar, N.; Gaur, S.N. The distribution pattern of Sarcocystis species, their transmission and pathogenesis in sheep in Fars Province of Iran. Vet. Res. Commun. 1996, 20, 243–253. [Google Scholar] [CrossRef]
- Savini, G.; Dunsmore, J.D.; Robertson, I.D.; Seneviratna, P. Sarcocystis spp in Western Australian sheep. Aust. Vet. J. 1993, 70, 152–154. [Google Scholar] [CrossRef]
- Mason, F.E. Sarcocystis in the camel in Egypt. J. Comp. Pathol. Ther. Edinb. 1910, 23, 168–176. [Google Scholar] [CrossRef]
- Fatani, A.; Hilali, M.; al-Atiya, S.; al-Shami, S. Prevalence of Sarcocystis in camels (Camelus dromedarius) from Al-Ahsa, Saudi Arabia. Vet. Parasitol. 1996, 62, 241–245. [Google Scholar] [CrossRef]
- Odening, K. The present state of species-systematics in Sarcocystis Lankester, 1882 (Protista, Sporozoa, Coccidia). Syst. Parasitol. 1998, 41, 209–233. [Google Scholar] [CrossRef]
- Boid, R.; Jones, T.; Luckins, A. 3. Protozoal diseases of camels. Br. Vet. J. 1985, 141, 87–105. [Google Scholar] [CrossRef]
- Rommel, M. Sarcocystosis of domestic animals and humans. In Pract. 1985, 7, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Shaapan, R.M. The common zoonotic protozoal diseases causing abortion. J. Parasit. Dis. 2016, 40, 1116–1129. [Google Scholar] [CrossRef] [PubMed]
- Stojecki, K.; Karamon, J.; Sroka, J.; Cencek, T. Molecular diagnostics of Sarcocystis spp. infections. Pol. J. Vet. Sci. 2012, 15, 589–596. [Google Scholar] [CrossRef]
- Beaver, P.; Jung, R.C.; Cupp, E.W. Clinical Parasitology. 9th Edition. J. Med Entomol. 1984, 21, 136. [Google Scholar] [CrossRef]
- Frenkel, J.K. Sarcocystosis. In Pathology of Infectious Diseases; Conner, D.H., Chandler, F.W., Schwartz, D.A., Manz, H.J., Lack, E.E., Eds.; Appleton and Lange: Stamford, CT, USA, 1997; Volume 1997, pp. 1253–1259. [Google Scholar]
- Jehle, C.; Dinkel, A.; Sander, A.; Morent, M.; Romig, T.; Luc, P.V.; De, T.V.; Thai, V.V.; Mackenstedt, U. Diagnosis of Sarcocystis spp. in cattle (Bos taurus) and water buffalo (Bubalus bubalis) in Northern Vietnam. Vet. Parasitol. 2009, 166, 314–320. [Google Scholar] [CrossRef][Green Version]
- Odening, K.; Stolte, M.; Bockhardt, I. On the diagnostics of Sarcocystis in cattle: Sarcocysts of a species unusual for Bos taurus in a dwarf zebu. Vet. Parasitol. 1996, 66, 19–24. [Google Scholar] [CrossRef]
- Beyazıt, A.; Yazıcıoğlu, Ö.; Karaer, Z. The prevalence of ovine Sarcocystis species in Izmir province. Vet. Fak. Derg. 2007, 54, 111–116. [Google Scholar]
- Wahba, A.; Ayoub, M.; Soliman, K. Light and ultrastructure of Sarcocystis spp. of camels and associated pathological changes. Anim. Health Res. J. 2014, 2, 143–158. [Google Scholar]
- Fukuyo, M.; Battsetseg, G.; Byambaa, B. Prevalence of Sarcocystis infection in meat-producing animals in Mongolia. Southeast Asian J. Trop. Med. Public Health 2002, 33, 490–495. [Google Scholar] [PubMed]
- Whaeeb, S.T.; Faraj, A.A. Molecular identification and phylogeny of microscopic Sarcocystis Sheep in Baghdad province. Int. J. Adv. Res. Biol. Sci. 2016, 3, 50–56. [Google Scholar]
- Arafa, M.; Monib, M.; Dyab, A.; Abdel-Ghaffar, S. Studies on ocular Sarcocystis in buffaloes in assiut governorate. Ass. Univ. Bull. Environ. Res. 2003, 6, 27–35. [Google Scholar]
- Pozio, E. Epidemiology and control prospects of foodborne parasitic zoonoses in the European Union. Parassitologia 2008, 50, 17–24. [Google Scholar]
- Spickler, A.R. Sarcocystosis. Available online: http://www.cfsph.iastate.edu/Factsheets/pdfs/sarcocystosis.pdf. (accessed on 21 August 2020).
