How Ultrasound Can Be Useful for Staging Chronic Kidney Disease in Dogs: Ultrasound Findings in 855 Cases
Abstract
1. Introduction
2. Materials and Methods
2.1. Ultrasonographic Procedures
- Renal Contour: regular or irregular contour.
- Cortico-medullary junction (C/M junction): normal or abnormal. C/M junction was considered abnormal for shaded or absent, enhanced or hyperechoic differentiation.
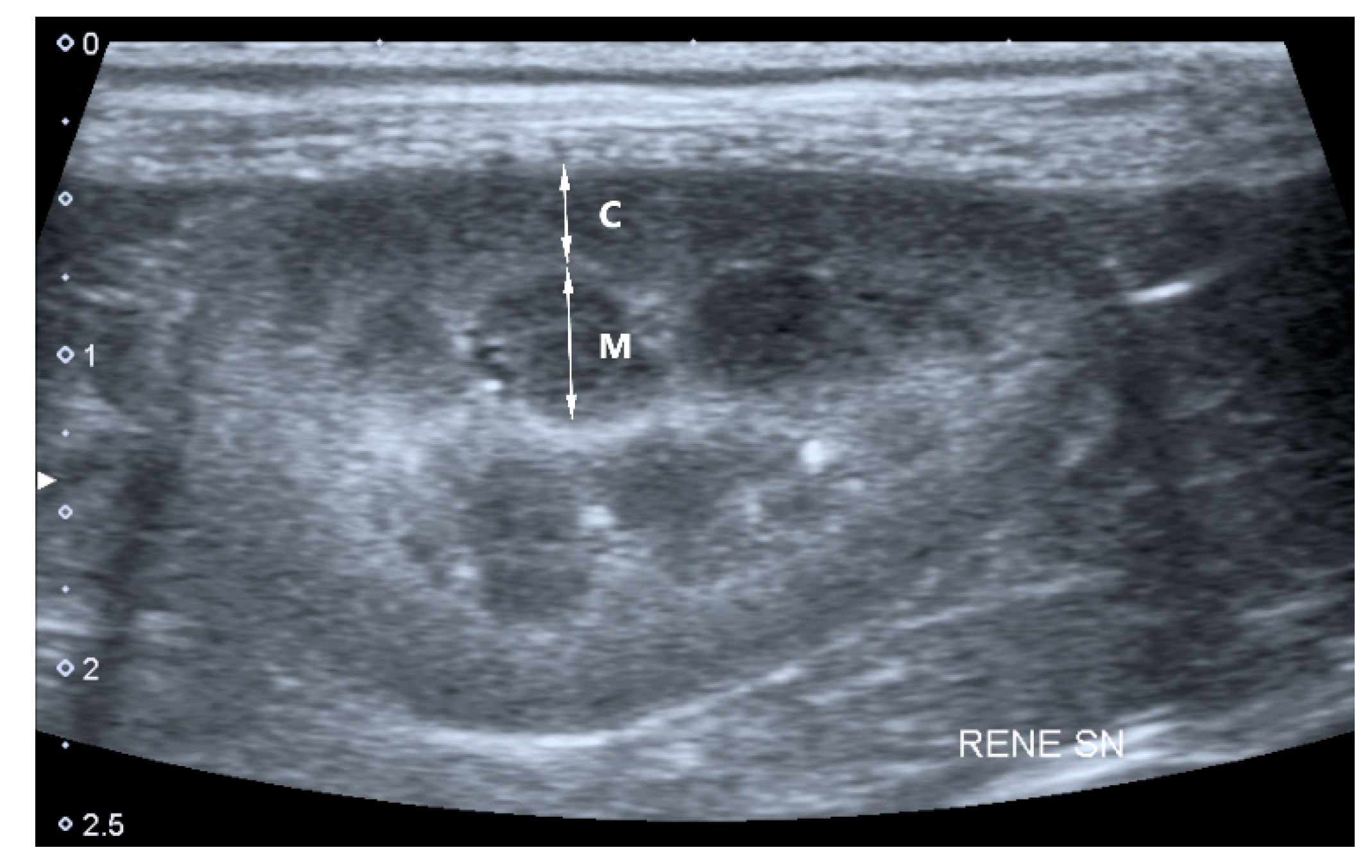
- Cortical and medullary ratio (C/M ratio): Normal (C/M ratio > 1:1) or abnormal (C/M ratio < 1:1). Cortical thickness was measured in the sagittal plane over a medullary pyramid, perpendicular to the capsule. Medullary thickness was measured from the renal hilum to the outer margin of the cortico-medullary junction (Figure 1).
