Abstract
We investigated the distribution of Dermacentor spp. and their infection by zoonotic bacteria causing SENLAT (scalp eschar neck lymphadenopathy) in Turin province, northwestern Italy. We collected ticks in a mountain and in a periurban park, from vegetation and different animal sources, and we sampled tissues from wild boar. Dermacentor marginatus (n = 121) was collected in both study areas, on vegetation, humans, and animals, while D. reticulatus (n = 13) was exclusively collected on wild boar from the periurban area. Rickettsia slovaca and Candidatus Rickettsia rioja infected 53.1% of the ticks, and R. slovaca was also identified in 11.3% of wild boar tissues. Bartonella spp. and Francisella tularensis were not detected, however, Francisella-like endosymbionts infected both tick species (9.2%). Our findings provide new insights on the current distribution of Dermacentor spp. and their infection with a spotted-fever group rickettsiae in the Alps region. Wild boar seem to play a major role in their eco-epidemiology and dispersion in the study area. Although further studies are needed to assess the burden of rickettsial diseases, our results highlight the risk of contracting SENLAT infection through Dermacentor spp. bites in the region.
1. Introduction
In the last few decades, human-induced changes in climate and land use have been favouring the geographic expansion of hard ticks, blood-feeding ectoparasites transmitting microorganisms of remarkable medical and veterinary importance [1]. In addition to Ixodes ricinus, the most widespread tick species and well-known disease vector in Europe [2], ticks of the genus Dermacentor have increasingly gained attention, both for their geographical spread and their vectorial role. In particular, Dermacentor reticulatus has been showing an intensive spread in areas of north-western, north-eastern, and central Europe [3,4,5,6,7,8]. Although the Alps region were considered a barrier for its southern spread [9], recent studies recorded D. reticulatus in the northern regions of Italy [10,11,12]. Dermacentor marginatus is, conversely, commonly distributed in the Mediterranean basin, including almost the entire Italian territory [13]. These three-host tick species parasitize a wide range of vertebrate hosts, with immatures feeding on small and medium-sized mammals, such as rodents and carnivores, and adult stages preferring larger animals including wild and domesticated ungulates and occasionally humans [7,13,14].
Dermacentor spp. are known to transmit a wide range of pathogens, including tick-borne encephalitis (TBE) and Omsk haemorrhagic fever viruses, Rickettsia spp., Francisella tularensis [15]. Interestingly, Crimean-Congo haemorrhagic fever virus was recently detected in D. marginatus in southern Spain [16], reinforcing the hypothesis of its possible involvement in the virus cycle [17]. With regard to the spotted-fever group (SFG) rickettsiae, Rickettsia slovaca, Candidatus Rickettsia rioja, and Rickettsia raoultii have often been reported in Dermacentor spp. [18]. These rickettsiae, together with other agents such as Bartonella henselae and Francisella tularensis, are the causative agents of scalp eschar neck lymphadenopathy—SENLAT in humans, also called tick-borne lymphadenopathy (TIBOLA) or Dermacentor-borne necrosis erythema and lymphadenopathy (DEBONEL) [19].
In Italy, a SENLAT outbreak linked to D. marginatus bites occurred in the late 2000 [20], in a rural area of Tuscany rich in wildlife, wild boar in particular. Wild boar (Sus scrofa), in fact, play an important role as hosts for adult D. marginatus, particularly in Mediterranean regions, and they may also feed adult D. reticulatus [21,22]. In addition to acting as a maintenance host for adult Dermacentor spp., wild boar possibly contribute to the maintenance of some rickettsia [23].
Spotted-fever group rickettsioses are notifiable diseases in Italy but are likely underdiagnosed and underreported. Most reported cases refer to the Mediterranean spotted fever in southern and insular Italy [24], caused by Rickettsia conorii and transmitted by Rhipicephalus sanguineus. However, diagnosis in humans is frequently based on the research of antibodies against Rickettsia spp. and no specific tests are performed to identify the causative rickettsial agent [25]. In northern Italy, data on rickettsioses are scarce; for example, in Piedmont region, the Regional Service for the Epidemiology of Infectious Diseases (SeREMI) registered only 15 human cases in the last decade (2009–2019; [26]), although SFG rickettsiae are commonly detected in ticks from the region and tick bites are increasingly reported [27].
In this study, we investigated the distribution of Dermacentor spp. in the Italian Alpine region, in a mountain and in a periurban natural area of Piedmont, and evaluated their infection by zoonotic tick-borne bacteria causing SENLAT. Finally, given the overabundance of wild boar in the periurban areas of Turin city, we tested tissue biopsies of culled animals for the SFG rickettsiae infection to investigate their possible role in the pathogens’ maintenance.
2. Materials and Methods
2.1. Study Area
The study was conducted in two natural areas in Turin province, northwestern Italy, differing in environmental characteristics, altitude, and abundance of wild ungulates (Supplementary Materials, Figure S1). The first is a mountain area located in the high Susa Valley, including Alpi Cozie regional park (45°03′ N, 6°54′ E) and surrounding areas belonging to the high Susa Valley hunting district. This Alpine valley is characterized by a xeric climate and abundant wild ungulates populations; details about climate, habitat characteristics, and wildlife composition are described in [27]. The second study site is a periurban area located nearby Turin city, belonging to the Po Torinese natural park (45°06′ N, 7°76′ E), which comprises several natural reserves highly fragmented by the presence of urban centers and crops. Po river and its tributaries shape a stepped landscape, where a great variety of ecosystems occur: marsh vegetation, such as reeds (Phragmites spp.), prevails along bank streams, together with alders (Alnus glutinosa), willows (Salix spp.), and black poplars (Populus nigra); in hilly areas, mixed broad-leave woods mainly compose the vegetation canopy, with a prevalence of deciduous oaks and sweet chestnuts (Castanea sativa). Wild ungulates are in expansion in the area; roe deer (Capreolus capreolus) are still rare, while wild boar (Sus scrofa) have become overabundant and are subjected to a management plan in order to contain the population.
2.2. Dermacentor spp. Ticks Collection
We carried out monthly collections of questing ticks during the spring-autumn seasons, by the dragging method on the ground vegetation in 100 m transects. In the mountain area, dragging was performed from 2016 to 2019 in 45 sites including open-exposed areas, conifers, and broad-leaves woods, ranging altitudes between 959 and 1884 m above sea level (asl), as described in [27]. Conversely, 11 transects from the periurban area were investigated in 2018 and 2019; they included deciduous woods at an altitude between 212 and 587 m asl.
In parallel, we actively conducted tick collection from hunted wild ungulates through skin inspection. The monitoring activity in the mountain area (October–December, 2017 to 2019) was carried out on a hunted game presented at the check station of the local hunting management unit [27]. Some specimens were also collected on owned dogs and livestock (cattle and horses) with the help of Alpi Cozie Park personnel, and on human patients that were visited at a local emergency unit (Susa hospital).
In the periurban area, wild boar subjected to controlled hunting and trapping by forestry authorities, were immediately inspected after culling (October–March, 2017 to 2020). Tissue biopsies (liver or ear tissue) were taken, wherever possible. Data regarding sex, age, and shooting location were recorded.
