Effects of Early Nutrition and Sanitary Conditions on Oral Tolerance and Antibody Responses in Broiler Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Designs
2.1.1. Experiment I
2.1.2. Experiment II
2.2. Sanitary Conditions
2.3. Induction of Oral Tolerance and Immunizations
2.4. Sample Collection
2.5. Bacterial Load in Cecum
2.6. Antibody Levels
2.7. Statistical Analyses
2.7.1. Experiment I
2.7.2. Experiment II
3. Results
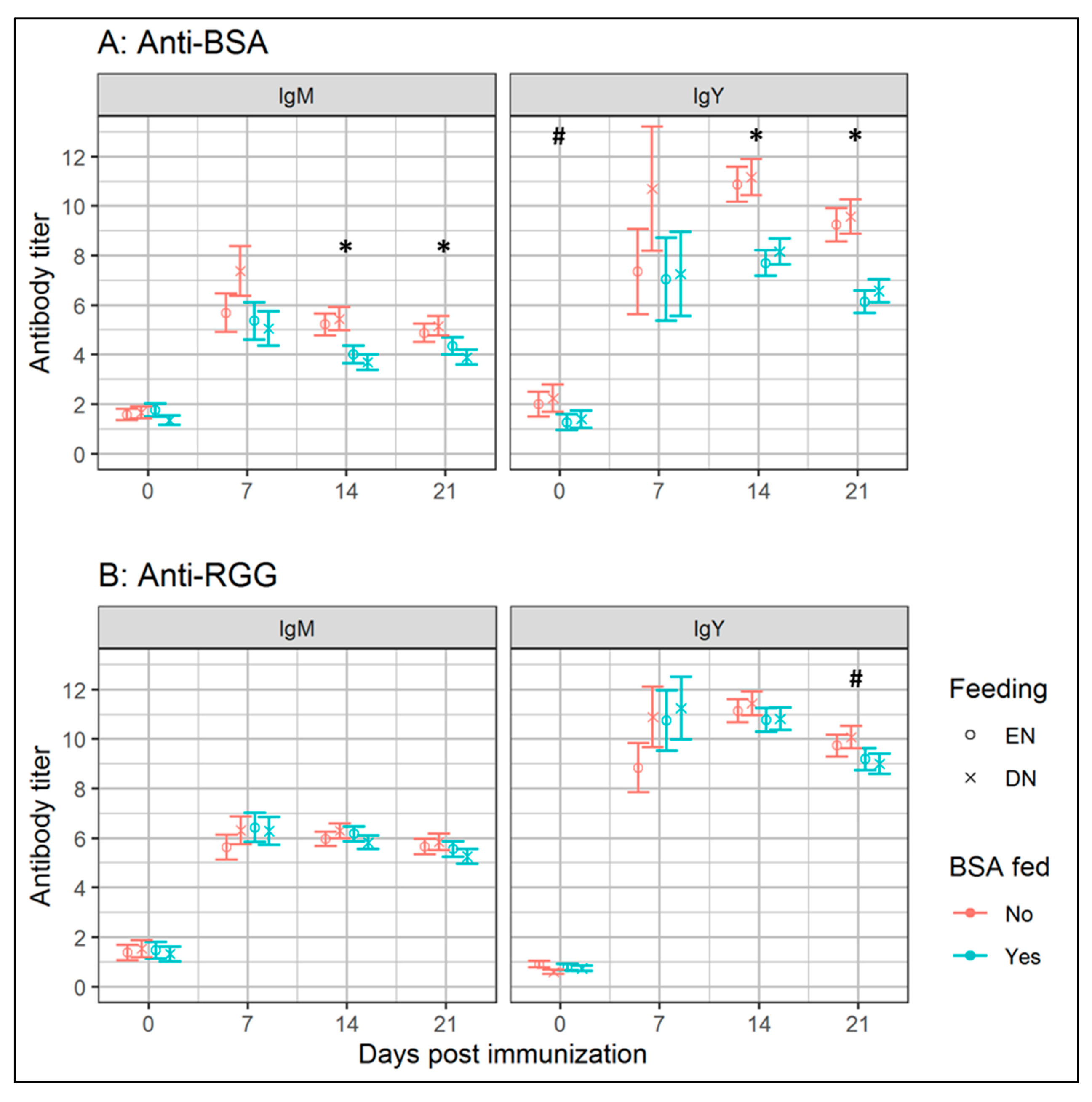
3.1. Experiment I: Effects of Early Antigenic Exposure on Later Life Antibody Responses
3.2. Experiment II: Effects of Early Life Feeding Strategy on Antibody Responses under Different Sanitary Conditions
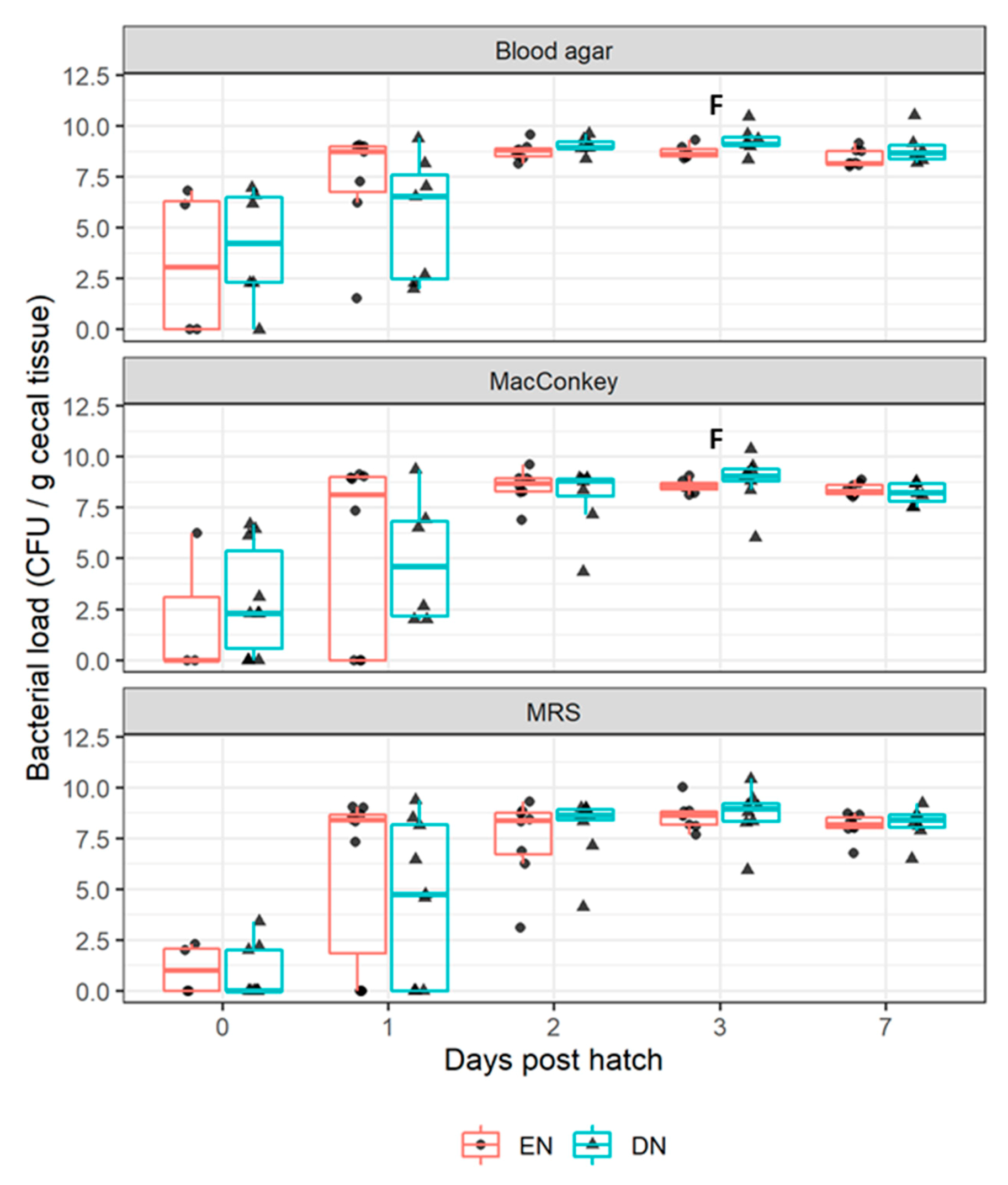
3.2.1. Bacterial Colonization
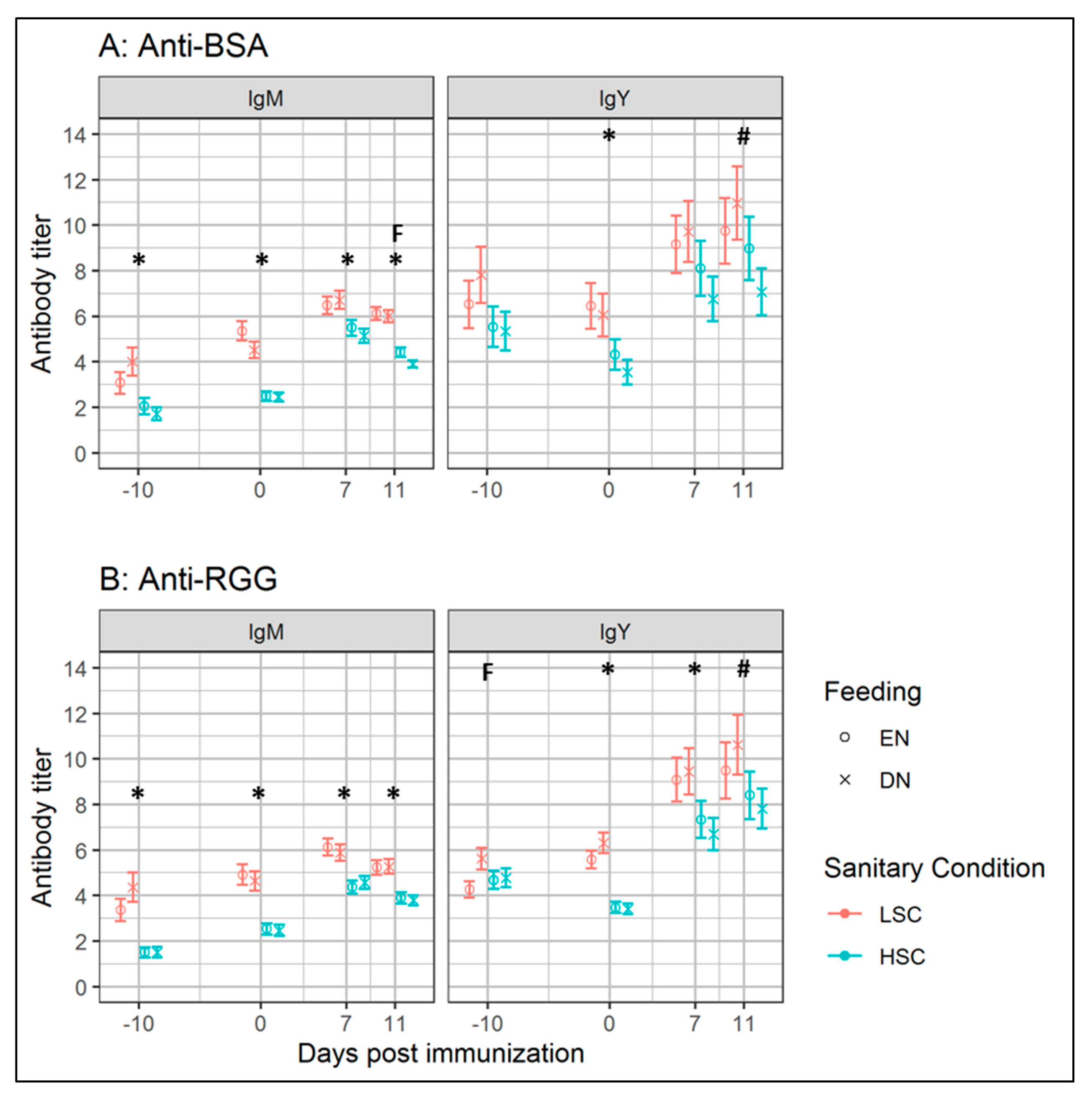
3.2.2. Antibody Levels and Responses
3.2.3. Growth Performance
4. Discussion
4.1. Aerobic Bacterial Colonization after Early Nutrition
4.2. Early-Life BSA Feeding Lowers Later-Life Antibody Responses Towards BSA under High Sanitary Conditions
4.3. Introduction of Low Sanitary Conditions
4.4. Antibody Responses and Performance after Early Nutrition under Different Sanitary Conditions
4.5. Low Sanitary Conditions Increased Antibody Levels
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Jong, I.C.; van Riel, J.; Bracke, M.B.M.; van den Brand, H. A “meta-analysis” of effects of post-hatch food and water deprivation on development, performance and welfare of chickens. PLoS ONE 2017, 12, e0189350. [Google Scholar] [CrossRef]
- Taha-Abdelaziz, K.; Hodgins, D.C.; Lammers, A.; Alkie, T.N.; Sharif, S. Effects of early feeding and dietary interventions on development of lymphoid organs and immune competence in neonatal chickens: A review. Vet. Immunol. Immunopathol. 2018, 201, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bar-Shira, E.; Sklan, D.; Friedman, A. Impaired immune responses in broiler hatchling hindgut following delayed access to feed. Vet. Immunol. Immunopathol. 