Unraveling Honey Bee–Varroa destructor Interaction: Multiple Factors Involved in Differential Resistance between Two Uruguayan Populations
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview
2.2. Estimation of the Honey Bee Population and Brood Area
2.3. Evaluation of Hygienic and Grooming Behaviors
2.4. Estimation of Mites in Honey Bees and in Brood Cells
2.5. Detection and Quantification of RNA Viruses in Honey Bees and Mites
2.6. DWV Variants
2.7. Molecular Characterization of Honey Bees
2.8. Molecular Characterization of V. destructor
2.9. Statistical Analysis
3. Results
3.1. Honey Bee Population and Brood Area
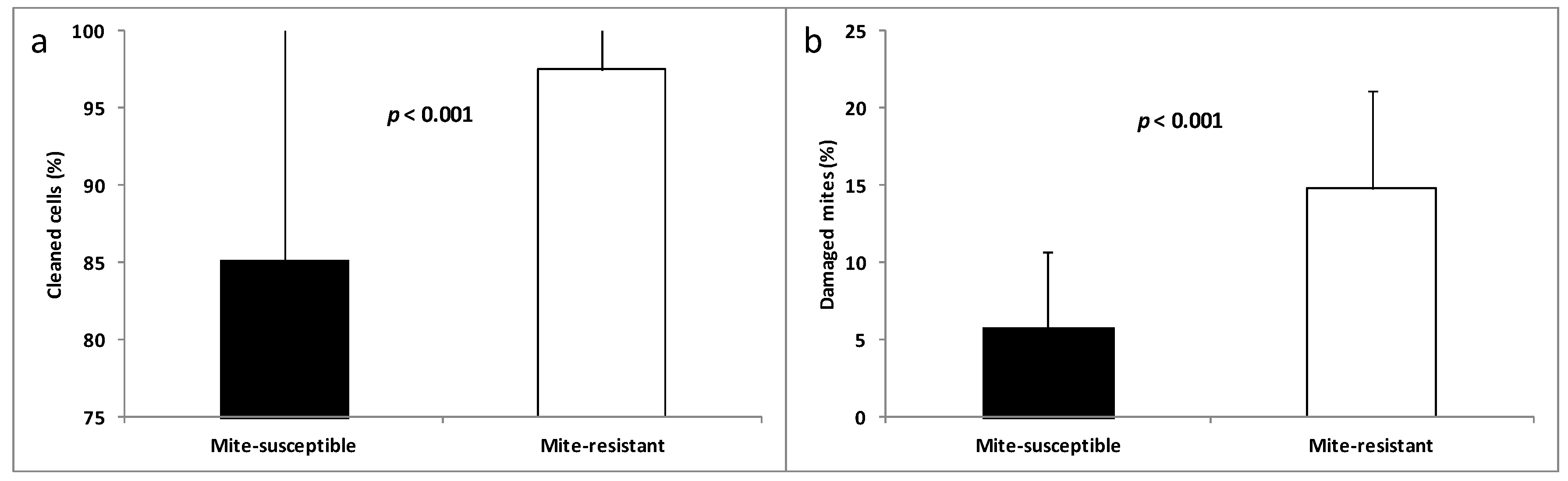
3.2. Hygienic and Grooming Behaviors
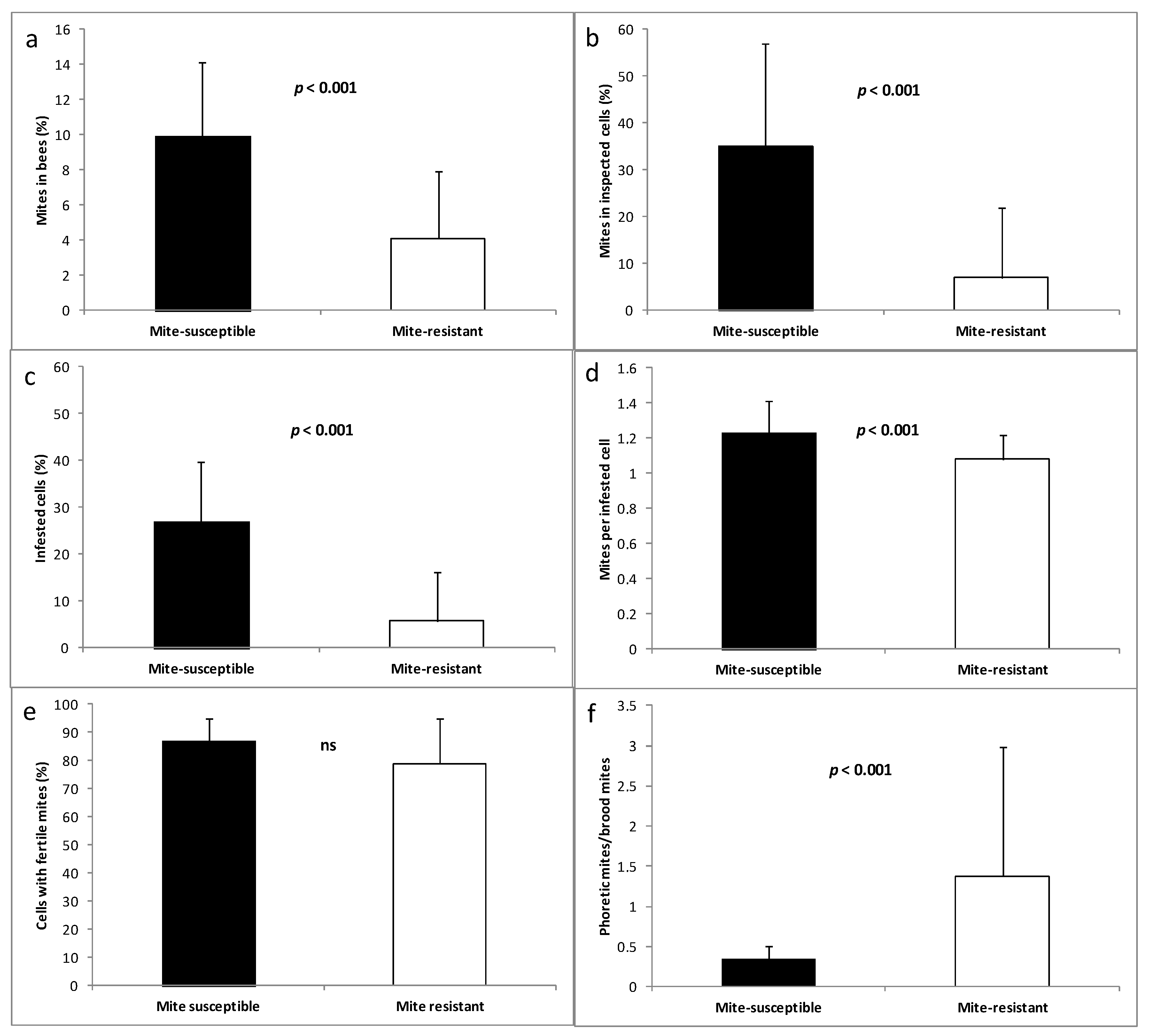
3.3. Mites in Honey Bees and Brood Cells
3.4. RNA Viruses in Honey Bees and Mites
3.5. Molecular Characterization of Honey Bees
3.6. Molecular Characterization of V. destructor
4. Discussion
4.1. Resistance Behaviors to V. destructor
4.2. Reproductive Aspects of V. destructor
4.3. Presence of Viruses in Honey Bees and Mites
4.4. Genetic Differences between Honey Bees
4.5. Genetic Differences between Mites
4.6. Final Considerations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef]
- Le Conte, Y.; Ellis, M.; Ritter, W. Varroa mites and honey bee health: Can Varroa explain part of the colony losses? Apidologie 2010, 41, 353–363. [Google Scholar] [CrossRef]
- Anderson, D.L.; Trueman, J.W.H. Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp. Appl. Acarol. 2000, 24, 165–189. [Google Scholar] [CrossRef]
- Boot, W.J.; Tan, N.Q.; Dien, P.C.; Huan, L.V.; Dung, N.V.; Long, L.T.; Beetsma, J. Reproductive success of Varroa jacobsoni in brood of its original host, Apis cerana, in comparison to that of its new host, Apis mellifera. Bull. Entomol. Res. 1996, 87, 119–126. [Google Scholar] [CrossRef]
- Rath, W. Co-adaptation of Apis cerana Fabr. and Varroa jacobsoni Oud. Apidologie 1999, 30, 97–110. [Google Scholar] [CrossRef]
- Yang, X.; Cox-Foster, D.L. Impact of an ectoparasite on the immunity and pathology of an invertebrate: Evidence for host immunosuppression and viral amplification. Proc. Natl. Acad. Sci. USA 2005, 102, 7470–7475. [Google Scholar] [CrossRef] [PubMed]
- Beaurepaire, A.; Piot, N.; Doublet, V.; Antunez, K.; Campbell, E.; Chantawannakul, P.; Chejanovsky, N.; Gajda, A.; Heerman, M.; Panziera, D.; et al. Diversity and global distribution of viruses of the western honey bee. Apis Mellifera Insects 2020, 11, 239. [Google Scholar] [CrossRef]
- De Miranda, J.R.; Genersch, E. Deformed wing virus. J. Invertebr. Pathol. 2010, 103, S48–S61. [Google Scholar] [CrossRef]
- Martin, S.J.; Brettell, L.E. Deformed wing virus in honeybees and other insects. Ann. Rev. Virol. 2019, 6, 49–69. [Google Scholar] [CrossRef]
- Guzmán-Novoa, E.; Eccles, L.; Calvete, Y.; McGowan, J.; Kelly, P.G.; Correa-Benítez, A. Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie 2010, 41, 443–450. [Google Scholar] [CrossRef]
