A One-Health Model for Reversing Honeybee (Apis mellifera L.) Decline
Abstract
1. Introduction
2. Materials and Methods
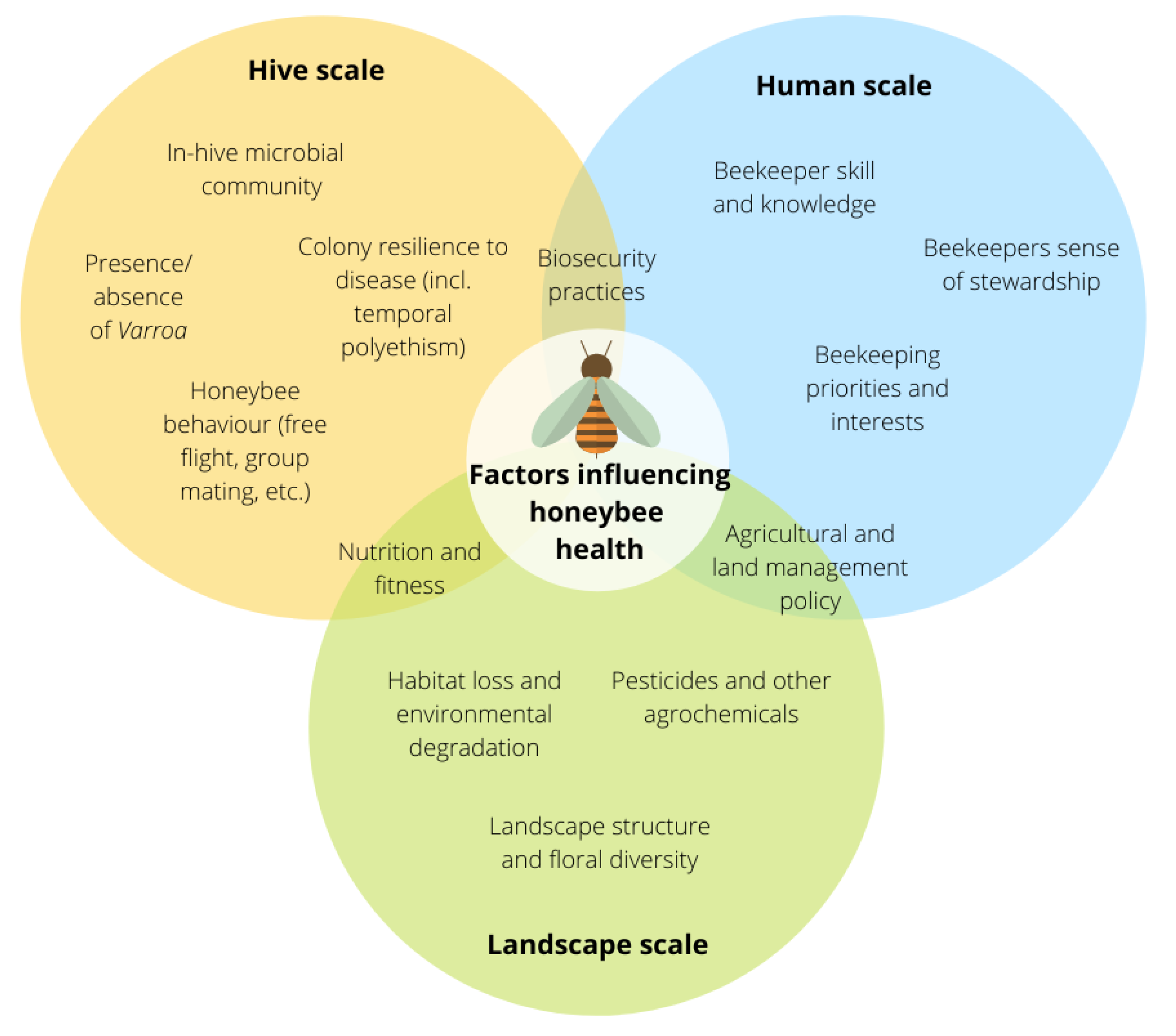
3. Hive-Scale Factors
4. Human-Scale Factors
5. Landscape-Scale Factors
6. Towards an Integrated Approach to Honeybee Health
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Organism | Type of Relationship | Reference |
|---|---|---|
| Lactobacillus spp. Firm-4 | Symbiotic/commensal – collectively, these species form the ‘core microbiome’ of the honeybee’s gut | [97,98] |
| Lactobacillus spp. Firm-5 (phylum Firmicutes) | ||
| Bifidobacterium spp. (phylum Actinobacteria) | ||
| Snodgrassella alvi | ||
| Frischella perrara | ||
| Gilliamella apicola | ||
| Bartonella apis | ||
| Alpha 2.1 (phylum Proteobacteria) | ||
| Black queen cell virus | Viral pathogen | [99,100] |
| Lake Sinai virus | ||
| Deformed wing virus B (VDV1) | ||
| Acute bee paralysis virus | ||
| Chronic bee paralysis virus | ||
| Sacbrood virus | ||
| Deformed wing virus A | ||
| Aphid lethal paralysis virus | ||
| Israeli acute paralysis virus | ||
| Iridescent invertebrate virus IV | ||
| Kashmir bee virus | ||
| Varroa destructor | Parasitic mite, viral vector | [32] |
| Frischella perrara | Bacterial pathogen/commensal | [101,102] |
| Paenibacillus larvae (c.a. American Foulbrood) | ||
| Melissococcus plutonius (c.a. European foul brood) | ||
| Nosema ceranae | Intestinal parasites | [102] |
| Nosema apis | ||
| Crithidia | ||
| Acarapis woodi |
References
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019. [Google Scholar] [CrossRef]
- Scheper, J.; Holzschuh, A.; Kuussaari, M.; Potts, S.G.; Rundlöf, M.; Smith, H.G.; Kleijn, D. Environmental factors driving the effectiveness of European agri-environmental measures in mitigating pollinator loss—A meta-analysis. Ecol. Lett. 2013, 16, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Mattila, H.R.; Rios, D.; Walker-Sperling, V.E.; Roeselers, G.; Newton, I.L.G. Characterization of the active microbiotas associated with honeybees reveals healthier and broader communities when colonies are genetically diverse. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Schmid, M.R.; Brockmann, A.; Pirk, C.W.W.; Stanley, D.W.; Tautz, J. Adult honeybees (Apis mellifera L.) abandon hemocytic, but not phenoloxidase-based immunity. J. Insect Physiol. 2008. [Google Scholar] [CrossRef]
- Saltykova, E.S.; Ben’kovskaya, G.V.; Gaifullina, L.R.; Novitskaya, O.P.; Poskryakov, A.V.; Nikolenko, A.G. Reaction of individual physiological barriers in bacterial infection in different races of the honeybee Apis mellifera. J. Evol. Biochem. Physiol. 2005. [Google Scholar] [CrossRef]
- vanEngelsdorp, D.; Meixner, M.D. A historical review of managed honeybee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 2010. [Google Scholar] [CrossRef]
- Amiri, E.; Strand, M.K.; Rueppell, O.; Tarpy, D.R. Queen quality and the impact of honeybee diseases on queen health: Potential for interactions between two major threats to colony health. Insects 2017, 8, 48. [Google Scholar] [CrossRef]
- Mutinelli, F. Veterinary medicinal products to control Varroa destructor in honeybee colonies (Apis mellifera) and related EU legislation—An update. J. Apic. Res. 2016. [Google Scholar] [CrossRef]
