Veterinary Diagnostic Approach of Common Virus Diseases in Adult Honeybees
Abstract
1. Introduction
2. Virus Diseases in Honeybees and Contributing Factors
2.1. Acute Bee Paralysis Virus—Kashmir Bee Virus—Israeli Acute Paralysis Virus—Complex
2.2. Chronic Bee Paralysis Virus
2.3. Deformed Wing Virus
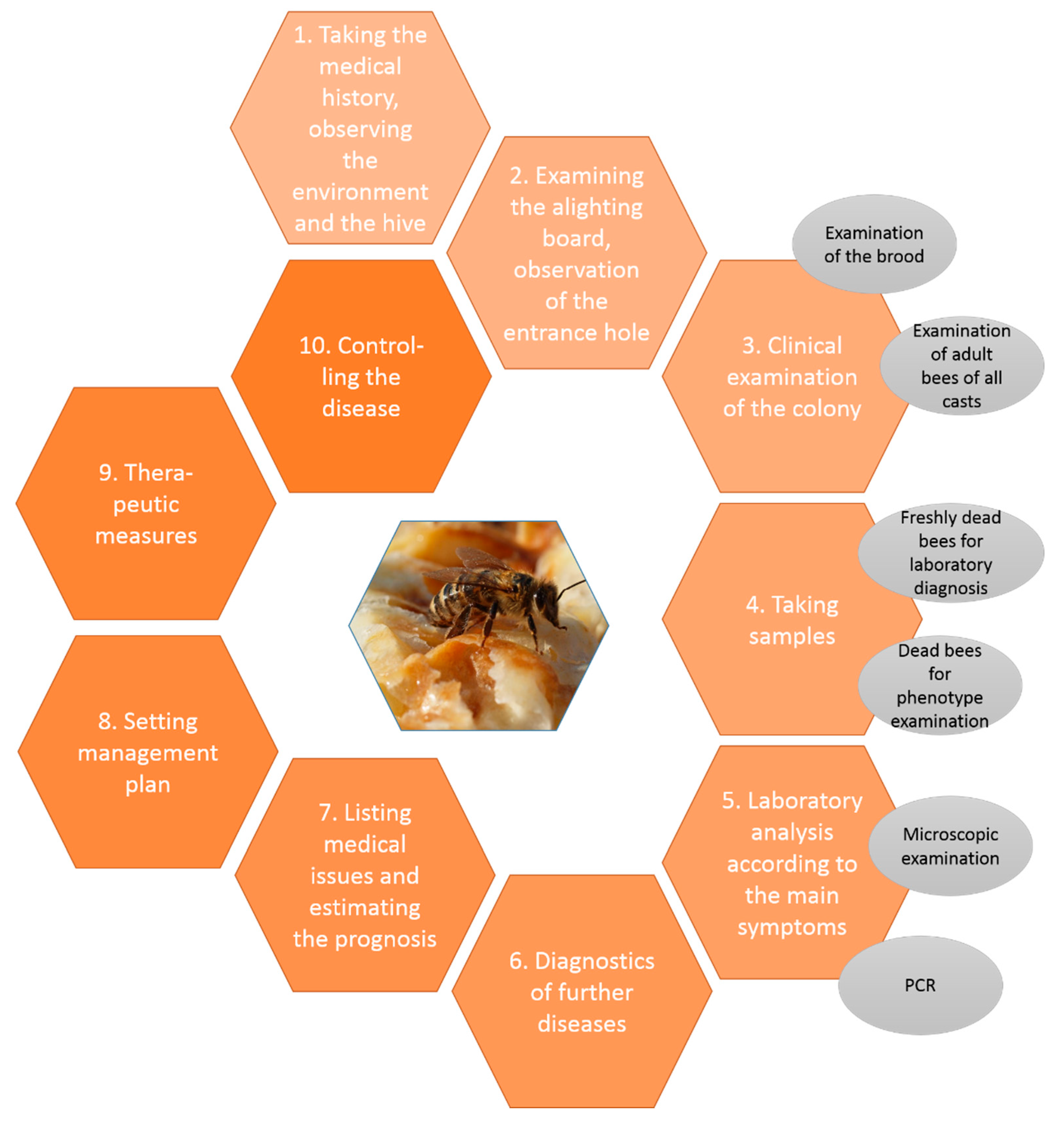
3. Veterinary Diagnostic Approach
3.1. Medical History, Appeareance of the Hive and Environment
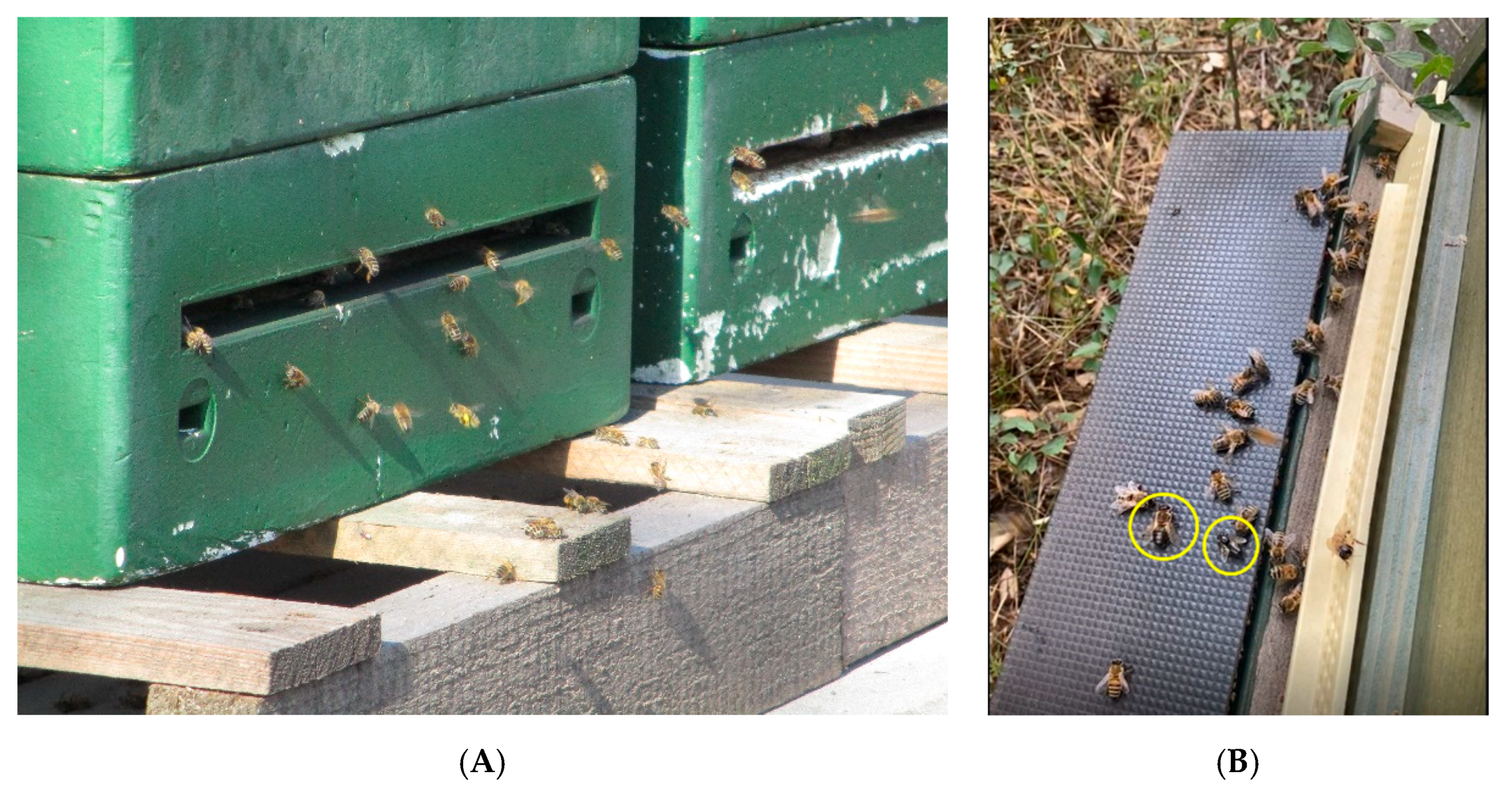
3.2. Examination of the Alighting Board and Observation of the Entrance Hole
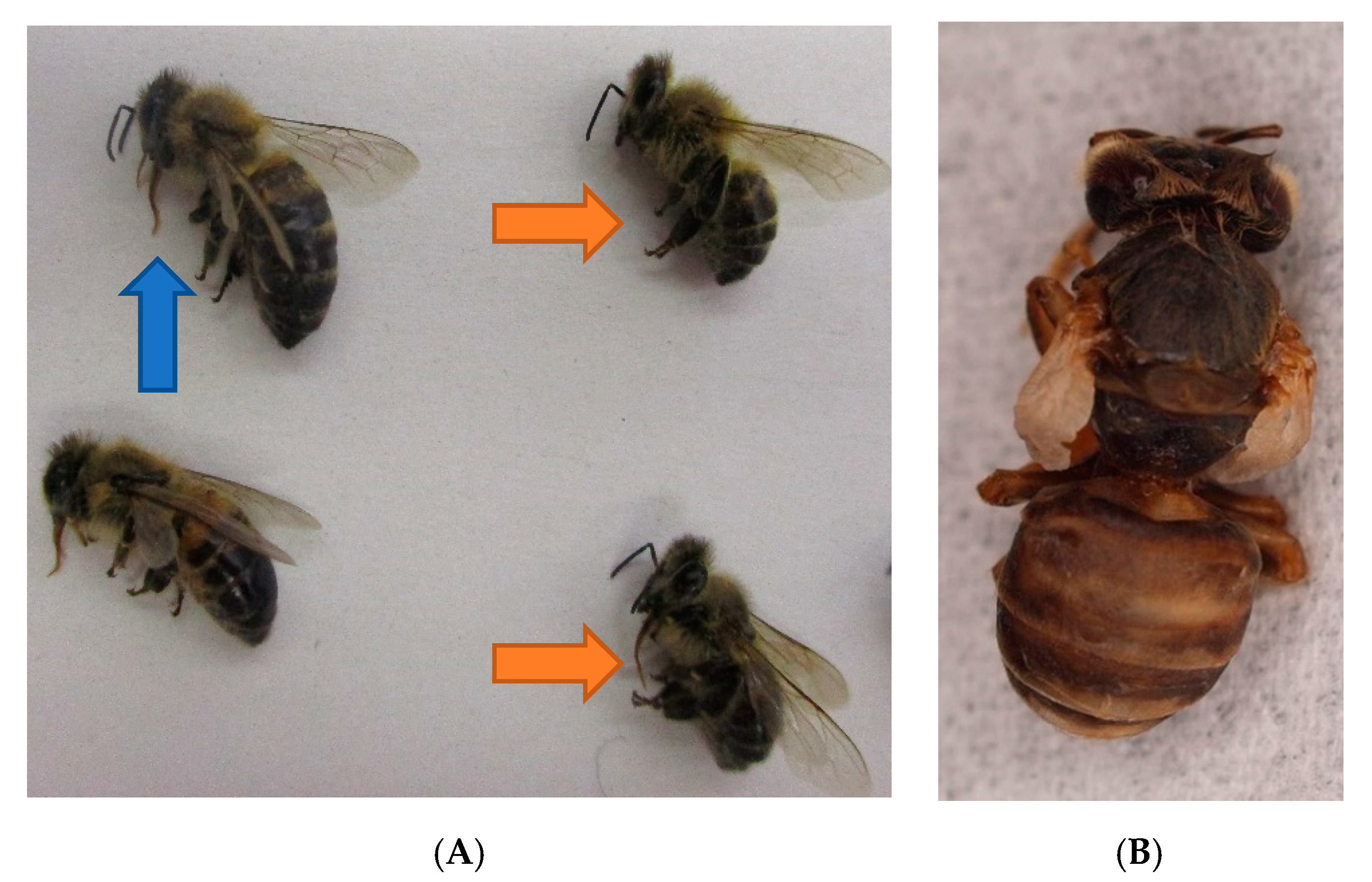
3.3. Clinical Examination of the Superorganism “Honeybee Colony” and Observations of Living Bees