All animals sampled in this study were culled by professional hunters in accordance with the Piedmont Regional Law no. 5 of 19 June, 2018 on the protection of fauna and wildlife management–hunting; a veterinarian inspector was always present when animal carcasses were brought at the check station of the local hunting management unit. No animal was harmed for the purpose of sample acquisition.
2.3. Tick-Borne Pathogens Detection
Collected ticks were stored in 70% ethanol at room temperature. By using the stereomicroscope, we classified ticks to the stage and species level [13,16], and measured the tick engorgement index—TEI of feeding ticks (index 2) according to [28]. Four D. reticulatus were not subjected to molecular analyses, but kept in our tick collection as reference specimens. Tissue biopsies were collected by using sterile scalpel blades and individually stored under RNAlater® Solution (Life Technologies Ltd., Warrington, UK), at −20 °C until the processing and analysis. All D. marginatus, nine D. reticulatus and tissues were individually homogenized, except for three adult D. marginatus that were analyzed in a pool; these were collected from a wild boar in the periurban area at the very beginning of the study. DNA was extracted by using the DNAzol reagent® (Life Technologies LTD, Warrington, UK), as described in [27]. The quality and quantity of extracted DNA samples were evaluated with a spectrophotometer (Nanodrop™ 2000, Thermo Fisher Scientific).
To detect the SFG rickettsiae infection, we primarily used a quantitative polymerase chain reaction (qPCR) assay specific for the detection of R. slovaca in ticks and tissues, as described by [29]. Negative samples were further tested by a conventional PCR assay targeting a fragment of gene coding citrate synthase (gltA; [30]), in order to identify SFG rickettsiae different from R. slovaca. All gltA-positive and qPCR-positive samples were eventually subjected to end-point nested-PCR targeting a fragment of OmpA gene [31,32], to obtain the nucleotide sequences of R. slovaca and other SFG rickettsiae. The infection by Bartonella spp. and Francisella tularensis/Francisella-like endosymbionts (FLEs) was investigated by PCR amplification of 16S-23S rRNA intergenic region [33] and tul4 gene (PCR and qPCR) [34], respectively. Positive controls and negative water controls were used on every (q)PCR assay performed in this study.
End-point PCRs positive sample amplicons were purified using the ExoSAP-IT™ PCR Product Clean-up Kit (GE Healthcare Limited, Chalfont, UK) and sent to an external service (BMR Genomics, Padua, Italy) for automatic sequencing.
2.4. Statistical Analyses
Data were analyzed by using the R software version 3.6.3 for Windows [35]. Prevalence and 95% exact binomial confidence intervals (CI) of tick infestation in wild ungulates and tick and tissue infection were calculated through a binomial exact test. To evaluate significant differences in tick infestation in wild boar according to the animal age and sex, we applied Pearson’s chi-squared test. For these purposes, we categorized the age variable into three groups: ‘Group 0’ included wild boar piglets under 6 months old; ‘Group 1’ included juvenile individuals from 6 to 18 months old; and ‘Group 2’ included adult individuals over 2 years old. Fisher’s exact test was used to compare the Rickettsia spp. infection according to the type of tissue analyzed, animal characteristics (age and sex), and shooting location. For all statistical tests, a p-value < 0.05 was considered statistically significant.
2.5. Phylogenetic Analyses
All nucleotide sequences were primarily handled by using BioEdit [36]. A multiple sequence alignment was performed by the ClustalW algorithm [37], which computes a distance matrix between each pair of sequences based on sequence pairwise comparisons. For bacterial identification, nucleotide sequences were then compared with reference sequences deposited in GenBank throughout BLAST® (Basic Local Alignment Search Tool). Phylogenetic analyses were conducted by applying the neighbour–joining method in MEGA X [38]. The stability of the trees obtained was estimated by a bootstrap analysis with 1000 replicates. Some representative sequences of the Rickettsia OmpA gene and FLEs tul4 gene were submitted to GenBank.
3. Results
3.1. Tick Collection
We collected 134 Dermacentor spp., namely 121 D. marginatus and 13 D. reticulatus.
Dermacentor marginatus was distributed in both study areas, on various hosts and vegetation (Table 1).

Table 1.
Dermacentor spp. ticks by geographic location and host source and their infection by the spotted-fever group (SFG) rickettsiae; Turin province, 2016–2020.
With regard to questing D. marginatus, immatures were found during summer (June–September) and prevailed over adults (Pearson’s Chi-squared test, p < 0.01), that were mainly sampled during spring (March–May) and early autumn (September–October). In the mountain area, D. marginatus (10 larvae, two nymphs and six adults) were collected in six dragging transects (13.3% of investigated sites; 95% CI = 5.1 − 26.8), located between 1014 to 1340 m asl [27]. In addition, we opportunistically collected five more adult D. marginatus questing on vegetation in a wet pasture frequented by wild boar at 1600 m asl. In the periurban area, D. marginatus (17 larvae, one nymph and one adult male) were collected in four dragging transects (36.4% of investigated sites; 95% CI = 10.9 − 69.2), corresponding to recreational hilly areas at altitudes between 278 and 587 m asl. We did not collect D. reticulatus on the vegetation in our study areas.
We gathered 92 feeding Dermacentor spp. from different animal sources, including wild ungulates, domestic animals, livestock, and humans (Table 1), namely 91 adults and one single nymph collected from a 4-year-old chamois. All feeding ticks had TEI > 2, and TEI was generally much higher in females compared with males.
In the mountain area, we inspected 373 carcasses of hunted wild ungulates [27] and a further moribund male roe deer found by a local veterinary officer. Dermacentor marginatus (n = 11) infested 6/207 red deer (Cervus elaphus; mean tick number per animal: 1.5, min–max = 1–3), 1/24 roe deer (one tick), and 2/143 of chamois (Rupicapra rupicapra; one tick per animal). Parasitized animals were culled at altitudes between 1000 and 1700 m asl. Hunters and forestry workers provided further 14 D. marginatus from two wild boar (mean tick number per animal: 7, min–max = 4–10), culled at 1600 m asl. Moreover, local farmers collected 10 more D. marginatus from dogs and livestock (Table 1) at an altitude of around 1700 m asl. Physicians of the Susa hospital emergency unit also provided two D. marginatus adults (one male and one female, in April–May 2019) feeding on two human patients residing in the valley; no data on the clinical status of the patients were provided.
In the periurban area, we exclusively collected feeding ticks from wild boar (Table 1): we visually inspected 102 culled animals and collected 53 adult ticks from 16 carcasses (infestation prevalence 15.7%, 95% CI = 9.2 − 24.2; mean tick number per animal: 3.3, min–max = 1–15). Dermacentor marginatus (20 females and 20 males) infested 12.7% (n = 13; 95% CI = 7.0 − 20.8) of wild boar (mean tick number/animal: 3.1, min–max = 1–14), while D. reticulatus (four females and nine males) parasitized 7.8% (n = 8; 95% CI = 3.4 − 14.9) of the animals (mean tick number/animal: 1.6, min–max = 1–5). The two Dermacentor species overlapped in time, especially during early spring (March-April) and autumn (November) when five individuals from different localities were found co-infested by both tick species. Tick infestation did not differ among animals according to age (Fisher’s exact test; p = 0.103), and sex (Pearson’s Chi squared test; p = 0.231). We biopsied liver (n = 18) and ear tissues (n = 62) from 80 wild boar, of which seven (8.8%; 95% CI = 3.6 − 17.2) were infested by ticks.