- Maggi, M.; Antúnez, K.; Invernizzi, C.; Aldea, P.; Vargas, M.; Negri, P.; Brasesco, C.; De Jong, D.; Message, D.; Teixeira, E.W.; et al. Honeybee health in South America. Apidologie 2016, 47, 835–854. [Google Scholar] [CrossRef]
- Pirk, C.W.W.; Strauss, U.; Yusuf, A.A.; Démares, F.; Human, H. Honey bee health in Africa—A review. Apidologie 2015, 47, 276–300. [Google Scholar] [CrossRef]
- Locke, B. Natural Varroa mite-surviving Apis mellifera honeybee populations. Apidologie 2016, 47, 467–482. [Google Scholar] [CrossRef]
- Fries, I.; Camazine, S. Implications of horizontal and vertical pathogen transmission for honey bee epidemiology. Apidologie 2001, 32, 199–214. [Google Scholar] [CrossRef]
- Neumann, P.; Blacquière, T. The Darwin cure for apiculture? Natural selection and managed honeybee health. Evol. Appl. 2017, 10, 226–230. [Google Scholar] [CrossRef]
- Dynes, T.L.; Berry, J.A.; Delaplane, K.S.; De Roode, J.C.; Brosi, B.J. Assessing virulence of Varroa destructor mites from different honey bee management regimes. Apidologie 2020, 51, 276–289. [Google Scholar] [CrossRef]
- Boecking, O.; Spivak, M. Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie 1999, 30, 141–158. [Google Scholar] [CrossRef]
- Büchler, R.; Berg, S.; Conte, Y.L. Breeding for resistance to Varroa destructor in Europe. Apidologie 2010, 41, 393–408. [Google Scholar] [CrossRef]
- Rinderer, T.E.; Harris, J.W.; Hunt, G.J.; de Guzman, L.I. Breeding for resistance to Varroa destructor in North America. Apidologie 2010, 41, 409–424. [Google Scholar] [CrossRef]
- Evans, J.D.; Spivak, M. Socialized medicine: Individual and communal disease barriers in honey bees. J. Invertebr. Pathol. 2010, 103, S62–S72. [Google Scholar] [CrossRef]
- Harbo, J.R.; Harris, J.W. Suppressed mite reproduction explained by the behaviour of adult bees. J. Apic. Res. 2005, 44, 21–23. [Google Scholar] [CrossRef]
- Arechavaleta-Velasco, M.E.; Guzman-Novoa, E. Relative effect of four characteristics that restrain the population growth of the mite Varroa destructor in honey bee (Apis mellifera) colonies. Apidologie 2001, 32, 157–174. [Google Scholar] [CrossRef]
- Mondragón, L.; Spivak, M.; Vandame, R. A multifactorial study of the resistance of honeybees Apis mellifera to the mite Varroa destructor over one year in Mexico. Apidologie 2005, 36, 345–358. [Google Scholar] [CrossRef]
- Guzman-Novoa, E.; Emsen, B.; Unger, P.; Espinosa Montaño, L.G.; Petukhova, T. Genotypic variability and relationships between mite infestation levels, mite damage, grooming intensity, and removal of Varroa destructor mites in selected strains of worker honey bees (Apis mellifera L.). J. Invertebr. Pathol. 2012, 110, 314–320. [Google Scholar] [CrossRef]
- Morfin, N.; Given, K.; Evans, M.; Guzmán-Novoa, E.; Hunt, G.J. Grooming behavior and gene expression of the Indiana “mite-biter” honey bee stock. Apidologie 2020, 51, 267–275. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Tewarson, N.C.; Singh, A.; Engels, W. Differential hygienic behaviour towards Varroa jacobsoni in capped worker brood of Apis cerana depends on alien scent adhering to the mites. J. Apicult. Res. 1993, 32, 89–93. [Google Scholar] [CrossRef]