3.4. Taking Samples for Laboratory Analysis
3.4.1. Examination of Dead Bees
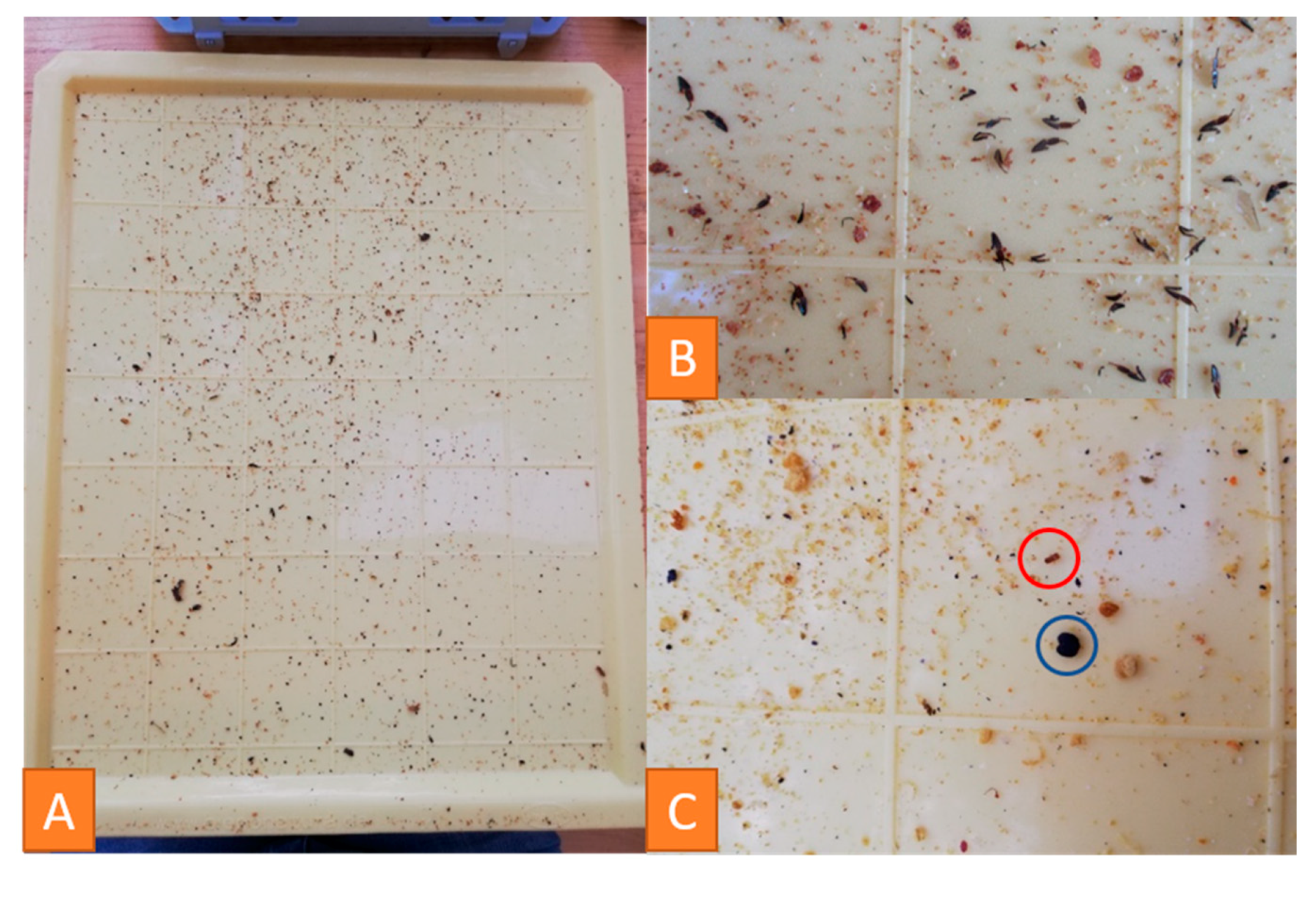
3.4.2. Examination of Debris
3.5. Laboratory Diagnosis
3.5.1. PCR to Detect Viral Diseases
3.5.2. Monitoring the Varroa infestation
3.5.3. Detection of Nosemosis
3.5.4. Intoxications
3.6. List of Medical Issues, Diagnoses and Prognosis
3.7. Outcome Control
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Iatridou, D.; Pohl, L.; Tlak Gajger, I.; De Briyne, N.; Bravo, A.; Saunders, J. Mapping the teaching of honeybee veterinary medicine in the European Union and European Free Trade Area. Vet. Rec. Open 2019, 6. [Google Scholar] [CrossRef]
- Available online: https://deutscherimkerbund.de/163-Bienen_Bestaeubung_Zahlen_die_zaehlen (accessed on 13 September 2020).