3.2. Tick-Borne Pathogen Infection
The spotted-fever group rickettsiae infected 53.1% (n = 68; 95% CI = 44.1 − 62.0) of Dermacentor spp. individually screened, and the pool of three D. marginatus collected from wild boar. Tick infection was similar in mountain and periurban areas (Pearson’s Chi squared test, p > 0.05), with an overall prevalence of 56.5% (95% CI = 43.3 − 69.0) and 50.0% (95% CI = 37.4 − 62.6) in individual ticks, respectively.
In the mountain area, the prevalence of SFG rickettsiae did not show significant differences between questing and feeding ticks (p > 0.05), although this latter group exhibited a greater infection prevalence (59.0% versus 52.2% of questing ticks). On the other hand, the infection prevalence significantly differed when considering the source of ticks in the periurban area (p < 0.01), where feeding ticks showed a rickettsial infection prevalence of 60.5% (95% CI = 49.3 − 70.8) versus 21.1% (95% CI = 6.1 – 45.6) in questing ticks.
With regard to the tick life stage, adult ticks (59.8%; 95% CI = 49.3 − 69.6) and nymphs (50.0%; 95% CI = 6.8 − 93.2) were more likely infected compared with larvae (29.6%; 95% CI= 13.8 − 50.2; p < 0.05).
Rickettsiae-infected feeding ticks were collected on all animal species, except for horses (Table 1). Dermacentor spp. ticks from wild boar showed high infection rates in both study areas (Table 1), with an overall prevalence of 61.5% (95% CI = 47.0 − 74.7) in D. marginatus and 55.6% (95% CI = 21.2 − 86.3) in D. reticulatus. We likewise recorded a high infection prevalence (75%, 95% CI = 42.8 − 94.5) in D. marginatus collected on wild ungulates from the mountain areas, in ticks from deer and chamois. The two D. marginatus from human patients also tested positive (Table 1).
Through molecular analyses (qPCR) and nucleotide sequencing, we identified R. slovaca in 75.0% (95% CI = 63.0 − 84.7) Dermacentor positive ticks, followed by the novel uncultured Ca. R. rioja (11.8%; 95% CI = 5.2 − 21.9). However, we failed to determine the rickettsiae species in eight PCR-amplicons given the poor quality of the resulting sequences. We recorded both SFG rickettsiae in questing and feeding ticks in the mountain and periurban areas (Table 1). Rickettsia slovaca infected D. marginatus from different sources, including questing adults (GenBank accession numbers: MT025712) and nymphs (MT330429), feeding ticks collected from wild ungulates (MT330430-3), and human patients (MT899421). In the periurban area, R. slovaca infected D. reticulatus ticks as well, in particular a female and two males collected from wild boar. By contrast, Ca. R. rioja infected only D. marginatus, including questing ticks (MT330435) and feeding ticks from wild boar (MT330436) in both study areas.
Overall, 11.3% (95% CI = 5.3 − 20.3) of the 80 wild boar tissues tested positive for SFG rickettsiae (gltA and qPCR). We identified R. slovaca in nine tissues, one liver and eight ear biopsies (GenBank accession numbers: MT330434). Two positive ear biopsies belonged to the infested wild boar, one parasitized by a D. marginatus male and one by a D. reticulatus male. No significant differences were observed in tissue infection according to the animal age, sex, and shooting location (Fisher’s exact test, p > 0.05).
Sequences of about 550 bp of the OmpA gene of R. slovaca showed 100% similarity with sequences identified in D. marginatus ticks from other Italian regions (MH532250-7, HM161786-8) and Turkey (MF379300-3-5-11). The sequences identified as Ca. R. rioja shared 99–100% identity to sequences of Ca. R. rioja (OmpA gene) detected in Spain—in the blood of SENLAT-human patient (EF028201), in a D. marginatus feeding on a SENLAT human patient (GQ404429), and in questing I. ricinus ticks (MK301593-4-5). The R. slovaca sequence obtained from our wild boar ear biopsy (GenBank accession number: MT330434), showed 98.6% similarity with the amplified OmpA gene detected in questing D. marginatus from Turkey (MK922644-53) (Figure 1).

Figure 1.
Phylogenetic tree of OmpA gene of Rickettsia spp. obtained from 32 nucleotide sequences (426 bp) from D. marginatus and D. reticulatus ticks collected in the study areas (AC: Alpi Cozie regional park; TP: Po Torinese natural park). Reference sequences are identified by the GenBank accession number enclosed in parentheses. Bootstrap values (1000 replications) above 70 are shown next to the internal nodes. Amplicons obtained in this study are indicated with a black symbol: ● Feeding ticks from wild boar, red deer, Northern chamois, and humans; ▲ Dermacentor ticks collected from the vegetation; and ■ Ear biopsy collected from wild boar.
We did not detect Bartonella spp. in our tick sample. Francisella tularensis was also absent, as confirmed by the specific qPCR targeting tul4 gene [34], however 9.2% (95% CI = 4.8 − 15.5) of the ticks tested positive for the tul4 gene end-point PCR. These positive samples were five D. marginatus from the mountain area, collected on a wild boar (n = 2) and a horse (n = 3), and seven D. reticulatus collected from three wild boar from the periurban area (GenBank accession numbers: MT899422-25). Nucleotide sequencing showed 98–100% similarity of our amplicons to Francisella-like endosymbionts identified in D. reticulatus from Portugal (MF497789-94) and Hungary (JQ942368) (Figure 2).
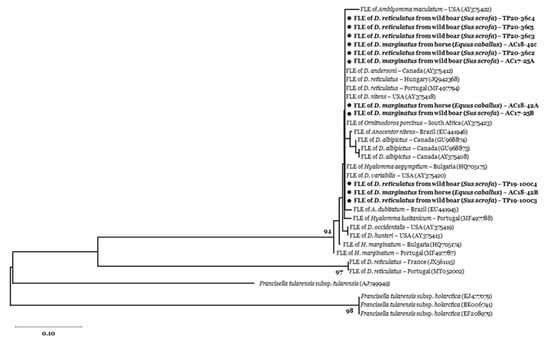
Figure 2.
Phylogenetic tree of tul4 (17 kDa lipoprotein) gene of Francisella spp. obtained from 11 nucleotide sequences (200 bp) identified in feeding D. marginatus and D. reticulatus ticks, respectively collected from horse and wild boar in our study areas (AC: Alpi Cozie regional park; TP: Po Torinese natural park). Reference sequences are identified by GenBank accession number enclosed in parentheses. Bootstrap values (1000 replications) above 70 are shown next to the internal nodes. Amplicons from this study are indicated with a black circle (●).
