The Influence of β-1,3-1,6-Glucans on Rabies Vaccination Titers in Cats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Design
2.3. Statistical Analysis
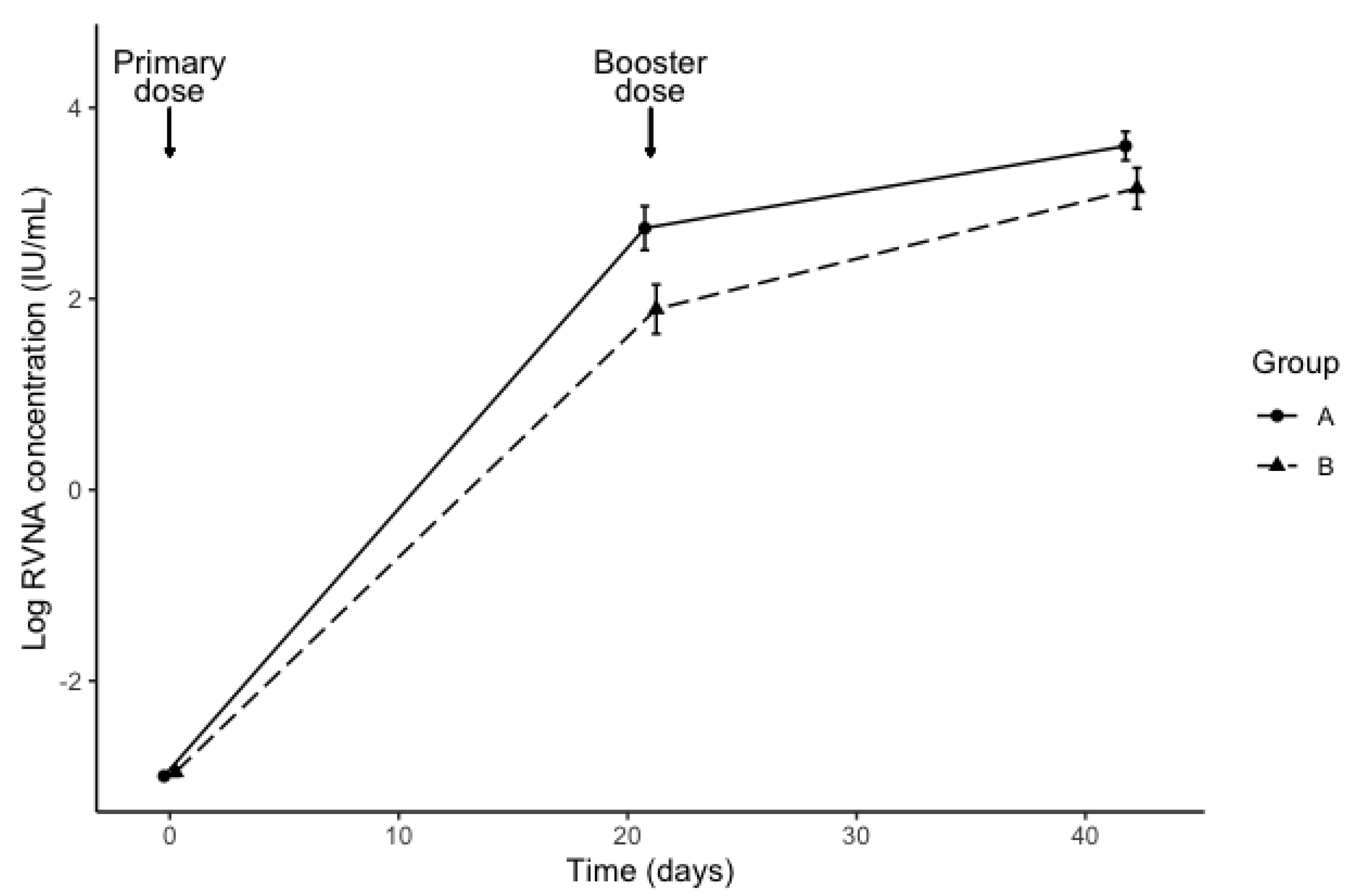
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DLA | Dog Leukocyte Antigen Complex |
| FLA | Feline Leukocyte Antigen Complex |
| GALT | Gut-Associated Lymphoid Tissue |
| MHC | Major Histocompatibility Complex |
| TLR | Toll-Like Receptors |
| RFFIT | Rapid Florescent Foci Inhibition Test |
| RVNA | Rabies Virus Neutralizing Antibody |
References
- Gibson, G.; Scott, K.; Rastall, R.; Tuohy, K.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 1–19. [Google Scholar] [CrossRef]
- Hong, F.; Hansen, R.D.; Yan, J.; Allendorf, D.J.; Baran, J.T.; Ostroff, G.R.; Ross, G.D. β-Glucan Functions as an Adjuvant for Monoclonal Antibody Immunotherapy by Recruiting Tumoricidal Granulocytes as Killer Cells. Am. Assoc. Cancer Res. 2003, 63, 9023–9031. [Google Scholar]
- Brown, G.D.; Taylor, P.R.; Reid, D.M.; Willment, J.A.; Williams, D.L.; Martinez-Pomares, L.; Wong, S.Y.C.; Gordon, S. Dectin-1 is a Major β-Glucan Receptor on Macrophages. J. Exp. Med. 2002, 196, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Baruah, K.; Cox, E.; Vanrompay, D.; Bossier, P. Structure-Functional Activity Relationship of b-Glucans From the Perspective of Immunomodulation: A Mini-Review. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352. [Google Scholar] [CrossRef]
- Marakalala, M.J.; Williams, D.L.; Hoving, J.C.; Engstad, R.; Netea, M.G.; Brown, G.D. Dectin-1 plays a redundant role in the immunomodulatory activities of b-glucan-rich ligands in vivo. Microbes Infect. 2013, 15, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Vojtek, B.; Mojžišová, J.; Smrčo, P.; Drážovská, M. Effects of orally administered β–1,3/1,6–glucan on vaccination responses and immunological parameters in dogs. Food Agric. Immunol. 2017, 28, 993–1002. [Google Scholar] [CrossRef]
- Hetland, G.; Sandven, P. β-1,3-glucan reduces growth of Mycobacterium tuberculosis in macrophage cultures. FEMS Immunol. Med. Microbiol. 2002, 33, 41–45. [Google Scholar] [CrossRef]
- Stuyven, E.; Cox, E.; Vancaeneghem, S.; Arnouts, S.; Deprez, P.; Goddeeris, B.M. Effect of β-glucans on an ETEC infection in piglets. Vet. Immunol. Immunopathol. 2009, 128, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vannucci, L.; Sima, P. The Effects of β-Glucan on Pig Growth and Immunity. Open Biochem. J. 2014, 8, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Procedure. Available online: https://www.rabieselisa.com/procedure/ (accessed on 4 September 2019).