- Dietemann, V.; Pflugfelder, J.; Anderson, D.; Charrière, J.-D.; Chejanovsky, N.; Dainat, B.; de Miranda, J.; Delaplane, K.; Dillier, F.-X.; Fuchs, S.; et al. Varroa destructor: Research avenues towards sustainable control. J. Apicult. Res. 2012, 51, 125–132. [Google Scholar] [CrossRef]
- Danka, R.G.; Rinderer, T.E.; Spivak, M.; Kefuss, J. Comments on: “Varroa destructor: Research avenues towards sustainable control”. J. Apicult. Res. 2013, 52, 69–71. [Google Scholar] [CrossRef]
- De Guzman, L.I.; Rinderer, T.E. Identification and comparison of Varroa species infesting honey bees. Apidologie 1999, 30, 85–95. [Google Scholar] [CrossRef]
- Garrido, C.; Rosenkranz, P.; Paxton, R.J.; Gonçalves, L.S. Temporal changes in Varroa destructor fertility and haplotype in Brazil. Apidologie 2003, 34, 535–541. [Google Scholar] [CrossRef]
- Solignac, M.; Cornuet, J.-M.; Vautrin, D.; Le Conte, Y.; Anderson, D.; Evans, J.; Cros-Arteil, S.; Navajas, M. The invasive Korea and Japan types of Varroa destructor, ectoparasitic mites of the Western honey bee (Apis mellifera), are two partly isolated clones. Proc. Roy. Soc. Lond. Ser. B Biol. Sci. 2005, 272, 411–419. [Google Scholar] [CrossRef]
- Dynes, T.L.; De Roode, J.C.; Lyons, J.I.; Berry, J.A.; Delaplane, K.S.; Brosi, B.J. Fine scale population genetic structure of Varroa destructor, an ectoparasitic mite of the honey bee (Apis mellifera). Apidologie 2017, 48, 9–101. [Google Scholar] [CrossRef]
- Beaurepaire, A.L.; Moro, A.; Mondet, F.; Le Conte, Y.; Neumann, P.; Locke, B. Population genetics of ectoparasitic mites suggest arms race with honeybee hosts. Sci. Rep. 2019, 9, 11355. [Google Scholar] [CrossRef]
- Cordara, J.J. La Historia de La Apicultura en Uruguay; Facultad de Ciencias Agrarias, Universidad de la Empresa: Montevideo, Uruguay, 2005. [Google Scholar]
- Diniz, N.M.; Soares, A.E.E.; Sheppard, W.S.; Del Lama, M.A. Genetic structure of honeybee populations from southern Brazil and Uruguay. Genet. Mol. Biol. 2003, 26, 47–52. [Google Scholar] [CrossRef]
- Branchiccela, B.; Aguirre, C.; Parra, G.; Estay, P.; Zunino, P.; Antúnez, K. Genetic changes in Apis mellifera after 40 years of Africanization. Apidologie 2014, 45, 752–756. [Google Scholar] [CrossRef][Green Version]
- Invernizzi, C.; Antúnez, K.; Campa, J.P.; Harriet, J.; Mendoza, Y.; Santos, E.; Zunino, P. Situación sanitaria de las abejas melíferas en Uruguay. Veterinaria 2011, 47, 15–27. [Google Scholar]
- Mendoza, Y.; Gramajo, E.; Invernizzi, C.; Tomasco, I.H. Mitochondrial haplotype analyses of the mite Varroa destructor (Acari: Varroidae) collected from honeybees Apis mellifera (Hymenoptera, Apidae) in Uruguay. Syst. Appl. Acarol. 2020, in press. [Google Scholar]
- Delaplane, K.S.; van der Steen, J.; Guzmán-Novoa, E. Standard methods for estimating strength parameters of Apis mellifera colonies. J. Apicult. Res. 2013, 52, 1–12. [Google Scholar] [CrossRef]