- Bundestierärztekammer, E.I.A. Statistik 2014: Tierärzteschaft in der Bundesrepublik Deutschland. DTÄB 2015, 5, 670–683. [Google Scholar]
- Bundestierärztekammer, E.I.A. Statistik 2019: Tierärzteschaft in der Bundesrepublik Deutschland. DTÄB 2020, 68, 860–870. [Google Scholar]
- Ritter, W. Bee diseases are a worldwide problem. Forum OiE 2014, 2, 5. [Google Scholar]
- Potts, S.G.; Roberts, S.P.M.; Dean, R.; Marris, G.; Brown, M.A.; Jones, R.; Neumann, P.; Settele, J. Declines of managed honey bees and beekeepers in Europe. J. Apic. Res. 2010, 49, 15–22. [Google Scholar] [CrossRef]
- Vidal-Naquet, N. Honeybee Veterinary Medicine: Apis mellifera L., 1st ed.; 5m Publisher: Sheffield, UK, 2015; pp. 71–75. [Google Scholar]
- Aupperle, H.; Genersch, E. Diagnostic Colour Atlas of Bee Pathology, 1st ed.; Publisher Verlag Laboklin: Bad Kissingen, Germany, 2016; p. 173. [Google Scholar]
- Available online: https://www.europarl.europa.eu/sides/getDoc.do?reference=P6-TA-2008-0567&type=TA&language=EN&redirect (accessed on 12 September 2020).
- Formato, G.; Smulders, F.J.M. Risk management in primary apicultural production. Part 1: Bee health and disease prevention and associated best practices. Vet. Q. 2011, 31, 29–47. [Google Scholar] [CrossRef]
- Available online: https://ec.europa.eu/food/animals/live_animals/bees/health_en (accessed on 12 September 2020).