- Tenter, A.M. Current research on Sarcocystis species of domestic animals. Int. J. Parasitol. 1995, 25, 1311–1330. [Google Scholar] [CrossRef]
- Hamidinejat, H.; Hekmatimoghaddam, S.; Jafari, H.; Sazmand, A.; Haddad Molayan, P.; Derakhshan, L.; Mirabdollahi, S. Prevalence and distribution patterns of Sarcocystis in camels (Camelus dromedarius) in Yazd province, Iran. J. Parasit. Dis. 2013, 37, 163–165. [Google Scholar] [CrossRef]
- Mandour, A.M.; Rabie, S.A.; Mohammed, N.I.; Hussein, N.M. On the presence of Sarcocystis miescheri sp. nov. in camels of Qena Governorate. Egypt. Acad. J. Biol. Sci. E. Med. Entomol. Parasitol. 2011, 3, 1–7. [Google Scholar] [CrossRef]
- Abdel Ghaffar, F.; Entzeroth, R.; Chobotar, B.; Scholtyseck, E. Ultastructural studies of Sarcocystis sp. from the camel (Camelus dromedarius) in Egypt. Tropenmed. Parasitol. 1979, 30, 434–438. [Google Scholar] [PubMed]
- Borrow Hagi, A.; Mohammed Hassan, A.; di Sacco, B. Sarcocystis in Somali camel. Parassitologia 1989, 31, 133–136. [Google Scholar]
- Latif, B.M.; Al-Delemi, J.K.; Mohammed, B.S.; Al-Bayati, S.M.; Al-Amiry, A.M. Prevalence of Sarcocystis spp. in meat-producing animals in Iraq. Vet. Parasitol. 1999, 84, 85–90. [Google Scholar] [CrossRef]
- Woldemeskel, M.; Gumi, B. Prevalence of Sarcocysts in one-humped camel (Camelus dromedarius) from southern Ethiopia. J. Vet. Med. B Infect. Dis Vet. Public Health 2001, 48, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghaffar, F.; Mehlhorn, H.; Bashtar, A.-R.; Al-Rasheid, K.; Sakran, T.; El-Fayoumi, H. Life cycle of Sarcocystis camelicanis infecting the camel (Camelus dromedarius) and the dog (Canis familiaris), light and electron microscopic study. Parasitol. Res. 2009, 106, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Hussein, S.H. The prevalence of Sarcocystis infection in Saudi Arabian Najdi sheep and camels. Biol. Sci. 1991, 1, 43–56. [Google Scholar]
- Kirmse, P.; Mohanbabu, B. Sarcocystis sp. in the one-humped camel (Camelus dromedarius) from Afghanistan. Br. Vet. J. 1986, 142, 73–74. [Google Scholar] [CrossRef]
- Kirmse, P. Sarcosporidiosis in equines of Morocco. Br. Vet. J. 1986, 142, 70–72. [Google Scholar] [CrossRef]
- Al-Ani, F.K.; Amr, Z. Sarcocystis spp Prevalence in Camel Meat in Jordan. Dairy Vet. Sci. J. 2017, 4, 1–3. [Google Scholar]
- El-Etreby, M. Myocardial sarcosporidiosis in the camel. Pathol. Vet. 1970, 7, 7–11. [Google Scholar] [CrossRef]
- El-Dakhly, K.M.; El-Nesr, K.A.; El-Nahass el, S.; Hirata, A.; Sakai, H.; Yanai, T. Prevalence and distribution patterns of Sarcocystis spp. in buffaloes in Beni-Suef, Egypt. Trop. Anim. Health Prod. 2011, 43, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- El-Bahy, N.; El-Bagory, A.-E.-R.; AbouLaila, M.; Elkhatam, A.; Mady, H.M. Prevalence of Sarcocystis fusiformis and Hydatid cyst among Different Ruminants at Menofia Governorate, Egypt. J. Curr. Vet. Res. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Omer, S.A.; Alzuraiq, A.A.; Mohammed, O.B. Prevalence and molecular detection of Sarcocystis spp. infection in the dromedary camel (Camelus dromedarius) in Riyadh city, Saudi Arabia. Biomed. Res. 2017, 28, 4962–4965. [Google Scholar]
- Romero, S.; Carletti, T.; Decker Franco, C.; More, G.; Schnittger, L.; Florin-Christensen, M. Seropositivity to Sarcocystis infection of llamas correlates with breeding practices. Vet. Parasitol. Reg. Stud. Rep. 2017, 10, 65–70. [Google Scholar] [CrossRef]
- Bucca, M.; Brianti, E.; Giuffrida, A.; Ziino, G.; Cicciari, S.; Panebianco, A. Prevalence and distribution of Sarcocystis spp. cysts in several muscles of cattle slaughtered in Sicily, Southern Italy. Food Control 2011, 22, 105–108. [Google Scholar] [CrossRef]