- Büchler, R.; Andonov, S.; Bienefeld, K.; Costa, C.; Hatjina, F.; Kezic, N.; Kryger, P.; Spivak, M.; Uzunov, A.; Wilde, J. Standard methods for rearing and selection of Apismellifera queens. J. Apicult. Res. 2013, 52, 1–30. [Google Scholar] [CrossRef]
- Invernizzi, C.; Zefferino, I.; Santos, E.; Sánchez, L.; Mendoza, Y. Multilevel assessment of grooming behaviour against Varroa destructor in Italian and Africanized honey bees. J. Apicult. Res. 2016, 54, 1–7. [Google Scholar]
- Dietemann, V.; Nazzi, F.; Martin, S.J.; Anderson, D.L.; Locke, B.; Delaplane, K.S.; Wauquiez, Q.; Tannahill, C.; Frey, E.; Ziegelmann, B.; et al. Standard methods for varroa research. J. Apicult. Res. 2013, 52, 1–54. [Google Scholar] [CrossRef]
- Antúnez, K.; D’Alessandro, B.; Corbella, E.; Zunino, P. Detection of Chronic bee paralysis virus and Acute bee paralysis virus in Uruguayan honeybees. J. Invertebr. Pathol. 2005, 90, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M.; Evans, J.D.; Robinson, G.E.; Berenbaum, M.R. Changes in transcript abundance relating to colony collapse disorder in honey bees (Apis mellifera). Proc. Natl. Acad. Sci. USA 2009, 106, 14790–14795. [Google Scholar] [CrossRef] [PubMed]
- Kukielka, D.; Esperón, F.; Higes, M.; Sánchez-Vizcaíno, J.M. A sensitive one-step real-time RT-PCR method for detection of deformed wing virus and black queen cell virus in honeybee Apis mellifera. J. Virol. Methods 2008, 147, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cox-Foster, D. Effects of parasitization by Varroa destructor on survivorship and physiological traits of Apis mellifera in correlation with viral incidence and microbial challenge. Parasitology 2007, 134, 405–412. [Google Scholar] [CrossRef]
- Anido, M.; Branchiccela, B.; Castelli, L.; Harriet, J.; Campá, J.; Zunino, P.; Antúnez, K. Prevalence and distribution of honey bee pathogens in Uruguay. J. Apicult. Res. 2016, 54, 532–540. [Google Scholar] [CrossRef]
- Chen, Y.P.; Pettis, J.S.; Feldlaufer, M.F. Detection of multiple viruses in queens of the honey bee, Apis mellifera L. J. Invertebr. Pathol. 2005, 90, 118–121. [Google Scholar] [CrossRef]
- Highfield, A.C.; El Nagar, A.; Mackinder, L.C.; Noel, L.M.; Hall, M.J.; Martin, S.J.; Schroeder, D.C. Deformed wing virus implicated in overwintering honeybee colony losses. Appl. Environ. Microbiol. 2009, 75, 7212–7220. [Google Scholar] [CrossRef]
- Kevill, J.L.; Highfield, A.; Mordecai, G.J.; Martin, S.J.; Schroeder, D.C. ABC assay: Method development and application to quantify the role of three DWV master variants in overwinter colony losses of European honey bees. Viruses 2017, 9, 314. [Google Scholar] [CrossRef]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F.R.N. A simple salting out procedure for extracting DNA from human nucleated cell. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef]
- Estoup, A.; Solignac, M.; Cornuet, J.-M. Precise assessment of the number of patrilines and of genetic relatedness in honeybee colonies. Proc. R. Soc. Lond. B 1994, 258, 1–7. [Google Scholar]