- Narjes, M.E.; Lippert, C. The Optimal Supply of Crop Pollination and Honey from Wild and Managed Bees: An Analytical Framework for Diverse Socio-Economic and Ecological Settings. Ecol. Econ. 2019. [Google Scholar] [CrossRef]
- Croft, S.; Brown, M.; Wilkins, S.; Hart, A.; Smith, G.C. Evaluating European Food Safety Authority Protection Goals for Honeybees (Apis mellifera): What Do They Mean for Pollination? Integr. Environ. Assess. Manag. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, E.P.J. The evolution of one health: A decade of progress and challenges for the future. Vet. Rec. 2014. [Google Scholar] [CrossRef] [PubMed]
- Destoumieux-Garzón, D.; Mavingui, P.; Boetsch, G.; Boissier, J.; Darriet, F.; Duboz, P.; Fritsch, C.; Giraudoux, P.; le Roux, F.; Morand, S.; et al. The one health concept: 10 years old and a long road ahead. Front. Vet. Sci. 2018. [Google Scholar] [CrossRef]
- Lerner, H.; Berg, C. A comparison of three holistic approaches to health: One health, ecohealth, and planetary health. Front. Vet. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hanley, N.; Breeze, T.D.; Ellis, C.; Goulson, D. Measuring the economic value of pollination services: Principles, evidence and knowledge gaps. Ecosyst. Serv. 2015. [Google Scholar] [CrossRef]
- Potts, S.G.; Roberts, S.P.M.; Dean, R.; Marris, G.; Brown, M.A.; Jones, R.; Neumann, P.; Settele, J. Declines of managed honeybees and beekeepers in Europe. J. Apic. Res. 2010. [Google Scholar] [CrossRef]
- Thompson, C.E.; Biesmeijer, J.C.; Allnutt, T.R.; Pietravalle, S.; Budge, G.E. Parasite pressures on feral honeybees (Apis mellifera sp.). PLoS ONE 2014. [Google Scholar] [CrossRef]
- Barr, J.J. A bacteriophages journey through the human body. Immunol. Rev. 2017. [Google Scholar] [CrossRef]
- Hinchliffe, S.; Allen, J.; Lavau, S.; Bingham, N.; Carter, S. Biosecurity and the topologies of infected life: From borderlines to borderlands. Trans. Inst. Br. Geogr. 2013. [Google Scholar] [CrossRef]
- Hinchliffe, S. More than one world, more than one health: Re-configuring interspecies health. Soc. Sci. Med. 2015. [Google Scholar] [CrossRef]
- Stärk, K.D.C.; Arroyo Kuribreña, M.; Dauphin, G.; Vokaty, S.; Ward, M.P.; Wieland, B.; Lindberg, A. One Health surveillance—More than a buzz word? Prev. Vet. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Lapinski, M.K.; Funk, J.A.; Moccia, L.T. Recommendations for the role of social science research in One Health. Soc. Sci. Med. 2015. [Google Scholar] [CrossRef]
- Craddock, S.; Hinchliffe, S. One world, one health? Social science engagements with the one health agenda. Soc. Sci. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.C. How to become a beekeeper: Learning and skill in managing honeybees. Cult. Geogr. 2018. [Google Scholar] [CrossRef]
- Donkersley, P.; Rhodes, G.; Pickup, R.W.; Jones, K.C.; Power, E.F.; Wright, G.A.; Wilson, K. Nutritional composition of honeybee food stores vary with floral composition. Oecologia 2017, 185, 749–761. [Google Scholar] [CrossRef]
- Maderson, S.; Wynne-Jones, S. Beekeepers’ knowledges and participation in pollinator conservation policy. J. Rural Stud. 2016. [Google Scholar] [CrossRef]