4. Discussion
Our results confirm the widespread distribution of D. marginatus and the local presence of D. reticulatus in Northwestern Italy. Interestingly, the two species overlap in periurban areas inhabited by wild boar. Moreover, we showed Dermacentor spp. infection by two SFG rickettsiae causing SENLAT in humans, R. slovaca and Ca. R. rioja, and by Francisella-like endosymbionts.
The finding of D. reticulatus feeding on wild boars was somewhat unexpected. This tick species is indeed widely distributed in central Europe [7,9], but its presence in Italy has been hitherto considered occasional [39,40]. In the Piedmont region, only Maurelli et al. had previously reported some specimens feeding on owned dogs [12]. However, in the bordering Lombardy region, D. reticulatus was recently reported in parks located in highly urbanized areas and their presence was linked to canine babesiosis cases [10,11]. Similarly, we detected D. reticulatus in the periurban park though not in the mountain study area. According to Estrada-Peña et al., this species prefers urban biotopes and is considered absent in high mountain regions [15]. However, its observation in warm Alpine valleys in France and Switzerland [7] indicate the need of monitoring its presence in the Italian Alpine areas such as Susa Valley, which are experiencing a rise in temperatures [41] and an increase in tick populations [27]. Dermacentor reticulatus were collected from wild boar mainly during spring and autumn, which is in line with the period of adults questing activity, also observed in our latitudes by [11]. The occurrence of D. reticulatus in close proximity to human settlements entails a potential risk of tick-borne diseases in humans and animals, since this species has a generalist feeding behaviour and is considered a competent vector of Babesia canis, SFG rickettsiae (R. slovaca and R. raoultii), Francisella tularensis, but also of TBE virus [7].
Dermacentor marginatus ticks, in contrast, are endemic in the Italian territory [13]. We recorded their occurrence from 200 m up to 1700 m asl, in the vegetation and on animals from mountain and periurban areas. Our results confirm the great plasticity of D. marginatus and adaptability to different environments. For example, Selmi et al. reported D. marginatus in different habitats (e.g., typical Mediterranean woods, meadows, and croplands), up to 1600 m asl [42]. Questing immature D. marginatus were the most common life stages encountered and prevailed during the summer, while the few adult ticks were mainly collected during spring, indicating a potential risk period for D. marginatus bites in humans. We unexpectedly analyzed also two D. marginatus collected on human patients at the local emergency unit. Dermacentor bites are uncommon in Piedmont region [43] and, in general, they are less frequent compared with bites of generalist ticks such as Ixodes ricinus [13,14]. This finding may indicate a significant presence of D. marginatus in the area, although with a focal distribution [27], and the chance for humans to be bitten.
Spotted-fever group rickettsiae infected around 50% of our D. marginatus sample. This prevalence is slightly lower than the infection prevalence observed in central Spain (63.9%) in questing and feeding D. marginatus collected from livestock and wild mammals [44]. Regard questing ticks, the SFG rickettsiae infection in our D. marginatus (38.1%) is similar to the infection prevalence observed in D. marginatus in Spain (35.8%) and Tuscany (36.4%) [45,46].
The overall occurrence of SFG rickettsiae in Dermacentor spp. was similar in the mountain and periurban areas. We observed a higher infection rate in feeding ticks compared to questing ticks, although the difference was significant in the periurban area only; this finding is in contrast with previous studies that reported similar Rickettsia-infection rates between Dermacentor spp. feeding on animals and questing [20,46,47]. In addition, adults and nymphs were significantly more infected than larvae. These results could be possibly due to the chance of ticks getting infected during the blood meal on the vertebrate hosts, either by systemic infection or by co-feeding [48]. Ticks are considered a reservoir for Rickettsia spp., thanks to transovarial and transstadial transmission [49]. Whether vertebrate hosts can serve as reservoir of SFG rickettsiae is still under debate [48]. Some studies previously suggested a possible role of small mammals [50] and wild boar [42,45,46] in the transmission cycle of R. slovaca. We indeed detected R. slovaca in wild boar tissues, with a prevalence of 11.3%, which is comparable with 11.1% of prevalence reported in skin biopsies from wild boar in Tuscany [46]. Our finding of rickettsiae-infected ear tissues suggests, at least, a local infection after the bite of infected ticks, so wild boar might behave as an amplifier host of the pathogen through co-feeding [48]. Nevertheless, if a local infection in the auricular tissues occurred, it persisted for a period time following the tick bite, since none of the wild boar with R. slovaca-positive tissues were found infested with ticks at the time of culling. In addition, the detection of R. slovaca in a liver sample might suggest a systemic circulation of the pathogen, which is in accordance with the finding of the pathogen in spleen tissues of wild boar from Algeria [51]. Further studies are needed to investigate this hypothesis and clarify whether the rickettsiemia in wild boar reaches a sufficient level for the bacterial transmission to ticks during the blood meals. Anyway, wild boar seems to play a role as maintenance host for Dermacentor adults in our study areas, including D. reticulatus. Wild boar were indeed the most infested animal species and showed higher tick loads compared to the other wild ungulates. Moreover, wild boar may disperse tick vectors in close proximity to human settlements, thanks to their ability to adapt and exploit even highly anthropized contexts [42]. In fact, we recovered questing Dermacentor spp. very close to urban areas in the Po Torinese natural park (Supplementary Materials, Figure S1).
Deer and chamois in the mountain area were also infested by D. marginatus, though with lower frequency and infestation burdens compared to I. ricinus [27]. Although we may have underestimated tick loads, since we only visually inspected the animal carcasses, our findings suggest a minor role of these ungulate species as maintenance hosts for D. marginatus compared to wild boar. Unfortunately, it was not possible to take biopsy samples to investigate their possible infection by Rickettsia spp.
Rickettsia slovaca was the most common rickettsia infecting our D. marginatus ticks (74.3% of mountain and 72.4% of periurban rickettsiae-positive sample). The pathogen infected 47.2% of D. marginatus collected from wild boar; this prevalence exceeds the infection rates previously observed in northeastern Spain (30.5%) and central Italy (32.1%) [45,46]. More recent studies, conducted in different regions of the Italian territory, reported a comparable prevalence of R. slovaca in D. marginatus collected from wild boar in Liguria (40.7%), but also lower infection rates in Sardinia (33.3%) and its absence in Elba Island, Tuscany [23]. Cicculli et al. [52] first recorded R. slovaca infecting D. marginatus from wild boar in Corsica, with a prevalence significantly lower than our report (15.4%).
Both D. marginatus from human patients tested positive for R. slovaca, and since both ticks were engorged, the transmission of the pathogen may have occurred; unfortunately, we did not get data on the health status of the patients. Infection by the SENLAT syndrome was reported in several European areas, such as Tuscany in Italy [46], Spain [53], and France [19,54]. In Piedmont region, Dutto and Selmi reported a case of disease in 2012, with symptoms compatible with the SENLAT syndrome, in a woodcutter bitten by D. marginatus in the parietal region [55]. This report indicates that the disease may have been present in the region for a long time.