- Estoup, A.; Garnery, L.; Solignac, M.; Cornuet, J.-M. Microsatellite variation in honey bee (Apis mellifera L.) populations: Hierarchical genetic structure and test of the infinite allele and stepwise mutation models. Genetics 1995, 140, 679–695. [Google Scholar]
- Oldroyd, B.P.; Smolenski, A.J.; Cornuet, J.; Wongsiri, S.; Estoup, A.; Rinderer, T.E.; Crozier, R.H. Levels of polyandry and intracolonial genetic relationships in Apis florea. Behav. Ecol. Sociobiol. 1995, 37, 329–335. [Google Scholar] [CrossRef]
- Rowe, D.; Rinderer, T.; Stelzer, J.; Oldroyd, B.P.; Crozier, R.H. Seven polymorphic microsatellite loci in honeybees (Apis mellifera). Insectes Soc. 1997, 44, 85–93. [Google Scholar] [CrossRef]
- Sanguinetti, C.J.; Días Neto, E.; Simpson, A.J. Rapid silver staining and recovery of PCR products separated on polyacrylamide gels. Biotechniques 1994, 17, 914–921. [Google Scholar]
- Pinto, M.A.; Rubink, W.L.; Patton, J.C.; Coulson, R.N.; Johnston, J.S. Africanization in the United States: Replacement of feral European honeybees (Apis mellifera L.) by an African hybrid swarm. Genetics 2005, 170, 1653–1665. [Google Scholar] [CrossRef]
- Evans, J.D. Microsatellite loci in the honey bee parasitic mite Varroa jacobsoni. Mol. Ecol. 2000, 9, 1436–1438. [Google Scholar] [CrossRef]
- Solignac, M.; Vautrin, D.; Pizzo, A.; Navajas, M.; Le Conte, Y.; Cornuet, J.-M. Characterization of microsatellite markers for the apicultural pest Varroa destructor (Acari: Varroidae) and its relatives. Mol. Ecol. Notes 2003, 3, 556–559. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Akaike, H. Autoregressive model fitting for control. Ann. Inst. Statist. Math. 1971, 23, 163–180. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Raymond, M.; Rousset, F. GENEPOP Version 1.2: Population genetics software for exact tests and ecumenicism. J. Hered. 1995, 86, 248–249. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [PubMed]
- Boecking, O.; Drescher, W. The removal response of Apis mellifera L. colonies to brood in wax and plastic cells after artificial and natural infestation with Varroa jacobsoni Oud. and to freeze killed brood. Exp. Appl. Acarol. 1992, 16, 321–329. [Google Scholar] [CrossRef]
- Spivak, M.; Reuter, G.S. Varroa jacobsoni infestation in untreated honey bee (Hymenoptera: Apidae) colonies selected for hygienic behavior. J. Econ. Entomol. 2001, 94, 326–331. [Google Scholar] [CrossRef]
- Ibrahim, A.; Reuter, G.S.; Spivak, M. Field trial of honey bee colonies bred for mechanisms of resistance against Varroa destructor. Apidologie 2007, 38, 67–76. [Google Scholar] [CrossRef]
- Leclercq, G.; Pannebakker, B.; Gengler, N.; Nguyen, B.K.; Francis, F. Drawbacks and benefits of hygienic behavior in honey bees (Apis mellifera L.): A review. J. Apic. Res. 2017, 56, 366–375. [Google Scholar] [CrossRef]