- Moritz, R.F.A.; Erler, S. Lost colonies found in a data mine: Global honey trade but not pests or pesticides as a major cause of regional honeybee colony declines. Agric. Ecosyst. Environ. 2016. [Google Scholar] [CrossRef]
- Watson, K.; Stallins, J.A. Honeybees and colony collapse disorder: A pluralistic reframing. Geogr. Compass 2016. [Google Scholar] [CrossRef]
- Donaldson, A. Biosecurity after event: Risk politics and animal disease. Environ. Plan. A 2008. [Google Scholar] [CrossRef]
- Jacques, A.; Laurent, M.; Ribière-Chabert, M.; Saussac, M.; Bougeard, S.; Budge, G.E.; Hendrikx, P.; Chauzat, M.P. A pan-European epidemiological study reveals honeybee colony survival depends on beekeeper education and disease control. PLoS ONE 2017. [Google Scholar] [CrossRef]
- Traver, B.E.; Fell, R.D. Nosema ceranae in drone honeybees (Apis mellifera). J. Invertebr. Pathol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Frey, E.; Rosenkranz, P. Autumn invasion rates of Varroa destructor (Mesostigmata: Varroidae) into honeybee (Hymenoptera: Apidae) colonies and the resulting increase in mite populations. J. Econ. Entomol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.E.; Sheehan, T.H.; Mott, B.M.; Maes, P.; Snyder, L.; Schwan, M.R.; Walton, A.; Jones, B.M.; Corby-Harris, V. Microbial ecology of the hive and pollination landscape: Bacterial associates from floral nectar, the alimentary tract and stored food of honeybees (Apis mellifera). PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Koeniger, N.; Koeniger, G.; Pechhacker, H. The nearer the better? Drones (Apis mellifera) prefer nearer drone congregation areas. Insectes Soc. 2005. [Google Scholar] [CrossRef]
- Johnson, B.R. Division of labor in honeybees: Form, function, and proximate mechanisms. Behav. Ecol. Sociobiol. 2010. [Google Scholar] [CrossRef]
- Perry, C.J.; Søvik, E.; Myerscough, M.R.; Barron, A.B. Rapid behavioral maturation accelerates failure of stressed honey bee colonies. Proc. Natl. Acad. Sci. USA 2015. [Google Scholar] [CrossRef]
- Gisder, S.; Aumeier, P.; Genersch, E. Deformed wing virus: Replication and viral load in mites (Varroa destructor). J. Gen. Virol. 2009. [Google Scholar] [CrossRef]
- Wu, J.Y.; Anelli, C.M.; Sheppard, W.S. Sub-lethal effects of pesticide residues in brood comb on worker honeybee (Apis mellifera) development and longevity. PLoS ONE 2011. [Google Scholar] [CrossRef]
- Stafford, K. Sheep veterinarians and the welfare of sheep: No simple matter. Small Rumin. Res. 2014. [Google Scholar] [CrossRef]
- Crist, E. Can an insect speak? The case of the honeybee dance language. Soc. Stud. Sci. 2004. [Google Scholar] [CrossRef]
- Smith, B.M.; Basu, P.C.; Chatterjee, A.; Chatterjee, S.; Dey, U.K.; Dicks, L.V.; Giri, B.; Laha, S.; Majhi, R.K.; Basu, P. Collating and validating indigenous and local knowledge to apply multiple knowledge systems to an environmental challenge: A case-study of pollinators in India. Biol. Conserv. 2017. [Google Scholar] [CrossRef]
- Hernández-Morcillo, M.; Hoberg, J.; Oteros-Rozas, E.; Plieninger, T.; Gómez-Baggethun, E.; Reyes-García, V. Traditional ecological knowledge in Europe: Status Quo and insights for the environmental policy agenda. Environment 2014. [Google Scholar] [CrossRef]
