Gut Microbiome of Healthy and Arthritic Dogs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Collection of Samples
2.3. Hematological Analysis
2.4. Fecal Short-Chain Fatty Acid and Lactic Acid Analysis
2.5. Fecal DNA Extraction, Sequencing, and Taxonomic Annotation
2.6. Statistical Analysis
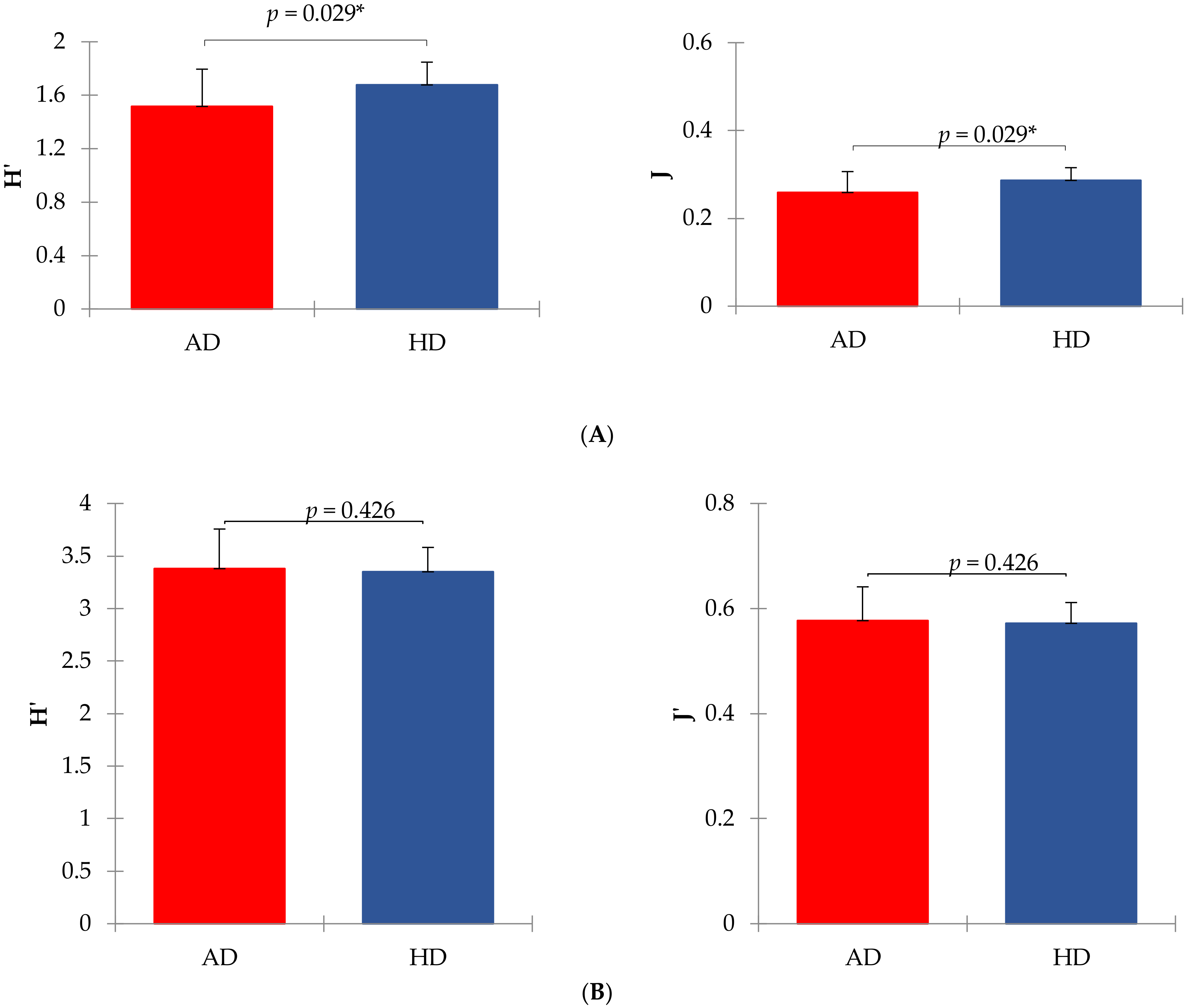
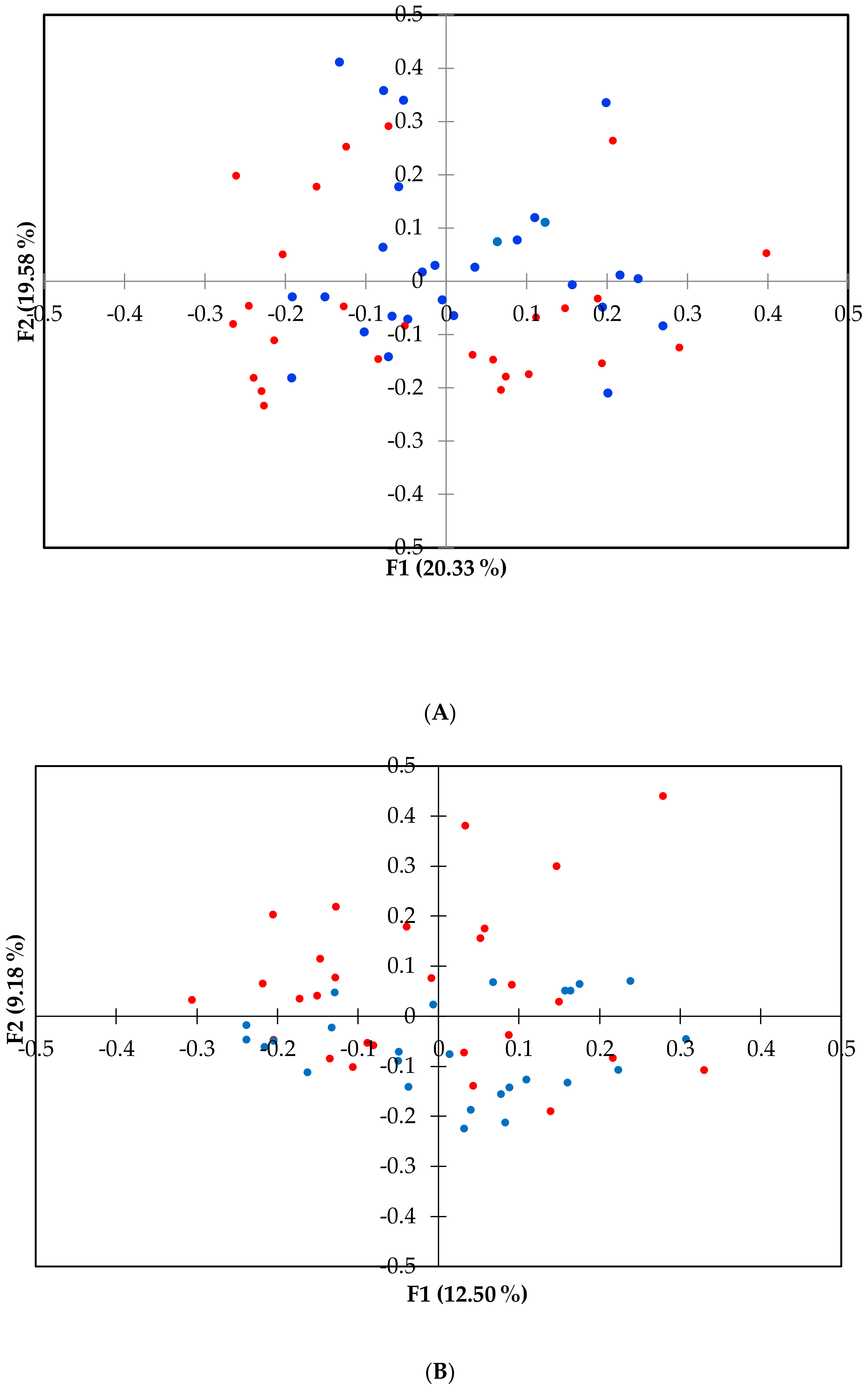
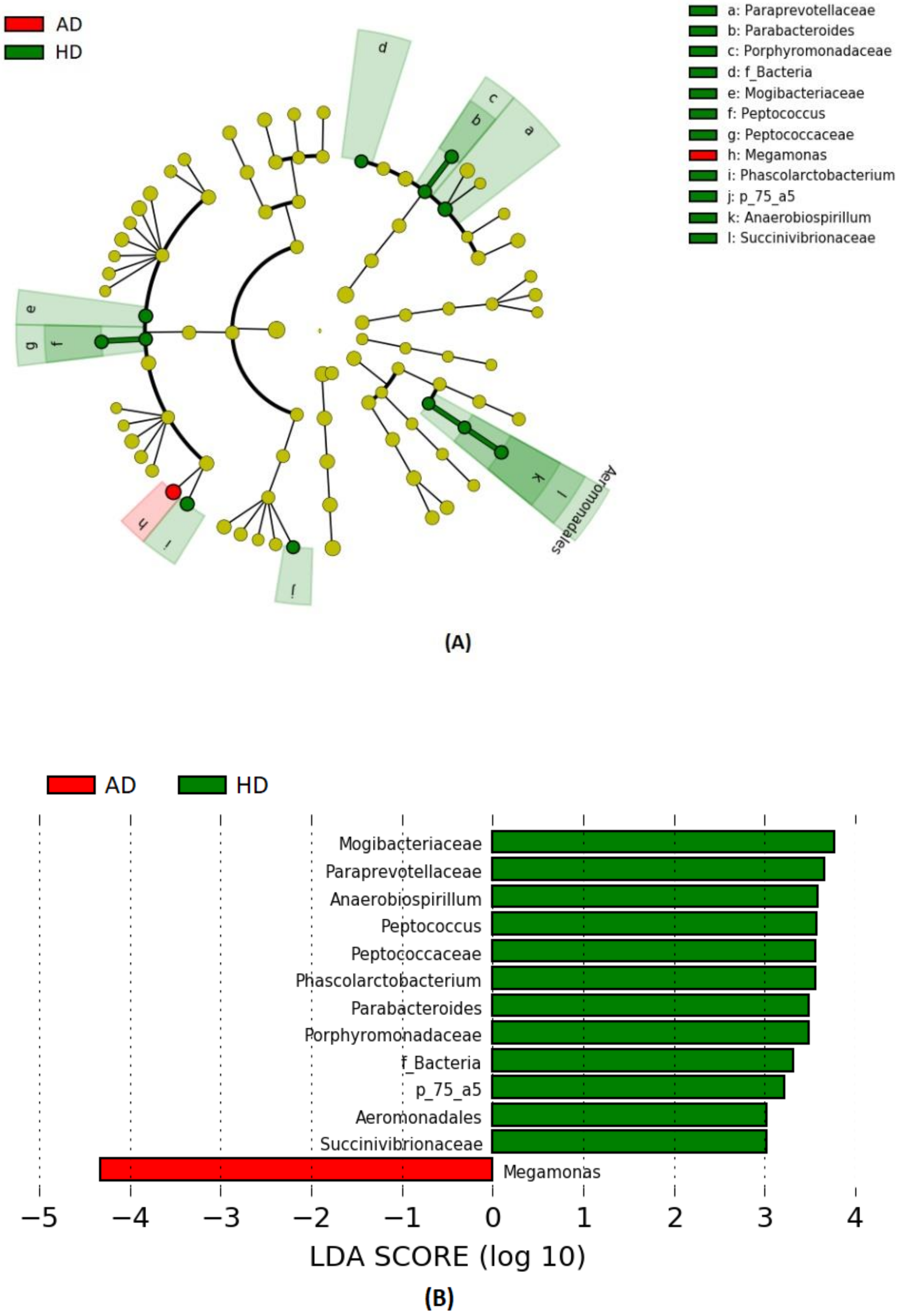
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Suchodolski, J.S.; Markel, M.E.; Garcia-Mazcorro, J.F.; Unterer, S.; Heilmann, R.M.; Dowd, S.E.; Kachroo, P.; Ivanov, I.; Minamoto, Y.; Dillman, E.M.; et al. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS ONE 2012, 7, e51907. [Google Scholar] [CrossRef]
- Al Shawaqfeh, M.K.; Wajid, B.; Minamoto, Y.; Markel, M.; Lidbury, J.A.; Steiner, J.M.; Serpedin, E.; Suchodolski, J.S. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef]
- Szychlinska, M.A.; Di Rosa, M.; Castorina, A.; Mobasheri, A.; Musumeci, G. A correlation between intestinal microbiota dysbiosis and osteoarthritis. Heliyon 2019, 5, e01134. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Scarsella, E.; Cintio, M.; Iacumin, L.; Ginaldi, F.; Stefanon, B. Interplay between neuroendocrine biomarkers and gut microbiota in dogs supplemented with grape proanthocyanidins: Results of dietary intervention study. Animals 2020, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.J. The Microbiome and Bone and Joint Disease. Curr. Rheumatol. Rep. 2018, 19, 77. [Google Scholar] [CrossRef]
- Hernandez, C.J.; Guss, J.D.; Luna, M.; Goldring, S.R. Links between the Microbiome and Bone. J. Bone Miner. Res. 2016, 31, 1638–1646. [Google Scholar] [CrossRef]
- Quach, D.; Britton, R.A. Gut microbiota and bone health. Understanding the gut-bone signaling axis. Adv. Exp. Med. Biol. 2017, 1033, 47–58. [Google Scholar]
- Berthelot, J.M.; Wendling, D. Translocation of dead or alive bacteria from mucosa to joints and epiphyseal bone-marrow: Facts and hypotheses. Jt. Bone Spine 2020, 87, 31–36. [Google Scholar] [CrossRef]
- Korotkyi, O.; Kyriachenko, Y.; Kobyliak, N.; Falalyeyeva, T.; Ostapchenko, L. Crosstalk between gut microbiota and osteoarthritis: A critical view. J. Funct. Foods 2020, 68, 103904. [Google Scholar] [CrossRef]