2005, 105, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Bar-Shira, E.; Friedman, A. Development and adaptations of innate immunity in the gastrointestinal tract of the newly hatched chick. Dev. Comp. Immunol. 2006, 30, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Binek, M.; Borzemska, W.; Pisarski, R.; Blaszczak, B.; Kosowska, G.; Malec, H.; Karpinska, E. Evaluation of the efficacy of feed providing on development of gastrointestinal microflora of newly hatched broiler chickens. Arch. Geflugelkd 2000, 64, 147–151. [Google Scholar]
- Karpinska, E.; Blaszczak, B.; Kosowska, G.; Degorski, A.; Binek, M.; Borzemska, W.B. Growth of the intestinal anaerobes in the newly hatched chicks according to the feeding and providing with normal gut flora. Bull. Vet. Inst. Pulawy 2001, 45, 105–109. [Google Scholar]
- Potturi, P.V.L.; Patterson, J.A.; Applegate, T.J. Effects of delayed placement on intestinal characteristics in turkey poults. Poult. Sci. 2005, 84, 816–824. [Google Scholar] [CrossRef]
- Klipper, E.; Sklan, D.; Friedman, A. Response, tolerance and ignorance following oral exposure to a single dietary protein antigen in Gallus domesticus. Vaccine 2001, 19, 2890–2897. [Google Scholar] [CrossRef]
- Simon, K.; de Vries Reilingh, G.; Bolhuis, J.E.; Kemp, B.; Lammers, A. Early feeding and early life housing conditions influence the response towards a noninfectious lung challenge in broilers. Poult. Sci. 2015, 94, 2041–2048. [Google Scholar] [CrossRef]
- Lamot, D.M.; van der Klein, S.A.S.; van de Linde, I.B.; Wijtten, P.J.A.; Kemp, B.; van den Brand, H.; Lammers, A. Effects of feed access after hatch and inclusion of fish oil and medium chain fatty acids in a pre-starter diet on broiler chicken growth performance and humoral immunity. Animal 2016, 10, 1409–1416. [Google Scholar] [CrossRef]