- Oteros-Rozas, E.; Ontillera-Sánchez, R.; Sanosa, P.; Gómez-Baggethun, E.; Reyes-García, V.; González, J.A. Traditional ecological knowledge among transhumant pastoralists in Mediterranean Spain. Ecol. Soc. 2013. [Google Scholar] [CrossRef]
- Suryanarayanan, S.; Kleinman, D.L. Be(e)coming experts: The controversy over insecticides in the honeybee colony collapse disorder. Soc. Stud. Sci. 2013. [Google Scholar] [CrossRef]
- Suryanarayanan, S. Balancing control and complexity in field studies of neonicotinoids and honeybee health. Insects 2013, 4, 153–167. [Google Scholar] [CrossRef]
- Lehébel-Péron, A.; Sidawy, P.; Dounias, E.; Schatz, B. Attuning local and scientific knowledge in the context of global change: The case of heather honey production in southern France. J. Rural Stud. 2016. [Google Scholar] [CrossRef]
- Neumann, P.; Blacquière, T. The Darwin cure for apiculture? Natural selection and managed honeybee health. Evol. Appl. 2017. [Google Scholar] [CrossRef]
- Loftus, J.C.; Smith, M.L.; Seeley, T.D. How honeybee colonies survive in the wild: Testing the importance of small nests and frequent swarming. PLoS ONE 2016. [Google Scholar] [CrossRef]
- Thoms, C.A.; Nelson, K.C.; Kubas, A.; Steinhauer, N.; Wilson, M.E. Beekeeper stewardship, colony loss, and Varroa destructor management. Ambio 2019. [Google Scholar] [CrossRef]
- Peck, D.T.; Seeley, T.D. Mite bombs or robber lures? The roles of drifting and robbing in Varroa destructor transmission from collapsing honeybee colonies to their neighbors. PLoS ONE 2019. [Google Scholar] [CrossRef]
- Fürst, M.A.; McMahon, D.P.; Osborne, J.L.; Paxton, R.J.; Brown, M.J.F. Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nature 2014. [Google Scholar] [CrossRef]
- Rinderer, T.E.; Harris, J.W.; Hunt, G.J.; de Guzman, L.I. Breeding for resistance to Varroa destructor in North America. Apidologie 2010. [Google Scholar] [CrossRef]
- De Mattos, I.M.; Soares, A.E.E.; Tarpy, D.R. Effects of synthetic acaricides on honeybee grooming behavior against the parasitic Varroa destructor mite. Apidologie 2017. [Google Scholar] [CrossRef]
- Sponsler, D.B.; Grozinger, C.M.; Hitaj, C.; Rundlöf, M.; Botías, C.; Code, A.; Lonsdorf, E.V.; Melathopoulos, A.P.; Smith, D.J.; Suryanarayanan, S.; et al. Pesticides and pollinators: A socioecological synthesis. Sci. Total Environ. 2019. [Google Scholar] [CrossRef]
- Tengö, M.; Brondizio, E.S.; Elmqvist, T.; Malmer, P.; Spierenburg, M. Connecting diverse knowledge systems for enhanced ecosystem governance: The multiple evidence base approach. Ambio 2014. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.J.F.; Paxton, R.J. The conservation of bees: A global perspective. Apidologie 2009. [Google Scholar] [CrossRef]
- Hall, D.M.; Steiner, R. Insect pollinator conservation policy innovations: Lessons for lawmakers. Environ. Sci. Policy 2019. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019. [Google Scholar] [CrossRef]
- Vanbergen, A.J.; Garratt, M.P.; Vanbergen, A.J.; Baude, M.; Biesmeijer, J.C.; Britton, N.F.; Brown, M.J.F.; Brown, M.; Bryden, J.; Budge, G.E.; et al. Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013. [Google Scholar] [CrossRef]