- Tomasello, G.; Tralongo, P.; Damiani, P.; Sinagra, E.; Di Trapani, B.; Zeenny, M.N.; Hussein, I.H.; Jurjus, A.; Leone, A. Dismicrobism in inflammatory bowel disease and colorectal cancer: Changes in response of colocytes. World J. Gastroenterol. 2014, 20, 18121–18130. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.M. The origin of human milk bacteria: Is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv. Nutr. 2014, 5, 779–784. [Google Scholar] [CrossRef]
- Ayral, X.; Pickering, E.H.; Woodworth, T.G.; Mackillop, N.; Dougados, M. Synovitis: A potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis–results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthr. Cartil. 2005, 13, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Dahaghin, S.; Bierma-Zeinstra, S.M.A.; Koes, B.W.; Hazes, J.M.W.; Pols, H.A.P. Do metabolic factors add to the effect of overweight on hand osteoarthritis? The Rotterdam Study. Ann. Rheum. Dis. 2007, 66, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B.; McDaniel, G.; Huebner, J.L.; Stabler, T.V.; Pieper, C.F.; Shipes, S.W.; Petry, N.A.; Low, P.S.; Shen, J.; McNearney, T.A.; et al. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Jubair, W.K.; Hendrickson, J.D.; Severs, E.L.; Schulz, H.M.; Adhikari, S.; Ir, D.; Pagan, J.D.; Anthony, R.M.; Robertson, C.E.; Frank, D.N.; et al. Modulation of inflammatory arthritis in mice by gut microbiota through mucosal inflammation and autoantibody generation. Arthritis Rheumatol. 2018, 70, 1220–1233. [Google Scholar] [CrossRef]
- Sandri, M.; Dal Monego, S.; Conte, G.; Sgorlon, S.; Stefanon, B. Raw meat-based diet influences faecal microbiome and end products of fermentation in healthy dogs. BMC Vet. Res. 2017, 13, 65. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Sandri, M.; Sgorlon, S.; Scarsella, E.; Stefanon, B. Effect of different starch sources in a raw meat-based diet on fecal microbiome in dogs housed in a shelter. Anim. Nutr. J. 2020. [Google Scholar] [CrossRef]
- Addinsoft. XLSTAT Statistical and Data Analysis Solution; Addinsoft: Boston, MA, USA, 2020. [Google Scholar]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.R.; Lee, H.B.; Park, S.Y.; Lee, H.Y.; Kim, I.S.; Kang, H.S.; Kim, N.S. Changes in the haematobiochemical parameters in experimental stifle osteoarthritis in dogs. Pak. J. Biol. Sci. 2006, 9, 2869–2874. [Google Scholar]