- Careghi, C.; Tona, K.; Onagbesan, O.; Buyse, J.; Decuypere, E.; Bruggeman, V. The effects of the spread of hatch and interaction with delayed feed access after hatch on broiler performance until seven days of age. Poult. Sci. 2005, 84, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Development Core Team: Vienna, Austria, 2018. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Lineair and Nonlinear Mixed Effects Models; R Development Core Team: Vienna, Austria, 2018. [Google Scholar]
- Reyneveld, G.I.; Savelkoul, H.F.J.; Parmentier, H.K. Current Understanding of Natural Antibodies and Exploring the Possibilities of Modulation Using Veterinary Models. A Review. Front. Immunol. 2020, 11, 2139. [Google Scholar] [CrossRef]
- Parmentier, H.K.; Lammers, A.; Hoekman, J.J.; de Vries Reilingh, G.; Zaanen, I.T.A.; Savelkoul, H.F.J. Different levels of natural antibodies in chickens divergently selected for specific antibody responses. Dev. Comp. Immunol. 2004, 28, 39–49. [Google Scholar] [CrossRef]
- Simon, K. Effects of Early Life Conditions on Immunity in Broilers and Layers. Ph.D. Thesis, Wageningen University & Research, Wageningen, The Netherlands, 2016. [Google Scholar]
- Apajalahti, J.; Kettunen, A.; Graham, H. Characteristics of the gastrointestinal microbial communities, with special reference to the chicken. World’s Poult. Sci. J. 2004, 60, 223–232. [Google Scholar] [CrossRef]
- Leshchinsky, T.V.; Klasing, K.C. Divergence of the inflammatory response in two types of chickens. Dev. Comp. Immunol. 2001, 25, 629–638. [Google Scholar] [CrossRef]
- Koenen, M.E.; Boonstra-Blom, A.G.; Jeurissen, S.H.M. Immunological differences between layer-and broiler-type chickens. Vet. Immunol. Immunopathol. 2002, 89, 47–56. [Google Scholar] [CrossRef]
- Simon, K.; de Vries Reilingh, G.; Kemp, B.; Lammers, A. Development of ileal cytokine and immunoglobulin expression levels in response to early feeding in broilers and layers. Poult. Sci. 2014, 93, 3017–3027. [Google Scholar] [CrossRef]
- Klipper, E.; Sklan, D.; Friedman, A. Maternal antibodies block induction of oral tolerance in newly hatched chicks. Vaccine 2004, 22, 493–502. [Google Scholar] [CrossRef]