- Ono, M.; Ohsumi, T. Prevalence of Sarcocystis spp. cysts in Japanese and imported beef (Loin: Musculus longissimus). Parasitol. Int. 1999, 48, 91–94. [Google Scholar] [CrossRef]
- Saglam, K.; Keles, H. Sarcocystosis in the Cremaster Muscle of an Infertile Bull, Spermiostasis and Orchitis. Kocatepe Vet. J. 2016, 9, 252–254. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Elshraway, N.T.; Youssef, A.I. Survey on Sarcocystis in bovine carcasses slaughtered at the municipal abattoir of El-Kharga, Egypt. Vet. World 2016, 9, 1461–1465. [Google Scholar] [CrossRef]
- Shekarforoush, S.S.; Shakerian, A.; Hasanpoor, M.M. Prevalence of Sarcocystis in slaughtered one-humped camels (Camelus dromedarius) in Iran. Trop. Anim. Health Prod. 2006, 38, 301–303. [Google Scholar] [CrossRef]
- Oryan, A.; Ahmadi, N.; Mousavi, S.M. Prevalence, biology, and distribution pattern of Sarcocystis infection in water buffalo (Bubalus bubalis) in Iran. Trop. Anim. Health Prod. 2010, 42, 1513–1518. [Google Scholar] [CrossRef]
- Metwally, D.M.; Al-Otaibi, T.T.; Al-Turaiki, I.M.; El-Khadragy, M.F.; Alajmi, R.A. Identification of Sarcocystis Spp. in One-humped Camels (Camelus dromedarius) from Riyadh and Dammam, Saudi Arabia, via Histological and Phylogenetic Approaches. Animals 2020, 10, 1108. [Google Scholar] [CrossRef] [PubMed]
- More, G.; Pardini, L.; Basso, W.; Marin, R.; Bacigalupe, D.; Auad, G.; Venturini, L.; Venturini, M.C. Seroprevalence of Neospora caninum, Toxoplasma gondii and Sarcocystis sp. in llamas (Lama glama) from Jujuy, Argentina. Vet. Parasitol. 2008, 155, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Castro-Forero, S.; Bulla-Castañeda, D.; Buitrago, H.; Díaz Anaya, A.; Madeira de Carvalho, L.; Pulido-Medellin, M. Sarcocystis spp., a parasite with zoonotic potential. Bulg. J. Vet. Med. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Beaver, P.C.; Gadgil, K.; Morera, P. Sarcocystis in man: A review and report of five cases. Am. J. Trop. Med. Hyg. 1979, 28, 819–844. [Google Scholar] [CrossRef]
- Nance, J.P.; Vannella, K.M.; Worth, D.; David, C.; Carter, D.; Noor, S.; Hubeau, C.; Fitz, L.; Lane, T.E.; Wynn, T.A.; et al. Chitinase dependent control of protozoan cyst burden in the brain. PLoS Pathog. 2012, 8, e1002990. [Google Scholar] [CrossRef]
- Guerrant, R.L.; Walker, D.H.; Weller, P.F. Tropical Infectious Diseases: Principles, Pathogens and Practice, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2011; p. 24. [Google Scholar]
- Vangeel, L. Bovine Sarcocystis Species and Their Role in Bovine Eosinophilic Myositis. Ph.D. Thesis, Ghent University, Ghend, Belgium, 2012. [Google Scholar]
- Hidron, A.; Vogenthaler, N.; Santos-Preciado, J.I.; Rodriguez-Morales, A.J.; Franco-Paredes, C.; Rassi, A., Jr. Cardiac involvement with parasitic infections. Clin. Microbiol. Rev. 2010, 23, 324–349. [Google Scholar] [CrossRef]
- Arness, M.K.; Brown, J.D.; Dubey, J.P.; Neafie, R.C.; Granstrom, D.E. An outbreak of acute eosinophilic myositis attributed to human Sarcocystis parasitism. Am. J. Trop. Med. Hyg. 1999, 61, 548–553. [Google Scholar] [CrossRef]
- McLeod, R.; Hirabayashi, R.N.; Rothman, W.; Remington, J.S. Necrotizing vasculitis and Sarcocystis: A cause-and-effect relationship? South. Med. J. 1980, 73, 1380–1383. [Google Scholar] [CrossRef]
- Italiano, C.M.; Wong, K.T.; AbuBakar, S.; Lau, Y.L.; Ramli, N.; Syed Omar, S.F.; Kahar Bador, M.; Tan, C.T. Sarcocystis nesbitti causes acute, relapsing febrile myositis with a high attack rate: Description of a large outbreak of muscular sarcocystosis in Pangkor Island, Malaysia, 2012. PLoS Negl. Trop. Dis. 2014, 8, e2876. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).