- Hotamisligil, G.S.; Erbay, E. Nutrient sensing and inflammation in metabolic diseases. Nat. Rev. Immunol. 2008, 8, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Barna, I.; Nyúl, D.; Szentes, T.; Schwab, R. Review of the relation between gut microbiome, metabolic disease and hypertension. Orv. Hetil. 2018, 159, 346–351. [Google Scholar] [CrossRef]
- Hillström, A.; Bylin, J.; Hagman, R.; Björhall, K.; Tvedten, H.; Königsson, K.; Fall, T.; Kjelgaard-Hansen, M. Measurement of serum C-reactive protein concentration for discriminating between suppurative arthritis and osteoarthritis in dogs. BMC Vet. Res. 2016, 12, 240. [Google Scholar] [CrossRef] [PubMed]
- Hindenberg, S.; Bauer, N.; Moritz, A. Extremely high canine C-reactive protein concentrations >100 mg/L—prevalence, etiology and prognostic significance. BMC Vet. Res. 2020, 16, 147. [Google Scholar] [CrossRef]
- Eirmann, L.A.; Freeman, L.M.; Laflamme, D.P.; Michel, K.E.; Satyaraj, E. Comparison of adipokine concentrations and markers of inflammation in obese versus lean dogs. Int. J. Appl. Res. Vet. Med. 2009, 7, 196–205. [Google Scholar]
- Yudkinm, J.; Kamari, M.; Humphries, S.; Mohamed-Ali, V. Inflammation, obesity, stress and coronary heart disease: Is interleukin-6 the link? Atherosclerosis 2000, 148, 209–241. [Google Scholar] [CrossRef]
- Dandona, P.; Aljada, A.; Chaudhuri, A.; Mohanty, P.; Garg, R. Metabolic syndrome: A comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 2005, 111, 1448–1454. [Google Scholar] [CrossRef]
- Fantuzzi, G. Adipose in the regulation of inflammation. Immunol. Endocr. Metab. Agents Med. Chem. 2007, 7, 129–136. [Google Scholar] [CrossRef]
- Farnaghi, S.; Crawford, R.; Xiao, Y.; Prasadam, I. Cholesterol metabolism in pathogenesis of osteoarthritis disease. Int. J. Rheum. Dis. 2017, 20, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, S.E.; Oh, J.H.; Seo, K.W.; Song, K.H. Leptin, adiponectin and serotonin levels in lean and obese dogs. BMC Vet. Res. 2014, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Wyckoff, H.W. Structure and function of alkaline phosphatases. Clin. Chim. Acta 1990, 186, 175–187. [Google Scholar] [CrossRef]
- Rodan, G.A.; Rodan, S.B. Expression of the osteoblastic phenotype. Bone Miner. Res. 1983, 2, 244–245. [Google Scholar]