- Kleijn, D.; van Langevelde, F. Interacting effects of landscape context and habitat quality on flower visiting insects in agricultural landscapes. Basic Appl. Ecol. 2006. [Google Scholar] [CrossRef]
- Klein, A.-M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Nottebrock, H.; Schmid, B.; Mayer, K.; Devaux, C.; Esler, K.J.; Böhning-Gaese, K.; Schleuning, M.; Pagel, J.; Schurr, F.M. Sugar landscapes and pollinator-mediated interactions in plant communities. Ecography Cop. 2017, 40, 1129–1138. [Google Scholar] [CrossRef]
- Odoux, J.F.; Feuillet, D.; Aupinel, P.; Loublier, Y.; Tasei, J.N.; Mateescu, C. Territorial biodiversity and consequences on physico-chemical characteristics of pollen collected by honeybee colonies. Apidologie 2012. [Google Scholar] [CrossRef]
- Donkersley, P.; Rhodes, G.; Pickup, R.W.; Jones, K.C.; Wilson, K. Honeybee nutrition is linked to landscape composition. Ecol. Evol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; Raubenheimer, D. The Nature of Nutrition: A Unifying Framework from Animal Adaptation to Human Obesity; Princeton University Press: Princeton, NJ, USA, 2012; ISBN 9781400842803. [Google Scholar]
- Kwang, P.L.; Simpson, S.J.; Clissold, F.J.; Brooks, R.; Ballard, J.W.O.; Taylor, P.W.; Soran, N.; Raubenheimer, D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. USA 2008. [Google Scholar] [CrossRef]
- Lee, K.P.; Cory, J.S.; Wilson, K.; Raubenheimer, D.; Simpson, S.J. Flexible diet choice offsets protein costs of pathogen resistance in a caterpillar. Proc. R. Soc. B Biol. Sci. 2006. [Google Scholar] [CrossRef]
- Ruedenauer, F.A.; Raubenheimer, D.; Kessner-Beierlein, D.; Grund-Mueller, N.; Noack, L.; Spaethe, J.; Leonhardt, S.D. Best be(e) on low fat: Linking nutrient perception, regulation and fitness. Ecol. Lett. 2020. [Google Scholar] [CrossRef]
- Donkersley, P. Trees for bees. Agric. Ecosyst. Environ. 2019. [Google Scholar] [CrossRef]
- Deguines, N.; Jono, C.; Baude, M.; Henry, M.; Julliard, R.; Fontaine, C. Large-scale trade-off between agricultural intensification and crop pollination services. Front. Ecol. Environ. 2014. [Google Scholar] [CrossRef]
- Holzschuh, A.; Dainese, M.; González-Varo, J.P.; Mudri-Stojnić, S.; Riedinger, V.; Rundlöf, M.; Scheper, J.; Wickens, J.B.; Wickens, V.J.; Bommarco, R.; et al. Mass-flowering crops dilute pollinator abundance in agricultural landscapes across Europe. Ecol. Lett. 2016. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.; et al. Country-specific effects of neonicotinoid pesticides on honeybees and wild bees. Science 2017. [Google Scholar] [CrossRef] [PubMed]
- Motta, E.V.S.; Raymann, K.; Moran, N.A. Glyphosate perturbs the gut microbiota of honeybees. Proc. Natl. Acad. Sci. USA 2018. [Google Scholar] [CrossRef]
- Zaluski, R.; Justulin, L.A.; Orsi, R.D.O. Field-relevant doses of the systemic insecticide fipronil and fungicide pyraclostrobin impair mandibular and hypopharyngeal glands in nurse honeybees (Apis mellifera). Sci. Rep. 2017. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.S.; Curzon, R. Stakeholder mapping for the governance of biosecurity: A literature review. J. Integr. Environ. Sci. 2015. [Google Scholar] [CrossRef]