- Nganso, B.; Fombong, A.; Yusuf, A.; Pirk, C.; Stuhl, C.; Torto, B. Low fertility, fecundity and numbers of mated female offspring explain the lower reproductive success of the parasitic mite Varroa destructor in African honeybees. Parasitology 2018, 145, 1633–1639. [Google Scholar] [CrossRef]
- Fries, I.; Rosenkranz, P. Number of reproductive cycles of Varroa jacobsoni in honey-bee (Apis mellifera) colonies. Exp. Appl. Acarol. 1996, 20, 103–112. [Google Scholar] [CrossRef]
- Martin, S.J.; Kemp, D. Average number of reproductive cycles performed by Varroa jacobsoni in honey bee (Apis mellifera) colonies. J. Apicult. Res. 1997, 36, 113–123. [Google Scholar] [CrossRef]
- Oddie, M.A.Y.; Büchler, R.; Dahle, B.; Kovacic, M.; Le Conte, Y.; Locke, B.; de Miranda, J.; Mondet, F.; Neumann, P. Rapid parallel evolution overcomes global honey bee parasite. Sci. Rep. 2018, 8, 7704. [Google Scholar] [CrossRef]
- Martin, S.J.; Hawkins, G.P.; Brettell, L.E.; Reece1, N.; Correia-Oliveira, M.E.; Allsopp, M.H. Varroa destructor reproduction and cell re-capping in mite-resistant Apis mellifera populations. Apidologie 2020, 51, 369–381. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Fries, I.; Boecking, O.; Stürmer, M. Damaged Varroa mites in the debris of honey bee (Apis mellifera L.) colonies with and without hatching brood. Apidologie 1997, 28, 427–437. [Google Scholar] [CrossRef]
- Lobb, N.; Martin, S. Mortality of Varroa jacobsoni Oudemans during or soon after the emergence of worker and drone honeybees Apis mellifera L. Apidologie 1997, 28, 367–374. [Google Scholar] [CrossRef]
- Tsuruda, J.M.; Harris, J.W.; Bourgeois, L.; Danka, R.G.; Hunt, G.J. High-resolution linkage analyses to identify genes that influence Varroa sensitive hygiene behavior in honey bees. PLoS ONE 2012, 7, e48276. [Google Scholar] [CrossRef] [PubMed]
- Spötter, A.; Gupta, P.; Mayer, M.; Reinsch, N.; Bienefeld, K. Genome-wide association study of a Varroa-specific defense behavior in honeybees (Apis mellifera). J. Hered. 2016, 107, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Holland, K.; Murray, M. Non-reproduction in the honeybee mite Varroa jacobsoni. Exp. Appl. Acarol. 1997, 21, 539–549. [Google Scholar] [CrossRef]
- Martin, S.J.; Medina, L.M. Africanized honey bees have unique tolerance to Varroa mites. Trends Parasitol. 2004, 20, 112–114. [Google Scholar] [CrossRef]
- Harris, J.W. Effect of brood type on Varroa-sensitive hygiene by worker honey bees (Hymenoptera: Apidae). Ann. Entomol. Soc. Am. 2008, 101, 1137–1144. [Google Scholar] [CrossRef]
- Fuchs, S. Preference for drone brood cells by Varroa jacobsoni Oud. in colonies of Apis mellifera carnica. Apidologie 1990, 21, 193–196. [Google Scholar] [CrossRef]
- Boot, W.J.; Schoenmaker, J.; Calis, J.N.M.; Beetsma, J. Invasion of Varroa jacobsoni into drone brood cells of the honey bee, Apis mellifera. Apidologie 1995, 26, 109–118. [Google Scholar] [CrossRef]