- Cortical echogenicity: normal or abnormal (increased cortical echogenicity compared to the echogenicity of the liver and spleen).
- Medullary echogenicity: normal or abnormal (increased medullary echogenicity compared to the echogenicity of the renal cortex).
- Cysts: presence or absence of cortical cysts that appear oval shaped, with anechoic content.
- Mineralization: presence or absence. Renal mineralization was defined as dispersed hyperechoic foci.
- Infarcts: presence or absence of renal infarcts. Infarcts were defined as wedge-shaped hyperechoic areas, with a broad base at the surface of the kidney, that narrows toward the corticomedullary junction, resulting in localized thinning of the cortex and renal contour defect.
- Pyelectasia: presence or absence. Pyelectasia was defined as a renal pelvis dilation > 3–4 mm.
- Peri-renal effusion: presence or absence of peri-renal fluid.
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Polzin, D.J. Chronic Kidney Disease. In Nephrology and Urology of Small Animals, 1st ed.; Bartges, J., Polzin, D.J., Eds.; Blackwell Publishing Ltd.: Chicester, UK, 2011; pp. 433–471. [Google Scholar]
- Bartges, J.W. Chronic kidney disease in dogs and cats. Vet. Clin. Small Anim. 2012, 42, 669–692. [Google Scholar] [CrossRef] [PubMed]
- IRIS Staging of CKD (Modified 2017). Available online: http://www.iris-kidney.com/pdf/IRIS_2017_Staging_of_CKD_09May18.pdf (accessed on 7 October 2018).
- Zotti, A.; Banzato, T.; Gelain, M.E.; Centelleghe, C.; Vaccaro, C.; Aresu, L. Correlation of renal histopathology with renal echogenicity in dogs and cats: An ex-vivo quantitative study. BMC Vet. Res. 2015, 11, 99. [Google Scholar] [CrossRef]
- Slike, H.; George, A.H. Ultrasonography of the urinary tract. In Nephrology and Urology of Small Animals, 1st ed.; Bartges, J., Polzin, D.J., Eds.; Blackwell Publishing Ltd.: Chicester, UK, 2011; pp. 128–145. [Google Scholar]
- Bragato, N.; Borges, N.C.; Fioravanti, M.C.S. B-mode and Doppler ultrasound of chronic kidney disease in dogs and cats. Vet. Res. Commun. 2017, 41, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Siddappa, J.K.; Singla, S.; Al Ameen, M.; Rakshit, S.C.; Kumar, N. Correlation of Ultrasonographic parameters with serum creatinine in chronic kidney disease. J. Clin. Imaging Sci. 2013, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Nyland, T.G.; Widmer, W.R.; Mattoon, J.S. Urinary Tract. In Small Animal Diagnostic Ultrasound, 3rd ed.; Mattoon, J., Nyland, T., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2015; pp. 557–607. [Google Scholar]
- Araújo, N.C.; Rioja, L.S.; Rebelo, M.A.P. A clinical predictor index for renal survival. J. Bras. Nefrol. 2010, 32, 27–32. [Google Scholar] [PubMed]