- Ifrah, M.E.; Perelman, B.; Finger, A.; Uni, Z. The role of the bursa of Fabricius in the immune response to vaccinal antigens and the development of immune tolerance in chicks (Gallus domesticus) vaccinated at a very young age. Poult. Sci. 2016, 96, 51–57. [Google Scholar] [CrossRef]
- Lammers, A.; Wieland, W.H.; Kruijt, L.; Jansma, A.; Straetemans, T.; Schots, A.; den Hartog, G.; Parmentier, H.K. Successive immunoglobulin and cytokine expression in the small intestine of juvenile chicken. Dev. Comp. Immunol. 2010, 34, 1254–1262. [Google Scholar] [CrossRef]
- Bar-Shira, E.; Cohen, I.; Elad, O.; Friedman, A. Role of goblet cells and mucin layer in protecting maternal IgA in precocious birds. Dev. Comp. Immunol. 2014, 44, 186–194. [Google Scholar] [CrossRef] [PubMed]
- den Hartog, G.; de Vries-Reilingh, G.; Wehrmaker, A.M.; Savelkoul, H.F.J.; Parmentier, H.K.; Lammers, A. Intestinal immune maturation is accompanied by temporal changes in the composition of the microbiota. Benef. Microbes 2016, 7, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, Y.; Lammers, A.; Jansman, A.J.M.; Rijnen, M.M.J.A.; Hendriks, W.H.; Gerrits, W.J.J. Performance of pigs kept under different sanitary conditions affected by protein intake and amino acid supplementation. J. Anim. Sci. 2016, 94, 4704–4719. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, Y.; Gerrits, W.J.J.; Jansman, A.J.M.; Kemp, B.; Bolhuis, J.E. A link between damaging behaviour in pigs, sanitary conditions, and dietary protein and amino acid supply. PLoS ONE 2017, 12, e0174688. [Google Scholar] [CrossRef]
- Iseri, V.J.; Klasing, K.C. Dynamics of the systemic components of the chicken (Gallus gallus domesticus) immune system following activation by Escherichia coli; implications for the costs of immunity. Dev. Comp. Immunol. 2013, 40, 248–257. [Google Scholar] [CrossRef]
- Van der Meer, Y. Nutrition of Pigs Kept under Low and High Sanitary Conditions. Ph.D. Thesis, Wageningen University & Research, Wageningen, The Netherlands, 2017. [Google Scholar]