Three out of nine D. reticulatus collected from wild boar also tested positive for R. slovaca. Previous studies report a R. slovaca prevalence of 28.8% in D. reticulatus feeding on horses, goats, and dogs in Slovakia [47], and in 25% questing ticks from Spain [56]. Dermacentor reticulatus is considered a competent vector for the SENLAT syndrome [19]. Hence, despite the modest presence of this tick vector in our study areas, our results highlight the potential risk for humans to contract the infection through D. reticulatus bites in Turin periurban areas.
To our knowledge, we first report Ca. Rickettsia rioja in Italy, which was detected in D. marginatus from wild boar and vegetation. Candidatus R. rioja was first identified in 2006 in Spain, on feeding ticks collected from SENLAT-human patients and subsequently characterized to the molecular level in 2009 [53,57], but it is still uncultured to date. Its pathogenicity has been recognized and the bacterium constitutes, alongside R. slovaca and R. raoultii, one of the causative agents of SENLAT syndrome [18]. The rickettsia was reported in human patients affected by SENLAT in Spain and France [19,58]. Notwithstanding, the similarity of the nucleotide sequence of Ca. R. rioja with that of R. raoultii hinders its identification, in particular when the OmpA gene is targeted for its amplification [56,57,59]. Upon discovery, Ca. R. rioja has been recorded in both feeding and questing ticks, including D. marginatus collected from a woman patient affected by SENLAT [58], but also in D. marginatus, D. reticulatus, and I. ricinus collected from the vegetation [56]. Given its recent discovery and the difficulty for its identification, it is conceivable that the prevalence and spread of Ca. R. rioja are underestimated in the literature. The prevalence of Ca. R. rioja (6.2%) in our study was significantly lower than that observed for R. slovaca (39.5%), however Remesar et al. [56] recorded a similar prevalence of both rickettsiae species in D. marginatus collected from the vegetation in northern Spain.
We did not find Bartonella spp. in our tick sample. In previous studies, Bartonella spp. were detected in 21.4% of D. reticulatus in Siberia [60], in 9% of D. marginatus, and 12% D. reticulatus in France [61], while it was not detected in D. marginatus from Sardinia, Italy [62,63]. The possible role of Dermacentor spp. as Bartonella vector deserves investigations since the B. henselae infection in humans was reported following an infected Dermacentor spp. bite [64] and is considered a SENLAT agent [19]; moreover, other tick species, such as I. ricinus, were recognized as vectors of Bartonella spp. [65,66].
We did not record the infection by F. tularensis in Dermacentor spp., as in other European studies [61,67]. The role of ticks in its transmission is indeed debated [68]. We instead identified FLEs in both D. marginatus and D. reticulatus. Infection prevalence was below 10%, comparable to studies carried out in Bulgaria [69] and Hungary [70], and lower than infection rates reported in Portugal [71] and France [34]. Studies carried out in northwestern and southern Italy have recently reported the circulation of FLEs in tick species different from Dermacentor spp., such as Hyalomma spp. and Rhipicephalus spp., collected from different animal hosts [63,72]. Occurrence of these maternally inherited symbionts seems to be crucial for tick survival since these bacteria may provide nutritional components, such as B-group vitamins, that normally lack in blood meals [73,74]. Co-speciation of FLEs and D. reticulatus has been previously suggested [34], however, our results on FLEs phylogeny do not support this hypothesis and are in line with previous studies that indicate a relatively recent association between the bacterium and Dermacentor ticks [75]. Future investigations will need to clarify if FLEs infection in ticks interferes with the prevalence of pathogenic Francisella strains, as previously suggested [71].
5. Conclusions
In southern Europe, the health threats posed by Dermacentor spp. ticks come abreast to those posed by other tick species such as I. ricinus, which shows a remarkable geographical expansion [27]. Our findings highlight the circulation of D. reticulatus in addition to D. marginatus in Piedmont region, and their infection with causative agents of the SENLAT syndrome (R. slovaca and Ca. Rickettsia rioja). Dermacentor spp. showed a wide distribution, from periurban to high mountain habitats, and parasitized a wide range of hosts, including humans. Wild boar, in particular, seem to play a major role in their eco-epidemiology in the study area. Therefore, we can state that northwestern Italy is at risk for SENLAT. A higher notification rate of tick-borne diseases to the health authorities, and the use of routine biomolecular diagnostic tests to confirm rickettsial infections and identify the specific causative agents, would help in assessing the effective burden of rickettsial diseases in the region.
Supplementary Materials
The following is available online at https://www.mdpi.com/2306-7381/7/4/157/s1. Figure S1: Study areas in Turin province: High Susa Valley (mountain; (A) and Po Torinese natural park (periurban area; (B). Maps illustrate dragging transects, land use characteristics, and the presence of questing Dermacentor marginatus in each location during the dragging sampling period (2016–2019).
Author Contributions
Conceptualization, A.G.-V. and L.T.; formal analysis, A.G.-V.; investigation, A.G.-V., G.G., L.T., E.R., and F.N.; resources, E.R. and F.N.; data curation, A.G.-V.; writing—original draft preparation, A.G.-V. and L.T.; writing—review and editing, G.G., E.R., F.N., and L.R.; visualization, A.G.-V.; supervision, L.T.; project administration, L.T.; funding acquisition, L.R. and L.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Ente di Gestione dei Parchi delle Alpi Cozie and the University of Turin.