- Kealy, J.K.; McAllister, H. The abdomen. In Diagnostic Radiology & Ultrasonography of the Dog and Cat, 4th ed.; Kealy, J.K., McAllister, H., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2005; pp. 21–172. [Google Scholar]
- Notomi, M.K.; Kogika, M.M.; Ikesaki, J.Y.H.; Monteiro, P.R.G.; Marquesi, M.L. Estudo retrospectivo de casos de insuficiência renal crônica em cães no período de 1999 a 2002. Braz. J. Vet. Res. Anim. 2006, 43, 12–22. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, M.J.; Kim, M.J.; Im, Y.J.; Kim, S.W.; Lim, N.L.; Han, S.W. Is increased echogenicity related to a decrease in glomerular filtration rate? Objective measurements in pediatric solitary kidney patients—A retrospective analysis. PLoS ONE 2015, 10, e0133577. [Google Scholar] [CrossRef]
- Yaprak, M.; Çakır, Ö.; Turan, M.N.; Dayanan, R.; Akın, S.; Değirmen, E.; Turgut, F.; Yıldırım, M. Role of ultrasonographic chronic kidney disease score in the assessment of chronic kidney disease. Int. Urol. Nephrol. 2017, 49, 123–131. [Google Scholar] [CrossRef]
- Koch, M.C.; Teixeira, M.A.; Alves, L. Análise comparativa entre a imagem ultrassonográfica renal e os valores de ureia e creatinina em 93 cães. Veterinária Foco. 2013, 11, 75–81. [Google Scholar]
- Mattei, C.; Pelander, L.; Hansson, K.; Uhlhorn, M.; Olsson, U.; Häggström, J.; Ljungvall, I.; Ley, C.J. Renal ultrasonographic abnormalities are associated with low glomerular filtration rate calculated by scintigraphy in dogs. Vet. Radiol. Ultrasound 2019, 60, 432–446. [Google Scholar] [CrossRef]
- Neuwirth, L.; Mahaffey, M.; Crowell, W.; Selcer, B.; Barsanti, J.; Cooper, R.; Brown, J. Comparison of excretory urography and ultrasonography for detection of experimentally induced pyelonephritis in dogs. Am. J. Vet. Res. 1993, 54, 660–669. [Google Scholar] [PubMed]
- Foster, J.D.; Krishnan, H.; Cole, S. Characterization of subclinical bacteriuria, bacterial cystitis, and pyelonephritis in dogs with chronic kidney disease. J. Am. Vet. Med. Assoc. 2018, 252, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Zwingenberger, A. Ultrasound of cats with chronic renal disease not always black and white. DVM Newsmag. 2008, 39, 44. [Google Scholar]
- Kruger, J.M.; Osborne, C.A.; Nachreiner, R.F.; Refsal, K.R. Hypecalcemia and renal failure: Etiology, pathophysisology, diagnosis, and treatment. Vet. Clin. N. Am. Small Anim. Pract. 1996, 26, 1417–1445. [Google Scholar] [CrossRef]
- Kruger, J.M.; Osborne, C.A. Calcium Disorder. In Nephrology and Urology of Small Animals, 1st ed.; Bartges, J., Polzin, D.J., Eds.; Blackwell Publishing Ltd.: Chicester, UK, 2011; pp. 642–656. [Google Scholar]