- Belsky, J.; Joshi, N.K. Impact of Biotic and Abiotic Stressors on Managed and Feral Bees. Insects 2019, 10, 233. [Google Scholar] [CrossRef]
- McMenamin, A.J.; Brutscher, L.M.; Glenny, W.; Flenniken, M.J. Abiotic and biotic factors affecting the replication and pathogenicity of bee viruses. Curr. Opin. Insect Sci. 2016, 16, 14–21. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Chen, Y.; Huang, E.; Huang, M.H. The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker honey bees (Apis mellifera L.). J. Insect Pathol. 2010, 56, 1184–1191. [Google Scholar] [CrossRef]
- Chen, Y.P.; Siede, R. Honey bee viruses. Adv. Virus Res. 2007, 70, 33–80. [Google Scholar]
- Genersch, E.; Aubert, M. Emerging and re-emerging viruses of the honey bee (Apis mellifera L.). Vet. Res. 2010, 41, 54. [Google Scholar] [CrossRef] [PubMed]
- Brutscher, L.M.; Daughenbaugh, K.F.; Flenniken, M.L. Antiviral defense mechanisms in honey bees. Curr. Opin. Insect Sci. 2015, 10, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Gisder, S.; Genersch, E. Special Issue: Honey Bee Viruses. Viruses 2015, 7, 5603–5608. [Google Scholar] [CrossRef]
- Vidal-Naquet, N. Les maladies de l´abeille domestique d´élevage Apis mellifera L. Bull. Acad. Vét. Fr. 2012, 165, 307–316. [Google Scholar] [CrossRef][Green Version]
- Hou, C.; Chejanovsky, N. Acute paralysis viruses of the honey bee. Virol. Sin. 2014, 29, 324–326. [Google Scholar] [CrossRef]
- Chen, Y.P.; Pettis, J.S.; Corona, M.; Chen, W.P.; Li, C.J. Israeli Acute Paralysis Virus: Epidemiology, Pathogenesis and Implications for Honey Bee Health. PLoS Pathog. 2014, 10, e1004261. [Google Scholar] [CrossRef]
- De Miranda, J.R.; Cordoni, G.; Budge, G. The Acute bee paralysis virus—Kashmir bee virus—Israeli acute paralysis virus complex. J. Invertebr. Pathol. 2010, 103, 30–47. [Google Scholar] [CrossRef]
- Ribière, M.; Olivier, V.; Blanchard, P. Chronic bee paralysis: A disease and a virus like no other? J. Invert. Pathol. 2010, 103, 120–131. [Google Scholar] [CrossRef]
- Dittes, J.; Schäfer, M.O.; Aupperle-Lellbach, H.; Mülling, C.K.W.; Emmerich, I.U. Overt infection with Chronic Bee Paralysis Virus (CBPV) in two honey bee colonies. Vet. Sci. 2020, 7, 142. [Google Scholar] [CrossRef]
- Campbell, E.M.; Budge, G.E.; Watkins, M.; Bowman, A.S. Transcriptome analysis of the synganglion from the honey bee mite, Varroa destructor and RNAi knockdown of neural peptide targets. Insect. Biochem. Molec. 2016, 70, 116–126. [Google Scholar] [CrossRef]
- Gisder, S.; Möckel, N.; Eisenhardt, D.; Genersch, E. In vivo evolution of viral virulence: Switching of deformed wing virus between hosts results in virulence changes and sequence shifts. Environ. Microbiol. 2018, 20, 4612–4628. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J.; Highfield, A.C.; Brettell, L.; Villalobos, E.M.; Budge, G.E.; Powell, M.; Nikaido, S.; Schroeder, D.C. Global Honey Bee Viral Landscape Altered by a Parasitic Mite. Science 2012, 336, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Jironkin, A.; Chandler, D.; Burroughs, N.; Evans, D.J.; Ryabov, E.V. Recombinants between Deformed wing virus and Varroa destructor virus-1 may prevail in Varroa destructor-infested honeybee colonies. J. Gen. Virol. 2011, 92, 156–161. [Google Scholar] [CrossRef]
- Koziy, R.V.; Wood, S.C.; Kozii, I.V.; van Rensburg, C.J.; Moshynkyy, I.; Dvylyuk, I.; Simko, E. Deformed Wing Virus Infection in Honey Bees (Apis mellifera L.). Vet. Pathol. 2019, 56, 636–641. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, J.R.; Genersch, E. Deformed wing virus. J. Invertebr. Pathol. 2010, 103, 48–61. [Google Scholar] [CrossRef]
- Roy, C. La sémiologie an apiculture. Journées Nationales Gtv—Nantes. 2011. Available online: https://www.researchgate.net/publication/343513678_La_semiologie_en_apiculture_-_JOURNEES_NATIONALES_GTV_-_NANTES_2011 (accessed on 20 September 2020).
- Imdorf, A.; Buehlmann, G.; Gerig, L.; Klichenmann, V.; Wille, H. Überprüfung der Schätzmethode zur Ermittlung der Brutfläche und der Anzahl Arbeiterinnen in freifliegenden Bienenvölkern. Apidologie 1987, 18, 137–146. [Google Scholar] [CrossRef]
- Deutsches Bienenjournal: Bienenvolk Schätzen: Volksstärken Genau Erfassen (20. Oktober 2016). Available online: https://www.bienenjournal.de/imkerpraxis/ratgeber/volksstaerken-erfassen-quiz/ (accessed on 24 May 2020).
- Horchler, L.; Gisder, S.; Boecking, O.; Genersch, E. Diagnostic value of faecal spots on and in honey bee (Apis mellifera) hives. Berl. Münch. Tierärztl. Wochenschr. 2018, 132, 41–48. [Google Scholar]
- Kevill, J.L.; Lee, K.; Goblirsch, M.; McDermott, E.; Tarpy, D.R.; Spivak, M.; Schroeder, D.C. The Pathogen Profile of a Honey Bee Queen Does Not Reflect That of Her Workers. Insects 2020, 11, 382. [Google Scholar] [CrossRef]
- Williams, G.R.; Rogers, R.E.L.; Kalkstein, A.L.; Taylor, B.A.; Shutler, D.; Ostiguy, N. Deformed wing virus in western honey bees (Apis mellifera) from Atlantic Canada and the first description of an overtly-infected emerging queen. J. Invertebr. Pathol. 2009, 101, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Hüsken, W.S. Gemülldiagnostik, Netstal, Presentation. 2019. Available online: http://www.imkerverband-sgap.ch/up/files/Vorderland/Gemuelldiagnose_Hysken17.pdf (accessed on 20 September 2020).