- Parmentier, H.K.; Bronkhorst, S.; Nieuwland, M.G.B.; de Vries Reilingh, G.; van der Linden, J.M.; Heetkamp, M.J.W.; Kemp, B.; Schrama, J.W.; Verstegen, M.W.A.; van den Brand, H. Increased fat deposition after repeated immunization in growing chickens. Poult. Sci. 2002, 81, 1308–1316. [Google Scholar] [CrossRef]
| Treatments | Fixed Effects 1 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early Nutrition PBS Fed | Early Nutrition BSA Fed | Delayed Nutrition PBS Fed | Delayed Nutrition BSA Fed | ||||||||||||
| Antigen | Mean | SEM | n2 | Mean | SEM | n | Mean | SEM | n | Mean | SEM | n | BSA | Feeding | BSA × Feeding |
| BSA | 5.1 | 0.38 | 11 | 6.4 | 0.55 | 8 | 4.4 | 0.33 | 12 | 5.0 | 0.36 | 13 | 0.05 | 0.05 | 0.47 |
| RGG | 5.2 | 0.18 | 11 | 5.6 | 0.23 | 8 | 5.0 | 0.17 | 12 | 5.2 | 0.17 | 13 | 0.18 | 0.18 | 0.68 |
| Parameter | Age (d) | Early Nutrition | Delayed Nutrition | Feeding Effect 1 | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SEM | n2 | Mean | SEM | n | |||
| Body weight (g) | 0 | 43.8 | 0.39 | 27 | 44.1 | 0.40 | 26 | 0.60 |
| 21 | 889 | 19.8 | 25 | 737 | 16.1 | 26 | 0.001 | |
| 24 | 1135 | 21.9 | 25 | 958 | 26.4 | 26 | 0.001 | |
| 42 | 3418 | 79.1 | 25 | 3202 | 78.2 | 26 | 0.09 | |
| Average daily gain (g/d) | 0–21 | 40.2 | 0.94 | 25 | 33.0 | 0.76 | 26 | 0.001 |
| 21–24 | 83 | 2.9 | 25 | 74 | 2.8 | 26 | 0.07 | |
| 24–42 | 125 | 3.9 | 25 | 124 | 3.9 | 26 | 0.79 | |
| 0–42 | 80 | 1.9 | 25 | 75 | 1.9 | 26 | 0.09 | |
| Antigen | Isotype | Early Nutrition LSC | Early Nutrition HSC | Delayed Nutrition LSC | Delayed Nutrition HSC | Fixed Effects 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | SC | Feeding | Batch 2 | SC × Feeding | ||
| BSA | IgM | 1.3 | 0.17 | 2.2 | 0.29 | 1.4 | 0.18 | 2.1 | 0.27 | 0.01 | 0.99 | 0.07 | 0.53 |
| BSA | IgY | 1.3 | 0.16 | 1.6 | 0.19 | 1.3 | 0.16 | 1.5 | 0.18 | 0.21 | 0.99 | 0.25 | 0.80 |
| RGG | IgM | 1.3 | 0.13 | 1.8 | 0.18 | 1.2 | 0.13 | 1.9 | 0.19 | <0.01 | 0.88 | 0.01 | 0.76 |
