Seroprevalence of Immunoglobulin G Antibodies Against Mycobacterium avium subsp. paratuberculosis in Dogs Bred in Japan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sera
2.2. Measurement of Titers against MAP
2.3. Statistical Analysis
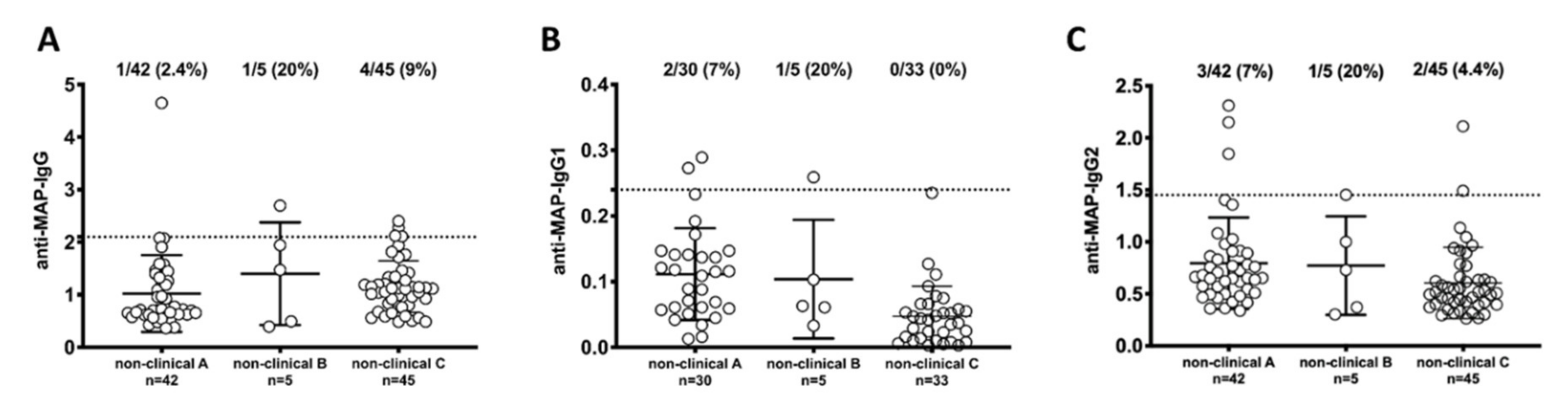
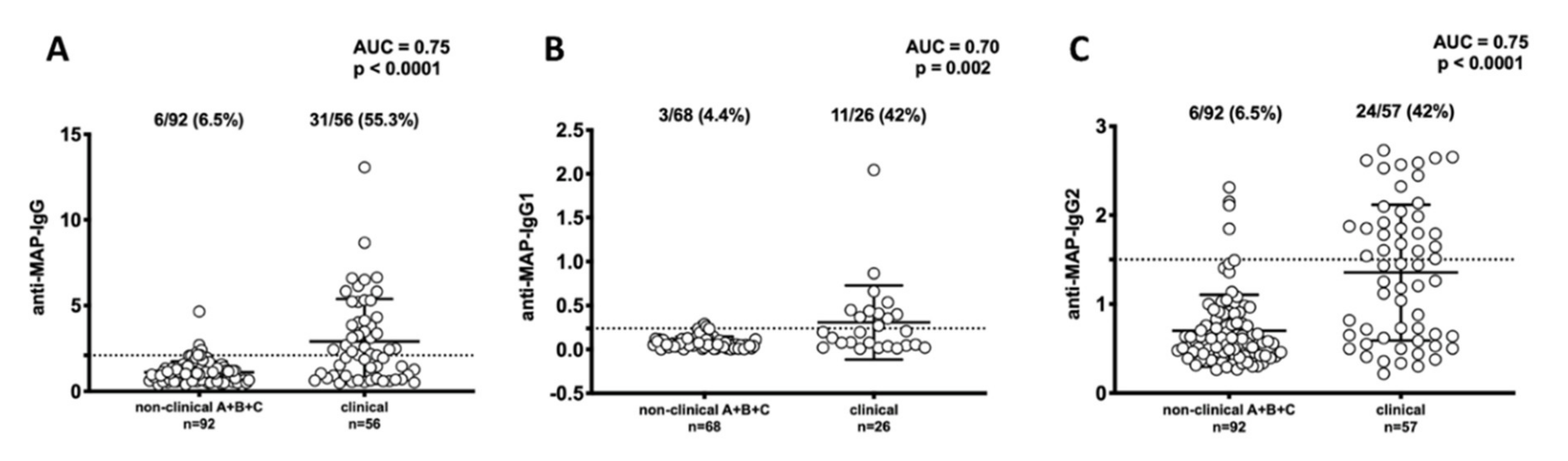
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Orpin, P.; Sibley, D. Johne’s disease control programmes. Vet. Rec. 2018, 183, 224–225. [Google Scholar] [CrossRef]
- Tewari, D.; Hovingh, E.; Linscott, R.; Martel, E.; Lawrence, J.; Wolfgang, D.; Griswold, D. Mycobacterium avium subsp. paratuberculosis antibody response, fecal shedding, and antibody cross-reactivity to Mycobacterium bovis in M. avium subsp. paratuberculosis-infected cattle herds vaccinated against Johne’s disease. Clin. Vaccine Immunol. 2014, 21, 698–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, N.B.; Barletta, R.G. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 2001, 14, 489–512. [Google Scholar] [CrossRef] [Green Version]
- Whittington, R.J.; Marshall, D.J.; Nicholls, P.J.; Marsh, I.B.; Reddacliff, L.A. Survival and dormancy of Mycobacterium avium subsp. paratuberculosis in the environment. Appl. Environ. Microbiol. 2004, 70, 2989–3004. [Google Scholar] [CrossRef] [Green Version]
- Garcia, A.B.; Shalloo, L. Invited review: The economic impact and control of paratuberculosis in cattle. J. Dairy Sci. 2015, 98, 5019–5039. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, S.; Tsutsui, T.; Yamamoto, T.; Nichiguchi, A. Epidemiologic indicators associated with within-farm spread of Johne’s disease in dairy farms in Japan. J. Vet. Med. Sci. 2007, 69, 1255–1258. [Google Scholar] [CrossRef] [Green Version]
- Momotani, E. Epidemiological situation and control strategies for paratuberculosis in Japan. Jpn. J. Vet. Res. 2012, 60, 19–29. [Google Scholar]
- William, C.D.; Sally, A.M.B. Crohn’s disease and Mycobacterium avium subsp. paratuberculosis: The need for a study is long overdue. Vet. Immunol. Immunopathol. 2012, 145, 1–6. [Google Scholar]
- Ami, P.; Nihir, S. Mycobacterium avium subsp paratuberculosis—Incidences in milk and milk products, their isolation, enumeration, characterization, and role in human health. J. Microbiol. Immunol. Infect. 2011, 44, 473–479. [Google Scholar]
- Bo, M.; Arru, G.; Niegowska, M.; Erre, G.L.; Manchia, P.A.; Sechi, L.A. Association between lipoprotein levels and humoral reactivity to Mycobacterium avium subsp. paratuberculosis in multiple sclerosis, Type 1 diabetes mellitus and rheumatoid arthritis. Microorganisms 2019, 7, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Over, K.; Crandall, P.G.; Bryan, C.A.O.; Ricke, S.C. Current perspectives on Mycobacterium avium subsp. paratuberculosis, Johne’s disease, and Crohn’s disease: A review. Crit. Rev. Microbiol. 2011, 37, 141–156. [Google Scholar] [CrossRef]