- Blanchard, P.; Ribière, M.; Celle, O.; Lallemand, P.; Schurr, F.; Olivier, V.; Iscache, A.L.; Faucon, J.P. Evaluation of a real-time two-step RT-PCR assay for quantitation of Chronic bee paralysis virus (CBPV) genome in experimentally-infected bee tissues and in life stages of a symptomatic colony. J. Virol. Methods 2007, 141, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ciglenečki, U.J.; Toplak, I. Development of a real-time RT-PCR assay with TaqMan probe for specific detection of acute bee paralysis virus. J. Virol. Meth. 2012, 184, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Schurr, F.; Tison, A.; Militano, L.; Cheviron, N.; Sircoulomb, F.; Rivière, M.P.; Ribière-Chabert, M.; Thiéry, R.; Dubois, E. Validation of quantitative real-time RT-PCR assays for the detection of six honeybee viruses. J. Virol. Meth. 2019, 270, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Depner, K.; Schirrmeier, H.; Beer, M. A universal heterologous internal control system for duplex real-time RT-PCR assays used in a detection system for pestiviruses. J. Virol. Meth. 2006, 136, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Higgins, J.A.; Feldlaufer, M.F. Quantitative Real-Time Reverse Transcription-PCR Analysis of Deformed Wing Virus Infection in the Honeybee (Apis mellifera L.). Appl. Environ. Microbiol. 2005, 71, 436–441. [Google Scholar] [CrossRef]
- Yang, X.; Cox-Foster, D.L. Impact of an ectoparasite of the immunity and pathology of an invertebrate: Evidence for host immunosuppression and viral amplification. Proc. Natl. Acad. Sci. USA 2005, 102, 7470–7475. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (OIE). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; Office International des Epizooties: Paris, France, 2008; pp. 1092–1106. [Google Scholar]
- Bak, B.; Wilde, J.; Siuda, M.; Kobylinska, M. Comparison of two methods of monitoring honeybee infestation with Varroa destructor mite. Anim. Sci. 2009, 46, 33–38. [Google Scholar]
- Brunnemann, G.; Poker, V.; Büchler, R. Bienenprobe mit Puderzucker—Die neue bienenschonende Varroa-Befallsmessung. ADIZ 2011, 8, 7–9. [Google Scholar]
- Toplak, I.; Ciglenečki, U.J.; Aronstein, K.; Gregorc, A. Chronic Bee Paralysis Virus and Nosema ceranae Experimental Co-Infection of Winter Honey Bee Workers. Viruses 2013, 5, 2282–2297. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (OIE). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; OIE: Paris, France, 2018. [Google Scholar]
- Gisder, S.; Genersch, E. Molecular differentiation of Nosema apis and Nosema ceranae based on species-specific sequence differences in protein coding gene. J. Invertebr. Pathol. 2013, 113, 1–6. [Google Scholar] [CrossRef]
- Ritter, W. Diagnostik und Bekämpfung der Bienenkrankheiten, 1st ed.; Gustav Fischer Verlag: Jena, Germany, 1996; p. 51. [Google Scholar]
- Available online: https://bienenuntersuchung.julius-kuehn.de/index.php?menuid=93 (accessed on 5 September 2020).
- Available online: https://www.julius-kuehn.de/en/ (accessed on 5 September 2020).
- Available online: https://bienenuntersuchung.julius-kuehn.de/index.php?menuid=39 (accessed on 8 September 2020).