- Rupprecht, C.E.; Fooks, A.R.; Abela-Ridder, B. Laboratory Techniques in Rabies, 15th ed.; World Health Organization: Geneva, Switzerland, 2018; Available online: https://apps.who.int/iris/bitstream/handle/10665/310836/9789241515153-eng.pdf?ua=1 (accessed on 8 July 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 5 May 2020).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Krakowski, L.; Krzyzanowski, J.; Wrona, Z.; Siwicki, A.K. The effect of nonspecific immunostimulation of pregnant mares with 1,3/1,6 glucan and levamisole on the immunoglobulins levels in colostrum, selected indices of nonspecific cellular and humoral immunity in foals in neonatal and postnatal period. Vet. Immunol. Immunopathol. 1999, 68, 1–11. [Google Scholar] [CrossRef]
- Ai, Q.; Mai, K.; Zhang, L.; Tan, B.; Zhang, W.; Xu, W.; Li, H. Effects of dietary β-1 3-glucan on innate immune response of large yellow croaker, Pseudosciaena crocea. Fish. Shellfish Immunol. 2007, 22, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Selim, K.M.; Reda, R.M. Beta-Glucans and Mannan Oligosaccharides Enhance Growth and Immunity in Nile Tilapia. N. Am. J. Aquac. 2015, 77, 22–30. [Google Scholar] [CrossRef]
- Soltanian, S.; Stuyven, E.; Cox, E.; Sorgeloos, P.; Bossier, P. Beta-glucans as immunostimulant in vertebrates and invertebrates. Crit. Rev. Microbiol. 2009, 35, 109–138. [Google Scholar] [CrossRef] [PubMed]
- Ewaschuk, J.B.; Johnson, I.R.; Madsen, K.L.; Vasanthan, T.; Ball, R.; Field, C.J. Barley-derived β-glucans increases gut permeability, ex vivo epithelial cell binding to E. coli, and naïve T-cell proportions in weanling pigs. J. Anim. Sci. 2012, 90, 2652–2662. [Google Scholar] [CrossRef]
- Zhang, M.; Kim, J.A.; Huang, A.Y.C. Optimizing Tumor Microenvironment for Cancer Immunotherapy: β-Glucan-Based Nanoparticles. Front. Immunol. 2018, 9, 341. [Google Scholar] [CrossRef]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Cota, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R.; et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef]
- Stuyven, E.; Verdonck, F.; Van Hoek, I.; Daminet, S.; Duchateau, L.; Remon, J.P.; Goddeeris, B.M.; Cox, E. Oral administration of β-1,3/1,6-glucan to dogs temporally changes total and antigen specific IgA and IgM. Clin. Vaccine Immunol. 2010, 17, 281–285. [Google Scholar] [CrossRef]
- Day, M. Cats are not small dogs: Is there an immunological explanation for why cats are less affected by arthropod-borne disease than dogs? Parasites Vectors 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Morris, K. The Feline Major Histocompatibility Complex. Univ. Syd. Undergrad. Res. J. 2009, 1, 1. [Google Scholar]
- Handl, S.; Dowd, S.E.; Garcia-Mazcorro, J.F.; Steiner, J.; Suchodolski, J.S. Massive parallel 16S rRNA gene pyrosequencing reveals highly diverse fecal bacterial and fungal communities in healthy dogs and cats. FEMS Microbiol. Ecol. 2011, 76, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Tizard, I.R.; Jones, S.W. The microbiota regulates immunity and immunologic diseases in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, J.W.S. The Evolutionary Basis for the Feeding Behavior of Domestic Dogs (Canis familiaris) and Cats (Felis catus). J. Nutr. 2006, 136, 1927S–1931S. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mazcorro, J.F.; Ishaq, S.L.; Rodriguez-Herrera, M.V.; Garcia-Hernandez, C.A.; Kawas, J.R.; Nagaraja, T.G. Review: Are there indigenous Saccharomyces in the digestive tract of livestock animal species? Implications for health, nutrition and productivity traits. Animal 2019, 14, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Cohn, D.A. Sensitivity to androgen. A possible factor in sex differences in the immune response. Clin. Exp. Immunol. 1979, 38, 218–227. [Google Scholar]