- Zinsstag, J. Convergence of ecohealth and one health. Ecohealth 2012. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, A.; Crowe, O.; Regan, E.; Begley, S.; Caffarra, A. The role of citizen science in monitoring biodiversity in Ireland. Int. J. Biometeorol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Jalbert, K.; Kinchy, A.J.; Perry, S.L. Civil society research and Marcellus Shale natural gas development: Results of a survey of volunteer water monitoring organizations. J. Environ. Stud. Sci. 2014. [Google Scholar] [CrossRef]
- van der Steen, J.S.; Brodschneider, R. Public participation in bee science: C.S.I. pollen. Bee World 2014. [Google Scholar] [CrossRef]
- Bonney, R.; Cooper, C.B.; Dickinson, J.; Kelling, S.; Phillips, T.; Rosenberg, K.V.; Shirk, J. Citizen science: A developing tool for expanding science knowledge and scientific literacy. Bioscience 2009. [Google Scholar] [CrossRef]
- Ellis, R.; Waterton, C. Environmental citizenship in the making: The participation of volunteer naturalists in UK biological recording and biodiversity policy. Sci. Public Policy 2004. [Google Scholar] [CrossRef]
- Tang, M.; Hardman, C.J.; Ji, Y.; Meng, G.; Liu, S.; Tan, M.; Yang, S.; Moss, E.D.; Wang, J.; Yang, C.; et al. High-throughput monitoring of wild bee diversity and abundance via mitogenomics. Methods Ecol. Evol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, M.; Espinosa, M.; Gomez y Paloma, S.; Paracchini, M.L.; Piorr, A.; Zasada, I. Agricultural landscapes as multi-scale public good and the role of the common agricultural policy. J. Environ. Plan. Manag. 2015, 58, 2088–2112. [Google Scholar] [CrossRef]
- Hadley, A.S.; Betts, M.G. The effects of landscape fragmentation on pollination dynamics: Absence of evidence not evidence of absence. Biol. Rev. 2012, 87, 526–544. [Google Scholar] [CrossRef] [PubMed]
- Eastburn, D.J.; O’Geen, A.T.; Tate, K.W.; Roche, L.M. Multiple ecosystem services in a working landscape. PLoS ONE 2017, 12, e0166595. [Google Scholar] [CrossRef]
- Hall, M.A.; Nimmo, D.G.; Cunningham, S.A.; Walker, K.; Bennett, A.F. The response of wild bees to tree cover and rural land use is mediated by species’ traits. Biol. Conserv. 2019. [Google Scholar] [CrossRef]
- Wilson, J.S.; Forister, M.L.; Carril, O.M. Interest exceeds understanding in public support of bee conservation. Front. Ecol. Environ. 2017. [Google Scholar] [CrossRef]
- Campbell, A.J.; Wilby, A.; Sutton, P.; Wäckers, F. Getting more power from your flowers: Multi-functional flower strips enhance pollinators and pest control agents in apple orchards. Insects 2017, 8, 101. [Google Scholar] [CrossRef]
- Padilla, F.M.; Pugnaire, F.I. Species identity and water availability determine establishment success under the canopy of Retama sphaerocarpa shrubs in a dry environment. Restor. Ecol. 2009, 17, 900–907. [Google Scholar] [CrossRef]
- Breeze, T.D.; Vaissière, B.E.; Bommarco, R.; Petanidou, T.; Seraphides, N.; Kozák, L.; Scheper, J.; Biesmeijer, J.C.; Kleijn, D.; Gyldenkærne, S.; et al. Agricultural policies exacerbate honeybee pollination service supply-demand mismatches across Europe. PLoS ONE 2014. [Google Scholar] [CrossRef]