- Jacques, D.; Cauzinille, L.; Bouvy, B.; Dupre, G. A retrospective study of 40 dogs with polyarthritis. Vet. Surg. 2002, 31, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Musco, N.; Vassalotti, G.; Mastellone, V.; Cortese, L.; della Rocca, G.; Molinari, M.L.; Calabrò, S.; Tudisco, R.; Cutrignelli, M.I.; Lombardi, P. Effects of a nutritional supplement in dogs affected by osteoarthritis. Vet. Med. Sci. 2019, 5, 325–335. [Google Scholar] [CrossRef]
- Ide, K.; Kato, K.; Sawa, Y.; Hayashi, A.; Takizawa, R.; Nishifuji, K. Comparison of the expression, activity, and fecal concentration of intestinal alkaline phosphatase between healthy dogs and dogs with chronic enteropathy. Am. J. Vet. Res. 2016, 77, 721–729. [Google Scholar] [CrossRef]
- Batt, R.M.; Morgan, J.O. Role of Serum Folate and Vitamin B12 Concentrations in the Differentiation of Small Intestinal Abnormalities in the Dog. Res. Vet. Sci. 1982, 32, 17–22. [Google Scholar] [CrossRef]
- Heilmann, R.M.; Steiner, J.M. Clinical utility of currently available biomarkers in inflammatory enteropathies of dogs. J. Vet. Intern. Med. 2018, 32, 1495–1508. [Google Scholar] [CrossRef]
- Allenspach, K.; Wieland, B.; Gröne, A.; Gaschen, F. Chronic enteropathies in dogs: Evaluation of risk factors for negative outcome. J. Vet. Intern. Med. 2007, 21, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, N.; Parnell, N.K.; Hill, S.L.; Suchodolski, J.S.; Steiner, J.M. Serum cobalamin and methylmalonic acid concentrations in dogs with chronic gastrointestinal disease. Am. J. Vet. Res. 2013, 74, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, R.M.; Berghoff, N.; Mansell, J.; Grützner, N.; Parnell, N.K.; Gurtner, C.; Suchodolski, J.S.; Steiner, J.M. Association of fecal calprotectin concentrations with disease severity, response to treatment, and other biomarkers in dogs with chronic inflammatory enteropathies. J. Vet. Intern. Med. 2018, 32, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Dossin, O.; Lavoué, R. Protein-losing enteropathies in dogs. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 399–418. [Google Scholar] [CrossRef]
- Wennogle, S.A.; Priestnall, S.L.; Webb, C.B. Histopathologic characteristics of intestinal biopsy samples from dogs with chronic inflammatory enteropathy with and without hypoalbuminemia. J. Vet. Intern. Med. 2017, 31, 371–376. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef]
- Ruaux, C.G. Laboratory testing for the diagnosis of intestinal disorders. In Small Animal Gastroenterology; Steiner, J.M., Ed.; Schlütersche: Hannover, Germany, 2008; pp. 50–55. [Google Scholar]