| RGG | IgY | 1.3 | 0.13 | 1.7 | 0.17 | 1.2 | 0.12 | 1.4 | 0.14 | 0.07 | 0.22 | 0.02 | 0.49 |
| Antigen | Early Nutrition LSC | Early Nutrition HSC | Delayed Nutrition LSC | Delayed Nutrition HSC | Fixed Effects 1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | n2 | Mean | SEM | n | Mean | SEM | n | Mean | SEM | n | Feeding | SC | Batch | SC × Feeding | Batch × Feeding | |
| BSA | 7.7 | 0.25 | 32 | 6.0 | 0.24 | 35 | 7.6 | 0.26 | 29 | 6.0 | 0.24 | 35 | 0.71 | <0.001 | 0.20 | 0.91 | 0.06 |
| RGG | 7.5 | 0.15 | 32 | 6.1 | 0.15 | 36 | 7.1 | 0.16 | 29 | 5.9 | 0.15 | 35 | 0.03 | <0.001 | 0.37 | 0.72 | - 3 |
| Parameter | Age | Early Nutrition LSC | Early Nutrition HSC | Delayed Nutrition LSC | Delayed Nutrition HSC | Fixed Effects 1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | n2 | Mean | SE | n | Mean | SE | n | Mean | SE | n | Feeding | SC | Batch | Feeding × SC | Batch × SC | ||
| Body weight | 3 | N/A 3 | 68.7 | 0.42 | 24 | N/A | 38.5 | 0.42 | 24 | <0.001 | - | <0.001 | - | - | ||||
| (g) | 33 | 1633 | 36.2 | 12 | 2071 | 36.2 | 12 | 1313 | 36.2 | 12 | 1771 | 36.2 | 12 | <0.001 | <0.001 | 0.02 | 0.78 | <0.01 |
| Average daily | 3–14 | 26 | 0.5 | 12 | 32 | 0.5 | 12 | 17 | 0.5 | 12 | 23 | 0.5 | 12 | <0.001 | <0.001 | <0.001 | 0.73 | <0.001 |
| gain (g/d) | 14–28 | 59 | 1.5 | 12 | 78 | 1.5 | 12 | 50 | 1.5 | 12 | 68 | 1.5 | 12 | <0.001 | <0.001 | 0.12 | 0.82 | 0.01 |
| 28–33 | 90 | 2.6 | 12 | 112 | 2.6 | 12 | 77 | 2.6 | 12 | 105 | 2.6 | 12 | <0.01 | <0.001 | 0.32 | 0.28 | 0.04 | |
| 3–33 | 52 | 1.2 | 12 | 67 | 1.2 | 12 | 42 | 1.2 | 12 | 58 | 1.2 | 12 | <0.001 | <0.001 | 0.03 | 0.72 | 0.01 | |
| Average daily | 3–14 | 32 b | 0.7 | 12 | 37 a | 0.7 | 12 | 21 d | 0.7 | 12 | 28 c | 0.7 | 11 | <0.001 | <0.001 | <0.001 | 0.03 | 0.07 |
| feed intake (g/d) | 14–28 | 86 | 2.9 | 12 | 108 | 2.9 | 12 | 70 | 2.9 | 12 | 90 | 3.0 | 9 | <0.001 | <0.001 | 0.09 | 0.83 | 0.04 |
| 28–33 | 149 | 4.8 | 10 | 173 | 5.0 | 11 | 121 | 4.8 | 12 | 160 | 4.8 | 10 | <0.001 | <0.001 | 0.95 | 0.14 | 0.13 | |