- Bixler, P.R.; Dell’Angelo, J.; Mfune, O.; Roba, H. The political ecology of participatory conservation: Institutions and discourse. J. Polit. Ecol. 2015. [Google Scholar] [CrossRef]
- Rauschmayer, F.; van den Hove, S.; Koetz, T. Participation in EU biodiversity governance: How far beyond rhetoric? Environ. Plan. C Gov. Policy 2009. [Google Scholar] [CrossRef]
- Naylor, R.; Hamilton-Webb, A.; Little, R.; Maye, D. The ‘good farmer’: Farmer identities and the control of exotic livestock disease in England. Sociol. Ruralis 2018. [Google Scholar] [CrossRef]
- Smith, T.J.; Saunders, M.E. Honeybees: The queens of mass media, despite minority rule among insect pollinators. Insect Conserv. Divers. 2016. [Google Scholar] [CrossRef]
- Rousham, E.K.; Unicomb, L.; Islam, M.A. Human, animal and environmental contributors to antibiotic resistance in low-resource settings: Integrating behavioural, epidemiological and one health approaches. Proc. R. Soc. B Biol. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ollerton, J. Pollinator diversity: Distribution, ecological function, and conservation. Annu. Rev. Ecol. Evol. Syst. 2017. [Google Scholar] [CrossRef]
- Engel, P.; Martinson, V.G.; Moran, N.A. Functional diversity within the simple gut microbiota of the honey bee. Proc. Natl. Acad. Sci. USA 2012. [Google Scholar] [CrossRef] [PubMed]
- Raymann, K.; Moran, N.A. The role of the gut microbiome in health and disease of adult honey bee workers. Curr. Opin. Insect Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M.; Evans, J.D.; Robinson, G.E.; Berenbaum, M.R. Changes in transcript abundance relating to colony collapse disorder in honey bees (Apis mellifera). Proc. Natl. Acad. Sci. USA 2009, 106, 14790–14795. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Chen, Y. Nutrition, immunity and viral infections in honey bees. Curr. Opin. Insect Sci. 2015, 10, 170–176. [Google Scholar] [CrossRef]
- Engel, P.; Kwong, W.K.; Moran, N.A. Frischella perrara gen. nov., sp. nov., a gammaproteobacterium isolated from the gut of the honeybee, Apis mellifera. Int. J. Syst. Evol. Microbiol. 2013. [Google Scholar] [CrossRef]
- D’Alvise, P.; Seeburger, V.; Gihring, K.; Kieboom, M.; Hasselmann, M. Seasonal dynamics and co-occurrence patterns of honey bee pathogens revealed by high-throughput RT-qPCR analysis. Ecol. Evol. 2019. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donkersley, P.; Elsner-Adams, E.; Maderson, S. A One-Health Model for Reversing Honeybee (Apis mellifera L.) Decline. Vet. Sci. 2020, 7, 119. https://doi.org/10.3390/vetsci7030119
Donkersley P, Elsner-Adams E, Maderson S. A One-Health Model for Reversing Honeybee (Apis mellifera L.) Decline. Veterinary Sciences. 2020; 7(3):119. https://doi.org/10.3390/vetsci7030119
Chicago/Turabian StyleDonkersley, Philip, Emily Elsner-Adams, and Siobhan Maderson. 2020. "A One-Health Model for Reversing Honeybee (Apis mellifera L.) Decline" Veterinary Sciences 7, no. 3: 119. https://doi.org/10.3390/vetsci7030119
APA StyleDonkersley, P., Elsner-Adams, E., & Maderson, S. (2020). A One-Health Model for Reversing Honeybee (Apis mellifera L.) Decline. Veterinary Sciences, 7(3), 119. https://doi.org/10.3390/vetsci7030119