- Zamani, S.; Zali, M.R.; Aghdaei, H.A.; Sechi, L.A.; Niegowska, M.; Caggiu, E.; Keshavarz, R.; Mosavari, N.; Feizabadi, M.M. Mycobacterium avium subsp. paratuberculosis and associated risk factors for inflammatory bowel disease in Iranian patients. Gut. Pathog. 2017, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Autschbach, F.; Eisold, S.; Hinz, U.; Zinser, S.; Linnebacher, M.; Giese, T.; Löffler, T.; Büchler, M.W.; Schmidt, J. High prevalence of Mycobacterium avium subspecies paratuberculosis IS900 DNA in gut tissues from individuals with Crohn’s disease. Gut 2005, 54, 944–999. [Google Scholar] [CrossRef] [Green Version]
- Saleh, A.N.; George, G.; Clavdia, R.; John, V. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn’s disease. Lancet 2004, 18, 1039–1044. [Google Scholar]
- Adrienne, L.M.; Diane, M.; Najah, R.Z.; David, Y.G. Mycobacterium paratuberculosis as a cause of Crohn’s disease. Expert Rev. Gastroenterol. Hapatol. 2015, 9, 1523–1534. [Google Scholar]
- Leonardo, A.S.; Coad, C.T. Mycobacterium avium ss. paratuberculosis Zoonosis - The hundred year war - beyond Crohn’s disease. Front Immunol. 2015, 6, 96. [Google Scholar]
- Nakase, H.; Nishio, A.; Tamaki, H.; Matsuura, M.; Asada, M.; Chiba, T.; Okazaki, K. Specific antibodies against recombinant protein of insertion element 900 of Mycobacterium avium subspecies paratuberculosis in Japanese patients with Crohn’s disease. Inflamm. Bowel Dis. 2006, 12, 62–69. [Google Scholar] [CrossRef]
- Otsubo, S.; Cossu, D.; Eda, S.; Otsubo, Y.; Sechi, L.A.; Suzuki, T.; Yumiko, I.; Yamamoto, S.; Kuribayashi, T.; Momotani, E. Seroprevalence of IgG1 and IgG4 class antibodies against Mycobacterium avium subsp. paratuberculosis in Japanese population. Foodborne Pathog. Dis. 2015, 12, 851–856. [Google Scholar] [CrossRef]
- McAloon, C.G.; Roche, S.; Ritter, C.; Barkema, H.W.; Whyte, P.; More, S.J.; O’Grady, L.; Green, M.J.; Doherty, M.L. A review of paratuberculosis in dairy herds—Part 2: On-farm control. Vet. J. 2019, 246, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Grant, I.R. Mycobacterium paratuberculosis and milk. Acta. Vet. Scand. 2003, 44, 261–266. [Google Scholar] [PubMed]
- Grant, I.R.; Foddai, A.C.G.; Tarrant, J.C.; Kunkel, B.; Hartmann, F.A.; McGuirk, S.; Hansen, C.; Talaat, A.M.; Collins, M.T. Viable Mycobacterium avium ssp. paratuberculosis isolated from calf milk replacer. J. Dairy Sci. 2017, 100, 9723–9735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whittington, R.; Donat, K.; Weber, M.F.; Kelton, D.; Nielsen, S.S.; Eisenberg, S.; Arrigoni, N.; Juste, R.; Sáez, J.L.; Dhand, N.; et al. Control of paratuberculosis: Who, why and how. A review of 48 countries. Vet. Res. 2019, 15, 198. [Google Scholar]