- Lippi, I.; Guidi, G.; Marchetti, V.; Tognetti, R.; Meucci, V. Prognostic role of the product of serum calcium and phosphorus concentrations in dogs with chronic kidney disease: 31 cases (2008–2010). J. Am. Vet. Med. Assoc. 2014, 245, 1135–1140. [Google Scholar] [CrossRef]
- Lucero, M.C.; Duque, F.J.; Gil, M.; Ruiz, P.; Macías-García, B.; Cristóbal, J.I.; Zaragoza, C.; Barrera, R. A plasma calcium-phosphorus product can be used to predict the lifespan of dogs with chronic kidney disease. Can. Vet. J. 2019, 60, 1319–1325. [Google Scholar]
- Mason, M.A.; Shepler, B.M. Evaluation of morbidity and mortality data related to cardiovascular calcification from calcium containing phosphate binder use in patients undergoing hemodialysis. Pharmacotherapy 2010, 30, 741–748. [Google Scholar] [CrossRef]
- Kowalewich, N.J.; Hawkins, E.C. Calcinosis circumscripta involving the metatarsal region in a dog with chronic renal failure. Can. Vet. J. 1992, 33, 465–466. [Google Scholar]
- Reiss, A.B.; Miyawaki, N.; Moon, J.; Kasselman, L.J.; Voloshyna, I.; D’Avino, R., Jr.; De Leon, J. CKD, arterial calcification, atherosclerosis and bone health: Inter-relationships and controversies. Atherosclerosis 2018, 278, 49–59. [Google Scholar] [CrossRef]
- Ganesh, S.K.; Stack, A.G.; Levin, N.W.; Hulbert-Shearon, T.; Port, F.K. Association of elevated serum PO, Ca X PO product and parathyroid hormone with cardiac 44 mortality risk in chronic hemodialysis patients. J. Am. Soc. Nephrol. 2001, 12, 2131–2138. [Google Scholar]
- Yamada, S.; Giachelli, C.M. Vascular calcification in CKD-MBD: Roles for phosphate, FGF23, and Klotho. Bone 2017, 100, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Moshar, S.; Bayesh, S.; Mohsenikia, M.; Najibpour, R. The Association of calcium-phosphorus product with the severity of cardiac valves failure in patients under chronic hemodialysis. Cardiol. Res. 2016, 7, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Brushinsky, D. Disorders of calcium and phosphorus homeostasis. In Primer on Kidney Diseases, 4th ed.; Greenberg, A., Ed.; Academic Press: San Diego, CA, USA, 2005; pp. 120–130. [Google Scholar]
- Polzin, D.J.; Osborne, C.A.; Ross, S. Chronic kidney disease. In Textbook of Veterinary Internal Medicine: Diseases of the Dog and the Cat, 7th ed.; Ettinger, S.J., Feldman, E.C., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2010; pp. 1756–1785. [Google Scholar]
| Parameter | IRIS 2 | IRIS 3 | IRIS 4 | Reference Range |
|---|---|---|---|---|
| Creatinine | 1.59 ± 0.17 | 3.10 ± 0.80 | 9.40 ± 3.80 | 0.60–1.50 |
| Urea | 71.80 ± 42 | 152.70 ± 78.90 | 328.10 ± 123.80 | 15–55 |
| Calcium | 10.30 ± 1.50 | 10.60 ± 1.80 | 10.50 ± 2.30 | 8.7–11.8 |