- Berényi, O.; Bakonyi, T.; Derakhshifar, I.; Köglberger, H.; Nowotny, N. Occurrence of Six Honeybee Viruses in Diseased Austrian Apiaries. Appl. Environ. Microbiol. 2006, 72, 2414–2420. [Google Scholar] [CrossRef]
- Gauthier, L.; Tentcheva, D.; Tournaire, M.; Dainat, B.; Cousserans, F.; Colin, M.E.; Bergoin, M. Viral load estimation in asymptomatic honey bee colonies using the quantitative RT-PCR technique. Apidologie 2007, 38, 426–435. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (OIE). Terrestrial Animal Health Code 2019; OIE: Paris, France, 2019. [Google Scholar]

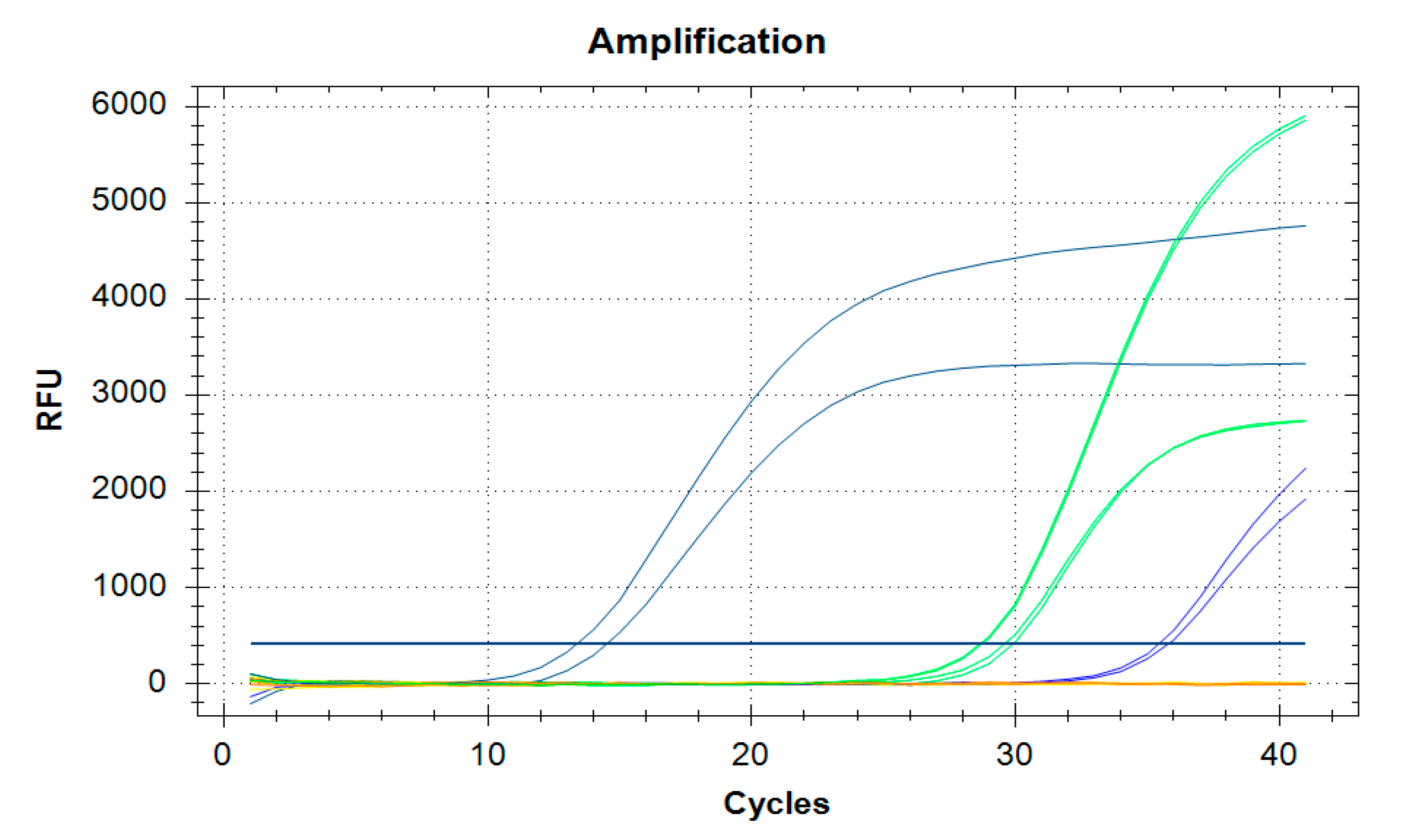





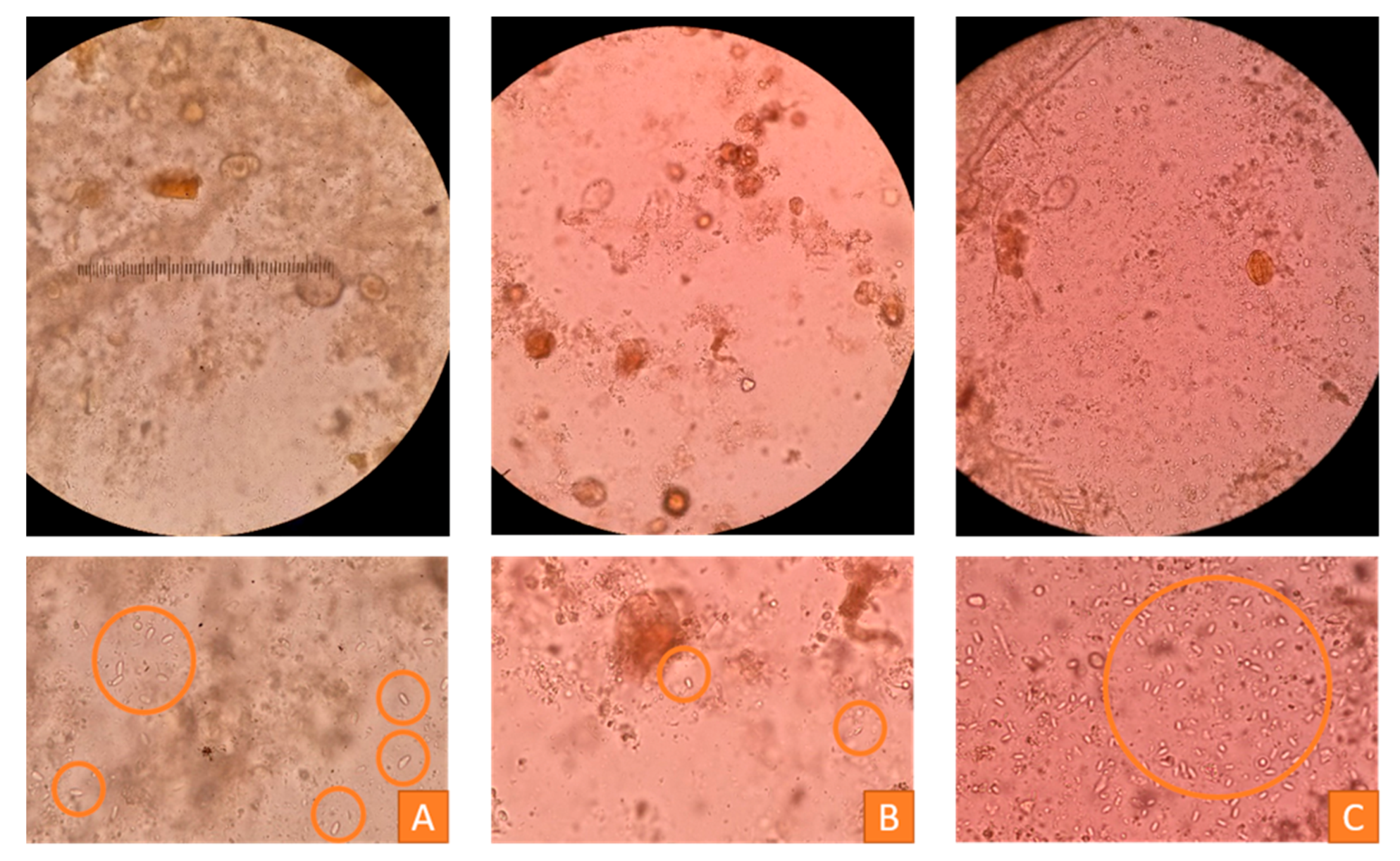
| Virus | Taxonomy | Symptoms in a Colony | Affected Castes | Transmission (Main Routes) |
|---|---|---|---|---|
| Chronic bee paralysis virus (CBPV) | unclassified (RNA) | hairless black syndrome and paralysis syndrome, high mortality after a few days | w, d, q | c, o |
| Acute bee paralysis virus (ABPV) | Dicistroviridae (RNA) | paralysis and high mortality after 1–2 days (experimentally) | w, d | vec, ver (v, to) |
| Kashmir bee virus (KBV) | Dicistroviridae (RNA) | mortality without other symptoms | w, d | vec, ver |
| Israeli acute paralysis virus (IAPV) | Dicistroviridae (RNA) | paralysis and death | w, d | vec, ver |
| Deformed Wing Virus (DWV) | Picorna-like virus (RNA) | crippled bees with deformed wings and shortened abdomens | w, d, q | vec, ver (v, to) |