| 3–33 | 74 | 2.0 | 12 | 90 | 2.1 | 11 | 58 | 2.0 | 12 | 77 | 2.1 | 11 | <0.001 | <0.001 | 0.16 | 0.42 | 0.09 | |
| Feed conversion ratio | 3–14 | 1.24 | 0.037 | 12 | 1.15 | 0.039 | 12 | 1.22 | 0.037 | 12 | 1.23 | 0.041 | 11 | 0.48 | 0.44 | <0.001 | 0.21 | <0.001 |
| 14–28 | 1.44 | 0.017 | 12 | 1.39 | 0.017 | 12 | 1.39 | 0.017 | 12 | 1.32 | 0.018 | 9 | 0.01 | <0.01 | 0.11 | 0.47 | 0.63 | |
| 28–33 | 1.60 | 0.023 | 10 | 1.54 | 0.022 | 11 | 1.57 | 0.020 | 12 | 1.51 | 0.022 | 10 | 0.26 | 0.03 | 0.09 | 0.89 | 0.50 | |
| 3–33 | 1.43 | 0.011 | 12 | 1.34 | 0.011 | 11 | 1.37 | 0.011 | 12 | 1.32 | 0.012 | 9 | <0.01 | <0.001 | 0.56 | 0.18 | 0.13 | |
| Corrected FCR 4 | 3–33 | 1.46 | 0.011 | 12 | 1.34 | 0.011 | 11 | 1.44 | 0.011 | 12 | 1.34 | 0.013 | 9 | 0.71 | <0.001 | 0.36 | 0.23 | 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hollemans, M.S.; Reilingh, G.d.V.; Vries, S.d.; Parmentier, H.K.; Lammers, A. Effects of Early Nutrition and Sanitary Conditions on Oral Tolerance and Antibody Responses in Broiler Chickens. Vet. Sci. 2020, 7, 148. https://doi.org/10.3390/vetsci7040148
Hollemans MS, Reilingh GdV, Vries Sd, Parmentier HK, Lammers A. Effects of Early Nutrition and Sanitary Conditions on Oral Tolerance and Antibody Responses in Broiler Chickens. Veterinary Sciences. 2020; 7(4):148. https://doi.org/10.3390/vetsci7040148
Chicago/Turabian StyleHollemans, Maarten S., Ger de Vries Reilingh, Sonja de Vries, Henk K. Parmentier, and Aart Lammers. 2020. "Effects of Early Nutrition and Sanitary Conditions on Oral Tolerance and Antibody Responses in Broiler Chickens" Veterinary Sciences 7, no. 4: 148. https://doi.org/10.3390/vetsci7040148
APA StyleHollemans, M. S., Reilingh, G. d. V., Vries, S. d., Parmentier, H. K., & Lammers, A. (2020). Effects of Early Nutrition and Sanitary Conditions on Oral Tolerance and Antibody Responses in Broiler Chickens. Veterinary Sciences, 7(4), 148. https://doi.org/10.3390/vetsci7040148




_Lammers.jpeg)