| Phosphuros | 4.70 ± 1.60 | 6.60 ± 3.10 | 14.20 ± 5.80 | 2.5–5.0 |
| sCaPP | 48.20 ± 15.80 | 69.70 ± 31.10 | 143.20 ± 55.40 | <70 |
| Parameter | IRIS 2 | IRIS 3 | IRIS 4 | p Value |
|---|---|---|---|---|
| Irregular contour | 33/337 (9.8%) | 52/295 (17.6%) | 54/223 (24.2%) | 0.0185 * |
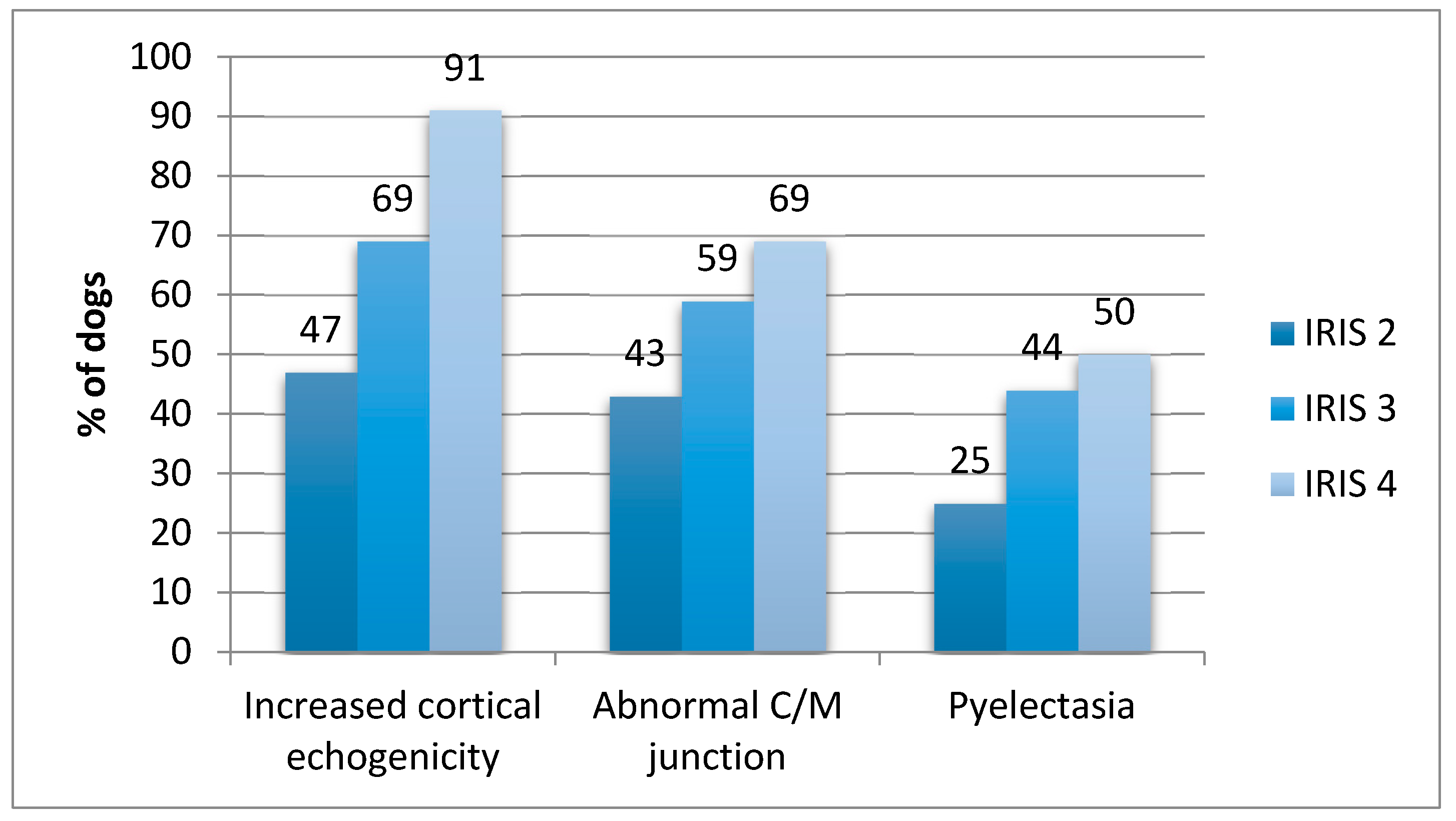
| Abnormal C/M junction | 144/337 (42.7%) | 174/295 (59%) | 153/223 (68.6%) | 0.0035 * |
| Abnormal C/M ratio | 30/337 (89%) | 68/295 (23%) | 66/223 (29.5%) | 0.0049 * |
| Increased cortical echogenicity | 158/337 (46.9%) | 203/295 (68.8%) | 203/223 (91%) | 0.0001 * |
| Abnormal medullary echogenicity | 59/337 (17.5%) | 90/295 (30.5%) | 95/223 (42.6%) | 0.0018 * |
| Pyelectasia | 85/337 (25.2%) | 131/295 (44.4%) | 111/223 (49.7%) | 0.0018 * |
| Cysts | 51/337 (15.1%) | 58/295 (19.7%) | 58/223 (26%) | 0.2096 |
| Mineralization | 59/337 (17.5%) | 74/295 (25.1%) | 54/223 (24.2) | 0.4688 |
| Infarcts | 10/337 (3%) | 9/295 (3%) | 6/223 (2.7%) | 0.9999 |
| Peri-renal effusion | 4/337 (1.2%) | 9/295 (3%) | 14/223 (6.3%) | 0.1401 |
| Number of US Abnormalities | IRIS 2 | IRIS 3 | IRIS 4 | p Value |
|---|---|---|---|---|
| 1 | 128/337 (38%) | 42/295 (14.2%) | 0/223 (0%) | 0.0001 * |
| 2–3 | 129/337 (38.3%) | 123/295 (41.7%) | 88/223 (39.5%) | |
| >3 | 80/337 (23.7%) | 130/295 (44.1%) | 135/223 (60.5%) |
| Number of US Abnormalities | IRIS 2 | IRIS 3 | IRIS 4 | p Value |
|---|---|---|---|---|
| sCaPP > 70 | 38/212 (17.9%) | 124/218 (56.7%) | 198/206 (96.1%) | 0.0001 * |
| Mineralization | 8/38 (21%) | 30/124 (24.2%) | 48/198 (24.2%) | 0.8442 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perondi, F.; Lippi, I.; Marchetti, V.; Bruno, B.; Borrelli, A.; Citi, S. How Ultrasound Can Be Useful for Staging Chronic Kidney Disease in Dogs: Ultrasound Findings in 855 Cases. Vet. Sci. 2020, 7, 147. https://doi.org/10.3390/vetsci7040147
Perondi F, Lippi I, Marchetti V, Bruno B, Borrelli A, Citi S. How Ultrasound Can Be Useful for Staging Chronic Kidney Disease in Dogs: Ultrasound Findings in 855 Cases. Veterinary Sciences. 2020; 7(4):147. https://doi.org/10.3390/vetsci7040147
Chicago/Turabian StylePerondi, Francesca, Ilaria Lippi, Veronica Marchetti, Barbara Bruno, Antonio Borrelli, and Simonetta Citi. 2020. "How Ultrasound Can Be Useful for Staging Chronic Kidney Disease in Dogs: Ultrasound Findings in 855 Cases" Veterinary Sciences 7, no. 4: 147. https://doi.org/10.3390/vetsci7040147
APA StylePerondi, F., Lippi, I., Marchetti, V., Bruno, B., Borrelli, A., & Citi, S. (2020). How Ultrasound Can Be Useful for Staging Chronic Kidney Disease in Dogs: Ultrasound Findings in 855 Cases. Veterinary Sciences, 7(4), 147. https://doi.org/10.3390/vetsci7040147