Acknowledgments
We thank the staff of Ente di Gestione dei Parchi delle Alpi Cozie and Ente di Gestione del Po Torinese for the logistic support during the fieldwork and financial contribution to this research. We especially thank the personnel of the natural areas studied, Massimo Rosso and Simona Molino from Alpi Cozie Natural Park, and Enrico Castello and Simona Zaghi from Po Torinese natural park together with the volunteer students who spent time and effort to collect ticks. Thank you to Elena Pujol and Claudio Berno for providing ticks from domestic and wild animals. We acknowledge the personnel of the Emergency Unit of Susa hospital for collecting ticks on human patients. We also thank Agustín Estrada-Peña, University of Zaragoza, Spain, for confirming the identification of our tick specimens. We thank the personnel of Comprensorio Alpino Alta Val di Susa (CATO 2) for their collaboration on tick monitoring in wild ungulates.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boulanger, N.; Boyer, P.; Talagrand-Reboul, E.; Hansmann, Y. Ticks and tick-borne diseases. Med. Mal. Infect. 2019, 49, 87–97. [Google Scholar] [CrossRef]
- Medlock, J.M.; Hansford, K.M.; Bormane, A.; Derdakova, M.; Estrada-Peña, A.; George, J.C.; Golovljova, I.; Jaenson, T.G.; Jensen, J.K.; Jensen, P.M.; et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit. Vectors. 2013, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Dautel, H.; Dippel, C.; Oehme, R.; Hartelt, K.; Schettler, E. Evidence for an increased geographical distribution of Dermacentor reticulatus in Germany and detection of Rickettsia sp. RpA4. Int. J. Med. Microbiol. 2006, 296, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Bullová, E.; Lukáň, M.; Stanko, M.; Petko, B. Spatial distribution of Dermacentor reticulatus tick in Slovakia in the beginning of the 21st century. Vet. Parasitol. 2009, 165, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Karbowiak, G. The occurrence of the Dermacentor reticulatus tick-its expansion to new areas and possible causes. Ann. Parasitol. 2014, 60, 37–47. [Google Scholar]
- Jongejan, F.; Ringenier, M.; Putting, M.; Berger, L.; Burgers, S.; Kortekaas, R.; Lenssen, J.; van Roessel, M.; Wijnveld, M.; Madder, M. Novel foci of Dermacentor reticulatus ticks infected with Babesia canis and Babesia caballi in the Netherlands and in Belgium. Parasit. Vectors. 2015, 8, 232. [Google Scholar] [CrossRef]
- Földvári, G.; Široký, P.; Szekeres, S.; Majoros, G.; Sprong, H. Dermacentor reticulatus: A vector on the rise. Parasit. Vectors. 2016, 9, 314. [Google Scholar] [CrossRef]
- Mierzejewska, E.J.; Estrada-Peña, A.; Alsarraf, M.; Kowalec, M.; Bajer, A. Mapping of Dermacentor reticulatus expansion in Poland in 2012–2014. Ticks Tick Borne Dis. 2016, 7, 94–106. [Google Scholar] [CrossRef]
- Rubel, F.; Brugger, K.; Pfeffer, M.; Chitimia-Dobler, L.; Didyk, Y.M.; Leverenz, S.; Dautel, H.; Kahl, O. Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick Borne Dis. 2016, 7, 224–233. [Google Scholar] [CrossRef]
- Olivieri, E.; Zanzani, S.A.; Latrofa, M.S.; Lia, R.P.; Dantas-Torres, F.; Otranto, D.; Manfredi, M.T. The southernmost foci of Dermacentor reticulatus in Italy and associated Babesia canis infection in dogs. Parasit. Vectors. 2016, 9, 213. [Google Scholar] [CrossRef]
- Olivieri, E.; Gazzonis, A.L.; Zanzani, S.A.; Veronesi, F.; Manfredi, M.T. Seasonal dynamics of adult Dermacentor reticulatus in a peri-urban park in southern Europe. Ticks Tick Borne Dis. 2017, 8, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Maurelli, M.P.; Pepe, P.; Colombo, L.; Armstrong, R.; Battisti, E.; Morgoglione, M.E.; Counturis, D.; Rinaldi, L.; Cringoli, G.; Ferroglio, E.; et al. A national survey of Ixodidae ticks on privately owned dogs in Italy. Parasit. Vectors. 2018, 11, 420. [Google Scholar] [CrossRef]
- Cringoli, G.; Iori, A.; Rinaldi, L.; Veneziano, V.; Genchi, C. Zecche. Mappe Parassitologiche; Ronaldo Editore: Napoli, Italy, 2005; pp. 144–151. [Google Scholar]
- Estrada-Peña, A.; Jongejan, F. Ticks feeding on humans: A review of records on human-biting Ixodoidea with special reference to pathogen transmission. Exp. Appl. Acarol. 1999, 23, 685–715. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Mihalca, A.D.; Petney, T.N. Ticks of Europe and North Africa: A Guide to Species Identification; Springer Nature: Cham, Switzerland, 2017; pp. 279–291. [Google Scholar] [CrossRef]
- Moraga-Fernández, A.; Ruiz-Fons, F.; Habela, M.A.; Royo-Hernández, L.; Calero-Bernal, R.; Gortazar, C.; de la Fuente, J.; Fernández de Mera, I.G. Detection of new Crimean-Congo haemorrhagic fever virus genotypes in ticks feeding on deer and wild boar, Spain. Transbound. Emerg. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gargili, A.; Estrada-Peña, A.; Spengler, J.R.; Lukashev, A.; Nuttall, P.A.; Bente, D.A. The role of ticks in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus: A review of published field and laboratory studies. Antiviral Res. 2017, 144, 93–119. [Google Scholar] [CrossRef] [PubMed]
- Portillo, A.; Santibáñez, S.; García-Álvarez, L.; Palomar, A.M.; Oteo, J.A. Rickettsioses in Europe. Microbes Infect. 2015, 17, 834–838. [Google Scholar] [CrossRef]
- Dubourg, G.; Socolovschi, C.; Del Giudice, P.; Fournier, P.E.; Raoult, D. Scalp eschar and neck lymphadenopathy after tick bite: An emerging syndrome with multiple causes. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1449–1456. [Google Scholar] [CrossRef]
- Selmi, M.; Bertolotti, L.; Tomassone, L.; Mannelli, A. Rickettsia slovaca in Dermacentor marginatus and tick-borne lymphadenopathy, Tuscany, Italy. Emerg. Infect. Dis. 2008, 14, 817–820. [Google Scholar] [CrossRef]
- Ruiz-Fons, F.; Fernández de Mera, I.G.; Acevedo, P.; Höfle, U.; Vicente, J.; de la Fuente, J.; Gortazar, C. Ixodid ticks parasitizing Iberian red deer (Cervus elaphus hispanicus) and European wild boar (Sus scrofa) from Spain: Geographical and temporal distribution. Vet. Parasitol. 2006, 140, 133–142. [Google Scholar] [CrossRef]
- Chitimia-Dobler, L. Spatial distribution of Dermacentor reticulatus in Romania. Vet Parasitol. 2015, 214, 219–223. [Google Scholar] [CrossRef]
- Selmi, M.; Ballardini, M.; Salvato, L.; Ricci, E. Rickettsia spp. in Dermacentor marginatus ticks: Analysis of the host-vector-pathogen interactions in a northern Mediterranean area. Exp. Appl. Acarol. 2017, 72, 79–91. [Google Scholar] [CrossRef]
- Gomez-Barroso, D.; Vescio, M.F.; Bella, A.; Ciervo, A.; Busani, L.; Rizzo, C.; Rezza, G.; Pezzotti, P. Mediterranean spotted fever rickettsiosis in Italy, 2001–2015: Spatio-temporal distribution based on hospitalization records. Ticks Tick Borne Dis. 2019, 10, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Rezza, G.; Ciervo, A. Le rickettsiosi in Italia: Diagnosi e sorveglianza. Notiziario dell’Istituto Superiore di Sanità 2015, 28, 3–8. [Google Scholar]
- Regional Service for the Epidemiology of Infectious Diseases (SeREMI). Available online: https://www.seremi.it/ (accessed on 20 August 2020).