| Phenotype Feature | Possible Causes (Selection) |
|---|---|
| shortened abdomens | DWV, Varroosis, CBPV |
| hairless, black abdomens | CBPV, black robbers for alimentary, genetic or mechanical reasons |
| crippled wings, legs, antennae | Varroosis, DWV, intoxication, CBPV |
| bloated abdomens and diarrhea (pressure on abdomen light-brown fluid) | Nosemosis, Malpighamoeba mellificae, CBPV |
| extended proboscis | virus diseases, intoxication, unspecific |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dittes, J.; Aupperle-Lellbach, H.; Schäfer, M.O.; Mülling, C.K.W.; Emmerich, I.U. Veterinary Diagnostic Approach of Common Virus Diseases in Adult Honeybees. Vet. Sci. 2020, 7, 159. https://doi.org/10.3390/vetsci7040159
Dittes J, Aupperle-Lellbach H, Schäfer MO, Mülling CKW, Emmerich IU. Veterinary Diagnostic Approach of Common Virus Diseases in Adult Honeybees. Veterinary Sciences. 2020; 7(4):159. https://doi.org/10.3390/vetsci7040159
Chicago/Turabian StyleDittes, Julia, Heike Aupperle-Lellbach, Marc O. Schäfer, Christoph K. W. Mülling, and Ilka U. Emmerich. 2020. "Veterinary Diagnostic Approach of Common Virus Diseases in Adult Honeybees" Veterinary Sciences 7, no. 4: 159. https://doi.org/10.3390/vetsci7040159
APA StyleDittes, J., Aupperle-Lellbach, H., Schäfer, M. O., Mülling, C. K. W., & Emmerich, I. U. (2020). Veterinary Diagnostic Approach of Common Virus Diseases in Adult Honeybees. Veterinary Sciences, 7(4), 159. https://doi.org/10.3390/vetsci7040159