- Calderone, N.W.; Kuenen, L.P.S. Effects of western honey bee (Hymenoptera: Apidae) colony, cell, type, and larval sex on host acquisition by female Varroa destructor (Acari: Varroidae). J. Econ. Entomol. 2001, 94, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Amdam, G.V.; Hartfelder, K.; Norberg, K.; Hagen, A.; Omholt, S.W. Altered physiology in worker honey bees (Hymenoptera: Apidae) infested with the mite Varroa destructor (Acari: Varroidae): A factor in colony loss during overwintering? J. Econ. Entomol. 2004, 97, 741–747. [Google Scholar] [CrossRef]
- Aldea, P.; Bozinovic, F. The energetic and survival costs of Varroa parasitism in honeybees. Apidologie 2020, in press. [Google Scholar] [CrossRef]
- Duay, P.; de Jong, D.; Engels, W. Decreased flight performance and sperm production in drones of the honey bee (Apis mellifera) slightly infested by Varroa destructor mites during pupal development. Genet. Mol. Res. 2002, 1, 227–232. [Google Scholar] [PubMed]
- Duay, P.; de Jong, D.; Engels, W. Weight loss in drone pupae (Apis mellifera) multiply infested by Varroa destructor mites. Apidologie 2003, 34, 61–65. [Google Scholar] [CrossRef]
- Fries, I.; Hansen, H.; Imdorf, A.; Rosenkranz, P. Swarming in honey bees (Apis mellifera) and Varroa destructor population development in Sweden. Apidologie 2003, 34, 389–398. [Google Scholar] [CrossRef]
- Villa, J.D.; Bustamante, D.M.; Dunkley, J.P.; Escobar, L.A. Changes in honey bee (Hymenoptera: Apidae) colony swarming and survival pre- and postarrival of Varroa destructor (Mesostigmata: Varroidae) in Louisiana. Ann. Entomol. Soc. Am. 2008, 101, 867–871. [Google Scholar] [CrossRef]
- Emsen, B.; Hamiduzzaman, M.M.; Goodwin, P.H.; Guzmán-Novoa, E. Lower virus infections in Varroa destructor-infested and uninfested brood and adult honey bees (Apis mellifera) of a low mite population growth colony compared to a high mite population growth colony. PLoS ONE 2015, 10, e0118885. [Google Scholar] [CrossRef]
- Locke, B.; Forsgren, E.; de Miranda, J.R. Increased tolerance and resistance to virus infections: A possible factor in the survival of Varroa destructor-resistant honey bees (Apis mellifera). PLoS ONE 2014, 9, e99998. [Google Scholar] [CrossRef]
- Martin, S.J.; Highfield, A.C.; Brettell, L.; Villalobos, E.M.; Budge, G.E.; Powell, M.; Nikaido, S.; Schroeder, D.C. Global honey bee viral landscape altered by a parasitic mite. Science 2012, 336, 1304–1306. [Google Scholar] [CrossRef]
- Riveros, G.; Arismendi, N.; Zapata, N.; Evans, D.; Pérez, I.; Aldea, P.; Vargas, M. Occurrence, prevalence and viral load of deformed wing virus variants in Apis mellifera colonies in Chile. J. Apicult. Res. 2020, 59, 63–68. [Google Scholar] [CrossRef]
- de Souza, F.S.; Kevill, J.L.; Correia-Oliveira, M.E.; de Carvalho, C.A.L.; Martin, S.J. Occurrence of deformed wing virus variants in the stingless bee Melipona subnitida and honey bee Apis mellifera populations in Brazil. J. Gen. Virol. 2019, 100, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Brasesco, C.; Quintana, S.; Di Gerónimo, V.; Genchi García, M.L.; Sguazza, G.; Bravi, M.E.; Fargnoli, L.; Reynaldi, F.J.; Eguaras, M.; Maggi, M. Deformed wing virus type a and b in managed honeybee colonies of Argentina. Bull. Entomol. Res. 2020, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.P.; Natsopoulou, M.E.; Doublet, V.; Fürst, M.; Weging, S.; Brown, M.J.; Gogol-Döring, A.; Paxton, R.J. Elevated virulence of an emerging viral genotype as a driver of honeybee loss. Proc. Biol. Sci. 2016, 283, 20160811. [Google Scholar] [CrossRef]