- Garcia-Vozmediano, A.; Krawczyk, A.I.; Sprong, H.; Rossi, L.; Ramassa, E.; Tomassone, L. Ticks climb the mountains: Ixodid tick infestation and infection by tick-borne pathogens in Western Alps. Ticks Tick Borne Dis. 2020, 11, 101489. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.T.; Bak, J.M.; Hu, R.; Nicholson, M.C.; Kelly, C.; Mather, T.N. Determining the duration of Ixodes scapularis (Acari: Ixodidae) attachment to tick-bite victims. J. Med. Entomol. 1995, 32, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Martello, E.; Mannelli, A.; Grego, E.; Ceballos, L.A.; Ragagli, C.; Stella, M.C.; Tomassone, L. Borrelia burgdorferi sensu lato and spotted fever group rickettsiae in small rodents and attached ticks in the Northern Apennines, Italy. Ticks Tick Borne Dis. 2019, 10, 862–867. [Google Scholar] [CrossRef]
- Labruna, M.B.; Whitworth, T.; Bouyer, D.H.; Mcbride, J.W.; Pinter, A.; Popov, V.; Gennari, S.M.; Walker, D.H. Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of Sao Paulo, Brazil, where brazilian Spotted Fever is endemic. J. Clin. Microbiol. 2004, 42, 90–98. [Google Scholar] [CrossRef]
- Fournier, P.E.; Roux, V.; Raoult, D. Phylogenetic analysis of spotted fever group rickettsiae by study of the outer surface protein rOmpA. Int. J. Syst. Bacteriol. 1998, 48, 839–849. [Google Scholar] [CrossRef]
- Sumner, J.W.; Durden, L.A.; Goddard, J.; Stromdahl, E.Y.; Clark, K.L.; Reeves, W.K.; Paddock, C.D. Gulf coast ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg. Infect. Dis. 2007, 13, 751–753. [Google Scholar] [CrossRef]
- Jensen, W.A.; Fall, M.Z.; Rooney, J.; Kordick, D.L.; Breitschwerdt, E.B. Rapid identification and differentiation of Bartonella species using a single-step PCR assay. J. Clin. Microbiol. 2000, 38, 1717–1722. [Google Scholar] [CrossRef]
- Michelet, L.; Bonnet, S.; Madani, N.; Moutailler, S. Discriminating Francisella tularensis and Francisella-like endosymbionts in Dermacentor reticulatus ticks: Evaluation of current molecular techniques. Vet. Microbiol. 2013, 163, 399–403. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.r-project.org/index.html (accessed on 5 September 2020).
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids. Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Manfredi, M.T. Dermacentor reticulatus Fabricius 1794 nel cane in Italia. Atti Soc. Ital. Sci. Vet. 1995, 49, 759–760. [Google Scholar]
- Iori, A.; Gabrielli, S.; Calderini, P.; Moretti, A.; Pietrobelli, M.; Tampieri, M.P.; Galuppi, R.; Cancrini, G. Tick reservoirs for piroplasms in central and northern Italy. Vet. Parasitol. 2010, 170, 291–296. [Google Scholar] [CrossRef]
- Acquaotta, F.; Fratianni, S.; Garzena, D. Temperature changes in the North-Western Italian Alps from 1961 to 2010. Theor. Appl. Climatol. 2015, 122, 619–634. [Google Scholar] [CrossRef]
- Selmi, M.; Tomassone, L.; Ceballos, L.A.; Crisci, A.; Ragagli, C.; Pintore, M.D.; Mignone, W.; Pautasso, A.; Ballardini, M.; Casalone, C.; et al. Analysis of the environmental and host-related factors affecting the distribution of the tick Dermacentor marginatus. Exp. Appl. Acarol. 2018, 75, 209–225. [Google Scholar] [CrossRef]
- Audino, T.; Pautasso, A.; Bellavia, V.; Carta, V.; Ferrari, A.; Verna, F.; Grattarola, C.; Iulini, B.; Pintore, M.; Bardelli, M.; et al. Ticks Infesting Humans in North-Western Italy and Associated Pathogens: A Cross-Sectional Study in a Three-Year Period (2017–2019) in North-Western Italy, PREPRINT (Version 1). 2020. [CrossRef]
- Toledo, A.; Olmeda, A.S.; Escudero, R.; Jado, I.; Valcárcel, F.; Casado-Nistal, M.A.; Rodríguez-Vargas, M.; Gil, H.; Anda, P. Tick-borne zoonotic bacteria in ticks collected from Central Spain. Am. J. Trop. Med. Hyg. 2009, 81, 67–74. [Google Scholar] [CrossRef]
- Ortuño, A.; Quesada, M.; López-Claessens, S.; Castellà, J.; Sanfeliu, I.; Antón, E.; Segura-Porta, F. The role of wild boar (Sus scrofa) in the eco-epidemiology of R. slovaca in Northeastern Spain. Vector Borne Zoonotic Dis. 2007, 7, 59–69. [Google Scholar] [CrossRef]
- Selmi, M.; Martello, E.; Bertolotti, L.; Bisanzio, D.; Tomassone, L. Rickettsia slovaca and Rickettsia raoultii in Dermacentor marginatus ticks collected on wild boars in Tuscany, Italy. J. Med. Entomol. 2009, 46, 1490–1493. [Google Scholar] [CrossRef] [PubMed]
- Špitálska, E.; Štefanidesová, K.; Kocianová, E.; Boldiš, V. Rickettsia slovaca and Rickettsia raoultii in Dermacentor marginatus and Dermacentor reticulatus ticks from Slovak Republic. Exp. Appl. Acarol. 2012, 57, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Tomassone, L.; Portillo, A.; Nováková, M.; de Sousa, R.; Oteo, J.A. Neglected aspects of tick-borne rickettsioses. Parasit. Vectors. 2018, 11, 263. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Raoult, D. Ticks and tickborne bacterial diseases in humans: An emerging infectious threat. Clin. Infect. Dis. 2001, 32, 897–928. [Google Scholar] [CrossRef]
- Martello, E.; Selmi, M.; Ragagli, C.; Ambrogi, C.; Stella, M.C.; Mannelli, A.; Tomassone, L. Rickettsia slovaca in immature Dermacentor marginatus and tissues from Apodemus spp. in the northern Apennines, Italy. Ticks Tick Borne Dis. 2013, 4, 518–521. [Google Scholar] [CrossRef]
- Zeroual, F.; Leulmi, H.; Bitam, I.; Benakhla, A. Molecular evidence of Rickettsia slovaca in spleen of wild boars in northeastern Algeria. New Microbes New Infect. 2018, 24, 17–20. [Google Scholar] [CrossRef]
- Cicculli, V.; Oscar, M.; Casabianca, F.; Villechenaud, N.; Charrel, R.; de Lamballerie, X.; Falchi, A. Molecular detection of spotted-fever group rickettsiae in ticks collected from domestic and wild animals in Corsica, France. Pathogens 2019, 8, 138. [Google Scholar] [CrossRef]
- Ibarra, V.; Oteo, J.A.; Portillo, A.; Santibáñez, S.; Blanco, J.R.; Metola, L.; Eiros, J.M.; Pérez-Martínez, L.; Sanz, M. Rickettsia slovaca infection: DEBONEL/TIBOLA. Ann. N. Y. Acad. Sci. 2006, 1078, 206–214. [Google Scholar] [CrossRef]