- McGraw, K.J.; Ardia, D.R. Sex differences in carotenoid status and immune performance in zebra finches. Evol. Ecol. Res. 2005, 7, 251–262. Available online: http://www.evolutionary-ecology.com/abstracts/v07/1807.html (accessed on 16 May 2020).
- Klein, S.; Flanagan, K. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Giefing-Kröll, C.; Berger, P.; Lepperdinger, G.; Grubeck-Loebenstein, B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell 2015, 14, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Roved, J.; Westerdahl, H.; Hasselquist, D. Sex differences in immune responses: Hormonal effects, antagonistic selection, and evolutionary consequences. Horm. Behav. 2017, 88, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Penhale, W.J.; Talal, N. Sex Hormones, Immune Responses, and Autoimmune Diseases Mechanisms of Sex Hormone Action. Am. J. Pathol. 1985, 121, 531–551. [Google Scholar]
- Fish, E.N. The X-files in immunity: Sex-based differences predispose immune responses. Nat. Rev. Immunol. 2008, 8, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Hohendorf, T.; Görlich, D.; Savola, P.; Kelkka, T.; Mustjoki, S.; Gross, C.C.; Owens, G.C.; Klotz, L.; Dornmair, K.; Wiendl, H.; et al. Sex bias in MHC I shaping of adaptive immunity. Proc. Natl. Acad. Sci. USA 2018, 115, 2168–2173. [Google Scholar] [CrossRef] [PubMed]
| Group A (Placebo) | Group B (Test) | |
|---|---|---|
| Sex | ||
| Male | 9 (50%) | 9 (53%) |
| Female | 9 (50%) | 8 (47%) |
| Age in months | ||
| 4–11 | 6 (33%) | 6 (35%) |
| 12–23 | 8 (44%) | 7 (41%) |
| 24–35 | 3 (17%) | 3 (18%) |
| 36+ | 1 (6%) | 1 (6%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byrne, J.; Knobel, D.; Moore, S.M.; Gatrell, S.; Butaye, P. The Influence of β-1,3-1,6-Glucans on Rabies Vaccination Titers in Cats. Vet. Sci. 2020, 7, 118. https://doi.org/10.3390/vetsci7030118
Byrne J, Knobel D, Moore SM, Gatrell S, Butaye P. The Influence of β-1,3-1,6-Glucans on Rabies Vaccination Titers in Cats. Veterinary Sciences. 2020; 7(3):118. https://doi.org/10.3390/vetsci7030118
Chicago/Turabian StyleByrne, John, Darryn Knobel, Susan M. Moore, Stephanie Gatrell, and Patrick Butaye. 2020. "The Influence of β-1,3-1,6-Glucans on Rabies Vaccination Titers in Cats" Veterinary Sciences 7, no. 3: 118. https://doi.org/10.3390/vetsci7030118
APA StyleByrne, J., Knobel, D., Moore, S. M., Gatrell, S., & Butaye, P. (2020). The Influence of β-1,3-1,6-Glucans on Rabies Vaccination Titers in Cats. Veterinary Sciences, 7(3), 118. https://doi.org/10.3390/vetsci7030118