- Xu, J.; Verbrugghe, A.; Lourenço, M.; Janssens, G.P.; Liu, D.J.; Van de Wiele, T.; Eeckhaut, V.; Van Immerseel, F.; Van de Maele, I.; Niu, Y.; et al. Does canine inflammatory bowel disease influence gut microbial profile and host metabolism? BMC Vet. Res. 2016, 12, 114. [Google Scholar] [CrossRef]
- Murugesan, S.; Nirmalkar, K.; Hoyo-Vadillo, C.; García-Espitia, M.; Ramírez-Sanchez, D.; García-Mena, J. Gut microbiome production of short chain fatty acids and obesity in children. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 621–625. [Google Scholar] [CrossRef]
- Macia, L.; Thorburn, A.N.; Binge, L.C.; Marino, E.; Rogers, K.E.; Maslowski, K.M.; Mackay, C.R. Microbial influences on epithelial integrity and immune function as a basis for inflammatory diseases. Immunol. Rev. 2012, 245, 164–176. [Google Scholar] [CrossRef]
- Li, R.W.; Li, C.-J. Enhancing butyrate biosynthesis in the gut for health benefits. In Butyrate: Food Sources, Functions and Health Benefits; Li, C.-J., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2014. [Google Scholar]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Ganapathy, V. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.-J. The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Young, P.; Holtrop, G.; Flint, H.J. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ. Microbiol. 2010, 12, 304–314. [Google Scholar] [CrossRef]
- Duncan, S.H.; Holtrop, G.; Lobley, G.E.; Calder, A.G.; Stewart, C.S.; Flint, H.J. Contribution of acetate to butyrate formation by human faecal bacteria. Br. J. Nutr. 2004, 91, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Louis, P.; Flint, H.J. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microbiol. 2004, 70, 5810–5817. [Google Scholar] [CrossRef]
- Minamoto, Y.; Otoni, C.C.; Steelman, S.M.; Büyükleblebici, O.; Steiner, J.M.; Jergens, A.E.; Suchodolski, J.S. Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microbes 2015, 6, 33–47. [Google Scholar] [CrossRef]
- Muñiz Pedrogo, D.A.; Chen, J.; Hillmann, B.; Jeraldo, P.; Al-Ghalith, G.; Taneja, V.; Davis, J.M.; Knights, D.; Nelson, H.; Faubion, W.A.; et al. An increased abundance of Clostridiaceae characterizes arthritis in inflammatory bowel disease and rheumatoid arthritis: A cross-sectional study. Inflamm. Bowel Dis. 2019, 25, 902–913. [Google Scholar] [CrossRef]