- Parola, P.; Rovery, C.; Rolain, J.M.; Brouqui, P.; Davoust, B.; Raoult, D. Rickettsia slovaca and R. raoultii in Tick-borne Rickettsioses. Emerg. Infect. Dis. 2009, 15, 1105–1108. [Google Scholar] [CrossRef]
- Dutto, M.; Selmi, M. Sindrome TIBOLA in Piemonte. Decidere Med. 2012, 12, 87–88. [Google Scholar]
- Remesar, S.; Díaz, P.; Portillo, A.; Santibáñez, S.; Prieto, A.; Díaz-Cao, J.M.; López, C.M.; Panadero, R.; Fernández, G.; Díez-Baños, P.; et al. Prevalence and molecular characterization of Rickettsia spp. in questing ticks from Northwestern Spain. Exp. Appl. Acarol. 2019, 79, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Portillo, A.; Ibarra, V.; Santibáñez, S.; Pérez-Martínez, L.; Blanco, J.R.; Oteo, J.A. Genetic characterisation of ompA, ompB and gltA genes from Candidatus Rickettsia rioja. Clin. Microbiol. Infect. 2009, 15, 307–308. [Google Scholar] [CrossRef]
- Pérez-Pérez, L.; Portillo, A.; Allegue, F.; Zulaica, A.; Oteo, J.A.; Caeiro, J.L.; Fabeiro, J.M. Dermacentor-borne Necrosis Erythema and Lymphadenopathy (DEBONEL): A case associated with Rickettsia rioja. Acta Derm. Venereol. 2010, 90, 214–215. [Google Scholar] [CrossRef] [PubMed]
- Santibáñez, S.; Portillo, A.; Santibáñez, P.; Palomar, A.M.; Oteo, J.A. Usefulness of rickettsial PCR assays for the molecular diagnosis of human rickettsioses. Enferm. Infecc. Microbiol. Clin. 2013, 31, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Rar, V.A.; Fomenko, N.V.; Dobrotvorsky, A.K.; Livanova, N.N.; Rudakova, S.A.; Fedorov, E.G.; Astanin, V.B.; Morozova, O.V. Tickborne pathogen detection, Western Siberia, Russia. Emerg. Infect. Dis. 2005, 11, 1708–1715. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; de la Fuente, J.; Nicollet, P.; Liu, X.; Madani, N.; Blanchard, B.; Maingourd, C.; Alongi, A.; Torina, A.; Fernández de Mera, I.G.; et al. Prevalence of tick-borne pathogens in adult Dermacentor spp. ticks from nine collection sites in France. Vector Borne Zoonotic Dis. 2013, 13, 226–236. [Google Scholar] [CrossRef]
- Satta, G.; Chisu, V.; Cabras, P.; Fois, F.; Masala, G. Pathogens and symbionts in ticks: A survey on tick species distribution and presence of tick-transmitted micro-organisms in Sardinia, Italy. J. Med. Microbiol. 2011, 60, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Chisu, V.; Foxi, C.; Mannu, R.; Satta, G.; Masala, G. A five-year survey of tick species and identification of tick-borne bacteria in Sardinia, Italy. Ticks Tick Borne Dis. 2018, 9, 678–681. [Google Scholar] [CrossRef]
- Angelakis, E.; Pulcini, C.; Waton, J.; Imbert, P.; Socolovschi, C.; Edouard, S.; Dellamoniaca, P.; Raoult, D. Scalp eschar and neck lymphadenopathy caused by Bartonella henselae after tick bite. Clin. Infect. Dis. 2010, 50, 549–551. [Google Scholar] [CrossRef]
- Cotté, V.; Bonnet, S.; Le Rhun, D.; Le Naour, E.; Chauvin, A.; Boulouis, H.J.; Lecuelle, B.; Lilin, T.; Vayssier-Taussat, M. Transmission of Bartonella henselae by Ixodes ricinus. Emerg. Infect. Dis. 2008, 14, 1074–1080. [Google Scholar] [CrossRef]
- Reis, C.; Cote, M.; Le Rhun, D.; Lecuelle, B.; Levin, M.L.; Vayssier-Taussat, M.; Bonnet, S.I. Vector competence of the tick Ixodes ricinus for transmission of Bartonella birtlesii. PLoS Negl. Trop. Dis. 2011, 5, e1186. [Google Scholar] [CrossRef]
- Sprong, H.; Fonville, M.; Docters van Leeuwen, A.; Devillers, E.; Ibañez-Justicia, A.; Stroo, A.; Hansford, K.; Cull, B.; Medlock, J.; Heyman, P.; et al. Detection of pathogens in Dermacentor reticulatus in northwestern Europe: Evaluation of a high-throughput array. Heliyon 2019, 5, e01270. [Google Scholar] [CrossRef] [PubMed]
- Michelet, L.; Delannoy, S.; Devillers, E.; Umhang, G.; Aspan, A.; Juremalm, M.; Chirico, J.; van der Wal, F.J.; Sprong, H.; Boye Pihl, T.P.; et al. High-throughput screening of tick-borne pathogens in Europe. Front. Cell. Infect. Microbiol. 2014, 4, 103. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.N.; Mitkova, N.; Reye, A.L.; Hubschen, J.M.; Vatcheva-Dobrevska, R.S.; Dobreva, E.G.; Kantardjiev, T.V.; Muller, C.P. Detection of new Francisella-like tick endosymbionts in Hyalomma spp. and Rhipicephalus spp. (Acari: Ixodidae) from Bulgaria. Appl. Environ. Microbiol. 2011, 77, 5562–5565. [Google Scholar] [CrossRef] [PubMed]
- Sréter-Lancz, Z.; Széll, Z.; Sréter, T.; Márialigeti, K. Detection of a novel Francisella in Dermacentor reticulatus: A need for careful evaluation of PCR-based identification of Francisella tularensis in Eurasian ticks. Vector Borne Zoonotic Dis. 2009, 9, 123–126. [Google Scholar] [CrossRef]
- de Carvalho, I.L.; Santos, N.; Soares, T.; Ze-Ze, L.; Nuncio, M.S. Francisella-like endosymbiont in Dermacentor reticulatus collected in Portugal. Vector Borne Zoonotic Dis. 2011, 11, 185–188. [Google Scholar] [CrossRef]
- Cerutti, F.; Modesto, P.; Rizzo, F.; Cravero, A.; Jurman, I.; Costa, S.; Giammarino, M.; Mandola, M.L.; Goria, M.; Radovic, S.; et al. The microbiota of hematophagous ectoparasites collected from migratory birds. PLoS ONE 2018, 13, e0202270. [Google Scholar] [CrossRef]
- Gerhart, J.G.; Dutcher, H.A.; Brenner, A.E.; Moses, A.S.; Grubhoffer, L.; Raghavan, R. Multiple Acquisitions of Pathogen-Derived Francisella Endosymbionts in Soft Ticks. Genome Biol. Evol. 2018, 10, 607–615. [Google Scholar] [CrossRef]
- Sperling, J.; MacDonald, Z.; Normandeau, J.; Merrill, E.; Sperling, F.; Magor, K. Within-population diversity of bacterial microbiomes in winter ticks (Dermacentor albipictus). Ticks Tick Borne Dis. 2020, 11, 101535. [Google Scholar] [CrossRef]
- Scoles, G.A. Phylogenetic Analysis of the Francisella-like Endosymbionts of Dermacentor ticks. J. Med. Entomol. 2004, 41, 277–286. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).