- Strain, S. Johne’s disease control: A challenging yet achievable goal. Vet. Rec. 2018, 182, 481–482. [Google Scholar] [CrossRef]
- Gautam, M.; Ridler, A.; Wilson, P.R.; Heuer, C. Control of clinical paratuberculosis in New Zealand pastoral livestock. N. Z. Vet. J. 2018, 66, 1–8. [Google Scholar]
- Ministry of Agriculture, Forestry and Fishers, Home Page. Available online: https://www.maff.go.jp/j/chikusan/sinko/lin/l.../H29pet_houkoku.pdf (accessed on 5 March 2020).
- Sweeney, R.W.; Whitlock, R.H.; Rosenberger, A.E. Mycobacterium paratuberculosis cultured from milk and supramammary lymph nodes of infected asymptomatic cows. J. Clin. Microbiol. 1992, 30, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Zara, E.G.; Benjamin, M.C.S.; George, B.; Ross, S.D.; Michael, R.H.; Jonathon, N.H.; Catherine, E.D.R. Survival of Mycobacterium avium subspecies paratuberculosis in retail pasteurised milk. Food Microbiol. 2018, 74, 57–63. [Google Scholar]
- McAloon, C.G.; Roche, S.; Ritter, C.; Barkema, H.W.; Whyte, P.; More, S.J.; O’Grady, L.; Green, M.J.; Doherty, M.L. A review of paratuberculosis in dairy herds—Part 1: Epidemiology. Vet. J. 2019, 246, 59–65. [Google Scholar] [CrossRef] [PubMed]
- CLEA Japan, Inc. Home Page. Available online: https://www.clea-japan.com/products/general_diet/item_d0020 (accessed on 4 March 2020).
- Japan SLC, Inc. Home Page. Available online: http://www.sankyolabo.co.jp/sales-pdf/animal_feeds.pdf (accessed on 4 March 2020).
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuribayashi, T.; Cossu, D.; Momotani, E. Seroprevalence of Immunoglobulin G Antibodies Against Mycobacterium avium subsp. paratuberculosis in Dogs Bred in Japan. Vet. Sci. 2020, 7, 93. https://doi.org/10.3390/vetsci7030093
Kuribayashi T, Cossu D, Momotani E. Seroprevalence of Immunoglobulin G Antibodies Against Mycobacterium avium subsp. paratuberculosis in Dogs Bred in Japan. Veterinary Sciences. 2020; 7(3):93. https://doi.org/10.3390/vetsci7030093
Chicago/Turabian StyleKuribayashi, Takashi, Davide Cossu, and Eiichi Momotani. 2020. "Seroprevalence of Immunoglobulin G Antibodies Against Mycobacterium avium subsp. paratuberculosis in Dogs Bred in Japan" Veterinary Sciences 7, no. 3: 93. https://doi.org/10.3390/vetsci7030093
APA StyleKuribayashi, T., Cossu, D., & Momotani, E. (2020). Seroprevalence of Immunoglobulin G Antibodies Against Mycobacterium avium subsp. paratuberculosis in Dogs Bred in Japan. Veterinary Sciences, 7(3), 93. https://doi.org/10.3390/vetsci7030093