- Coulson, S.; Butt, H.; Vecchio, P.; Gramotnev, H.; Vitetta, L. Green-lipped mussel extract (Perna canaliculus) and glucosamine sulphate in patients with knee osteoarthritis: Therapeutic efficacy and effects on gastrointestinal microbiota profiles. Inflammopharmacology 2013, 21, 79–90. [Google Scholar] [CrossRef]
- Wolfe, F.; Kong, S.X.; Watson, D.J. Gastrointestinal symptoms and health related quality of life in patients with arthritis. J. Rheumatol. 2000, 27, 1373–1378. [Google Scholar]
- Britton, R.A.; Irwin, R.; Quach, D.; Schaefer, L.; Zhang, J.; Lee, T.; Mccabe, L.R. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J. Cell. Phys. 2014, 229, 1822–1830. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, W.; Luo, W.; Zeng, C.; Deng, Z.; Ren, W.; Wu, G.; Lei, G. Alterations of amino acid metabolism in osteoarthritis: Its implications for nutrition and health. Amino Acids 2016, 48, 907–914. [Google Scholar] [CrossRef]
- McCabe, L.R.; Irwin, R.; Schaefer, L.; Britton, R.A. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J. Cell. Phys. 2013, 228, 1793–1798. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.; Omata, Y.; Hofmann, J.; Böttcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Krönke, G.; et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Kieler, I.N.; Shamzir Kamal, S.; Vitger, A.D.; Nielsen, D.S.; Lauridsen, C.; Bjornvad, C.R. Gut microbiota composition may relate to weight loss rate in obese pet dogs. Vet. Med. Sci. 2017, 3, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Chen, G.; Wan, P.; Dai, Z.; Hu, B.; Chen, L.; Ou, S.; Zeng, X.; Sun, Y. Modulating effects of dicaffeoylquinic acids from Ilex kudingcha on intestinal microecology in vitro. J. Agric. Food Chem. 2017, 65, 10185–10196. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Shu, S.; Dong, J.; Li, H.; Xu, C.; Han, Y.; Hu, J.; Han, Y.; Yang, R.; Cheng, N. Association study of gut flora in Wilson’s disease through high-throughput sequencing. Medicine 2018, 97, e11743. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S.; Dowd, S.E.; Wilke, V.; Steiner, J.M.; Jergens, A.E. 16S rRNA gene pyrosequencing reveals bacterial dysbiosis in the duodenum of dogs with idiopathic inflammatory bowel disease. PLoS ONE 2012, 7, e39333. [Google Scholar] [CrossRef]
- Omori, M.; Maeda, S.; Igarashi, H.; Ohno, K.; Sakai, K.; Yonezawa, T.; Horigome, A.; Odamaki, T.; Matsuki, N. Fecal microbiome in dogs with inflammatory bowel disease and intestinal lymphoma. J. Vet. Med. Sci. 2017, 79, 1840–1847. [Google Scholar] [CrossRef]
- Nakano, V.; Ignacio, A.; Fernandes, M.R.; Fukugaiti, M.H.; Avila-Campos, M.J. Intestinal Bacteroides and Parabacteroides species producing antagonistic substances. Microbiology 2006, 1, 61–64. [Google Scholar]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD-what role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef]
- Malnick, H.; Williams, K.; Phil-Ebosie, J.; Levy, A.S. Description of a medium for isolating Anaerobiospirillum spp., a possible cause of zoonotic disease, from diarrheal feces and blood of humans and use of the medium in a survey of human, canine, and feline feces. J. Clin. Microbiol. 1990, 28, 1380–1384. [Google Scholar] [CrossRef] [PubMed]
- Kempsell, K.E.; Cox, C.J.; Hurle, M.; Wong, A.; Wilkie, S.; Zanders, E.D.; Gaston, J.S.; Crowe, J.S. Reverse transcriptase-PCR analysis of bacterial rRNA for detection and characterization of bacterial species in arthritis synovial tissue. Infect. Immun. 2000, 68, 6012–6026. [Google Scholar] [CrossRef] [PubMed]
- Katz, K.D.; Hollander, D. Intestinal mucosal permeability and rheumatological diseases. Bailliere’s Clin. Rheumatol. 1989, 3, 271–284. [Google Scholar] [CrossRef]
- Berthelot, J.M.; Claudepierre, P. Trafficking of antigens from gut to sacroiliac joints and spine in reactive arthritis and spondyloarthropathies: Mainly through lymphatics? Jt. Bone Spine 2016, 83, 485–490. [Google Scholar] [CrossRef] [PubMed]
| Item | Unit | T0 | T45 | SEM | Effects | ||||
|---|---|---|---|---|---|---|---|---|---|
| AD | HD | AD | HD | Time | Status | T x S | |||
| Glucose | mg/dL | 93.14 | 94.23 | 92.86 | 95.77 | 1.20 | NS | NS | NS |
| Fructosamine | umol/L | 204.93 | 191.23 | 215.21 | 219.77 | 5.14 | NS | NS | NS |
| Urea | mg/dL | 35.50 | 36.28 | 36.71 | 35.08 | 1.38 | NS | * | NS |
| Creatine | mg/dL | 1.28 | 1.13 | 1.42 | 1.36 | 0.04 | * | NS | NS |
| Total bilirubin | mg/dL | 0.20 | 0.26 | 0.16 | 0.19 | 0.01 | * | * | NS |
| Total proteins | g/dL | 6.84 | 6.85 | 6.91 | 6.92 | 0.05 | NS | NS | NS |
| Albumin | g/dL | 3.04 | 2.98 | 3.01 | 2.91 | 0.04 | NS | * | NS |
| Globulin | g/dL | 3.81 | 3.87 | 3.90 | 4.02 | 0.06 | NS | NS | NS |
| Albumin/Globulin | g/dL | 0.81 | 0.78 | 0.77 | 0.74 | 0.02 | NS | NS | NS |
| Cholesterol | mg/dL | 238.29 | 228.31 | 266.57 | 242.15 | 7.07 | NS | NS | * |
| Triglycerides | mg/dL | 63.29 | 68.23 | 53.14 | 58.46 | 3.28 | NS | NS | NS |
| Lipase | u/L | 118.43 | 86.92 | 88.57 | 64.23 | 8.39 | NS | * | NS |
| C-Reactive Protein | mg/dL | 0.41 | 0.15 | 0.33 | 0.12 | 0.03 | NS | * | NS |
| α-Amylase | u/L | 819.71 | 771.77 | 727.07 | 767.92 | 34.51 | NS | NS | NS |
| AST (GOT) | u/L | 30.79 | 32.23 | 21.64 | 25.08 | 0.99 | ** | NS | NS |
| ALT (GPT) | u/L | 56.29 | 29.31 | 31.86 | 22.46 | 2.69 | ** | * | * |
| ALP | u/L | 59.57 | 69.62 | 52.14 | 46.69 | 3.15 | * | NS | NS |
| GGT | u/L | 6.93 | 6.46 | 7.07 | 7.46 | 0.20 | * | NS | * |
| CK | u/L | 102.43 | 158.92 | 90.29 | 97.69 | 8.82 | NS | NS | NS |
| LDH | u/L | 42.36 | 67.37 | 52.50 | 59.46 | 6.28 | NS | NS | NS |
| Cholinesterase | mg/dL | 6000.50 | 6232.00 | 6301.64 | 6472.95 | 231.02 | NS | NS | NS |
| Ca | mg/dL | 10.14 | 10.02 | 10.91 | 10.44 | 0.10 | ** | NS | NS |
| P-in | mg/dL | 3.49 | 5.03 | 3.89 | 3.98 | 0.16 | NS | NS | * |
| Mg | mg/dL | 2.25 | 2.32 | 2.18 | 2.39 | 0.03 | NS | * | NS |
| Fe | µg/dL | 154.79 | 175.69 | 148.07 | 188.54 | 7.70 | NS | NS | NS |
| Cl | mEq/L | 109.64 | 109.11 | 111.07 | 110.53 | 0.52 | * | NS | NS |
| K | mEq/L | 4.45 | 4.76 | 4.53 | 4.91 | 0.06 | NS | * | NS |
| Na | mEq/L | 146.86 | 149.20 | 148.86 | 149.92 | 0.41 | ** | * | NS |
| TLI | ng/mL | 37.74 | 26.61 | 48.62 | 38.25 | 5.37 | NS | NS | NS |
| Folates | ug/L | 10.74 | 11.63 | 11.52 | 12.17 | 0.64 | NS | * | NS |
| Vitamin B12 | pg/mL | 326.64 | 500.69 | 360.79 | 451.46 | 29.07 | NS | * | NS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cintio, M.; Scarsella, E.; Sgorlon, S.; Sandri, M.; Stefanon, B. Gut Microbiome of Healthy and Arthritic Dogs. Vet. Sci. 2020, 7, 92. https://doi.org/10.3390/vetsci7030092
Cintio M, Scarsella E, Sgorlon S, Sandri M, Stefanon B. Gut Microbiome of Healthy and Arthritic Dogs. Veterinary Sciences. 2020; 7(3):92. https://doi.org/10.3390/vetsci7030092
Chicago/Turabian StyleCintio, Michela, Elisa Scarsella, Sandy Sgorlon, Misa Sandri, and Bruno Stefanon. 2020. "Gut Microbiome of Healthy and Arthritic Dogs" Veterinary Sciences 7, no. 3: 92. https://doi.org/10.3390/vetsci7030092
APA StyleCintio, M., Scarsella, E., Sgorlon, S., Sandri, M., & Stefanon, B. (2020). Gut Microbiome of Healthy and Arthritic Dogs. Veterinary Sciences, 7(3), 92. https://doi.org/10.3390/vetsci7030092