- Kevill, J.L.; de Souza, F.S.; Sharples, C.; Oliver, R.; Schroeder, D.C.; Martin, S.J. DWV-A lethal to honey bees (Apis mellifera): A colony level survey of DWV variants (A, B, and C) in England, Wales, and 32 states across the US. Viruses 2019, 11, 426. [Google Scholar] [CrossRef]
- Sheppard, W.S.; Rinderer, T.E.; Garnery, L.; Shimanuki, H. Analysis of Africanized honey bee mitochondrial DNA reveals further diversity of origin. Gen. Mol. Biol. 1999, 22, 73–75. [Google Scholar] [CrossRef]
- Abrahamovich, A.H.; Atela, O.; De la Rúa, P.; Galián, J. Assessment of the mitochondrial origin of honey bees from Argentina. J. Apicul. Res. 2007, 46, 191–194. [Google Scholar] [CrossRef]
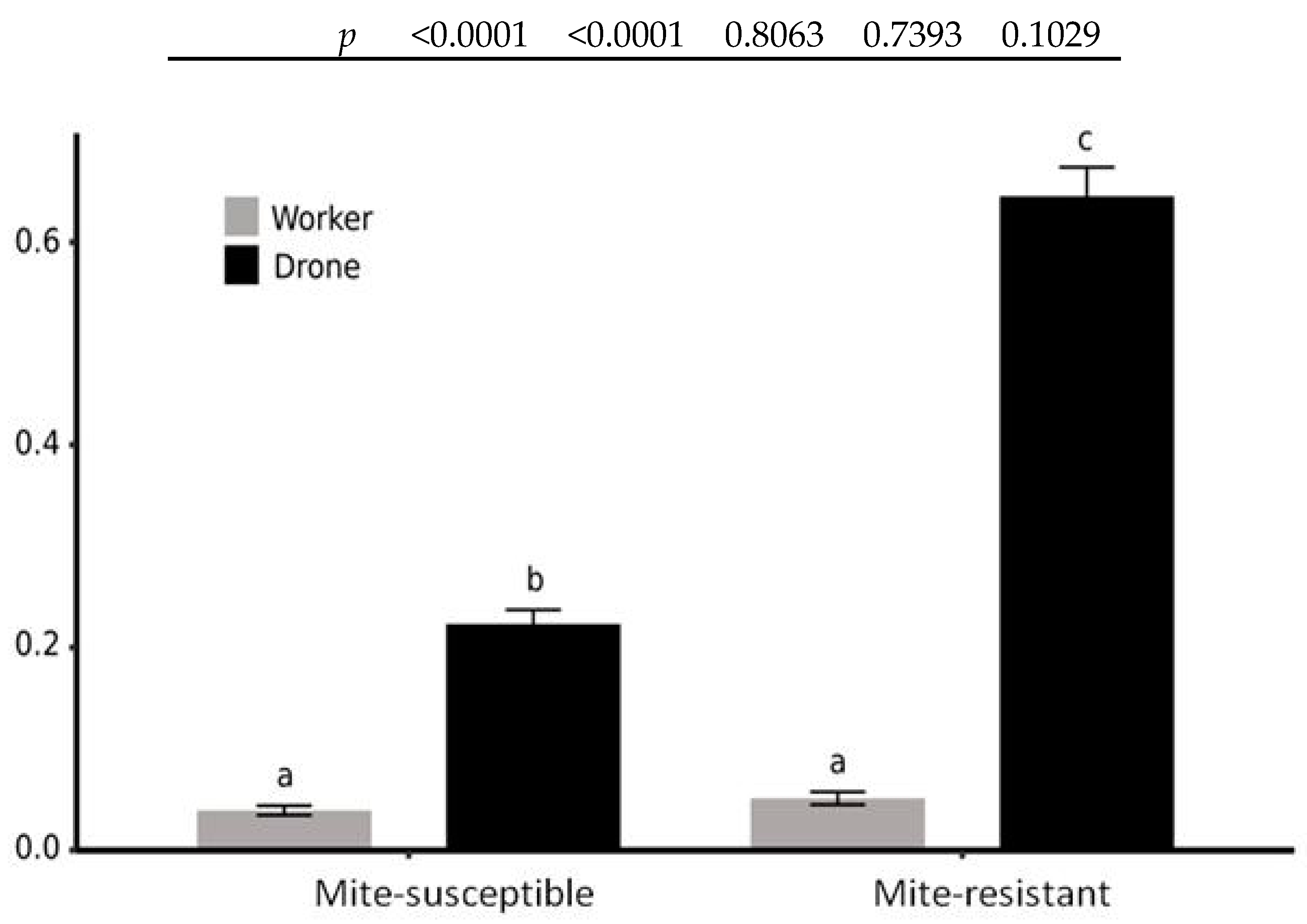


| Colonies | Mite-Susceptible | Mite-Resistant | |
|---|---|---|---|
| Drones | Inspected cells | 887 | 282 |
| Mite-infested cells | 176 | 101 | |
| Total mites | 198 | 182 | |
| Prevalence | 19.8% | 35.8% | |
| Abundance | 22.3% | 64.5% | |
| Workers | Inspected cells | 1710 | 1210 |
| Mite-infested cells | 67 | 60 | |
| Total mites | 67 | 62 | |
| Prevalence | 3.9% | 5.0% | |
| Abundance | 3.9% | 5.1% | |
| Ratio of mite distribution between drone and worker cells | 5.70 | 12.60 | |
| Locus | Mite-R | Mite-S | EU | AF | BR | |
|---|---|---|---|---|---|---|
| A43 | N | 32 | 22 | 50 | 41 | 32 |
| Na | 9 | 7 | 6 | 16 | 13 | |
| Ho | 0.063 | 0.682 | 0.640 | 0.854 | 0.875 | |
| He | 0.769 | 0.766 | 0.615 | 0.881 | 0.851 | |
| p | <0.0001 | <0.0001 | 0.2545 | 0.4865 | 0.9758 | |
| A88 | N | 32 | 23 | 50 | 41 | 32 |
| Na | 7 | 3 | 6 | 14 | 12 | |
| Ho | 0.125 | 0.043 | 0.700 | 0.878 | 0.750 | |
| He | 0.771 | 0.463 | 0.629 | 0.878 | 0.853 | |
| p | <0.0001 | <0.0001 | 0.6378 | 0.8287 | 0.1182 | |
| A28 | N | 31 | 39 | 50 | 41 | 32 |
| Na | 10 | 2 | 2 | 9 | 10 | |
| Ho | 0.065 | 0.000 | 0.380 | 0.805 | 0.813 | |
| He | 0.803 | 0.099 | 0.413 | 0.833 | 0.806 | |
| p | <0.0001 | <0.0001 | 0.2363 | 0.4356 | 0.4059 | |
| A8 | N | 38 | 37 | 50 | 41 | 32 |
| Na | 8 | 4 | 6 | 9 | 9 | |
| Ho | 0.211 | 0.081 | 0.800 | 0.829 | 0.688 | |
| He | 0.780 | 0.681 | 0.801 | 0.838 | 0.802 | |
| p | <0.0001 | <0.0001 | 0.0825 | 0.6681 | 0.0440 | |
| A113 | N | 34 | 39 | 50 | 41 | 32 |
| Na | 9 | 8 | 11 | 12 | 11 | |
| Ho | 0.382 | 0.769 | 0.640 | 0.854 | 0.875 | |
| He | 0.788 | 0.799 | 0.650 | 0.858 | 0.858 | |
| p | <0.0001 | <0.0001 | 0.8063 | 0.7393 | 0.1029 |
| Locus | Mite-R | Mite-S | |
|---|---|---|---|
| VD112 | N | 29 | 33 |
| Na | 2 | 3 | |
| He | 0.068 | 0.222 | |
| Ho | 0.000 | 0.242 | |
| p | 0.0175 | 1.000 | |
| VD001 | N | 29 | 26 |
| Na | 3 | 2 | |
| He | 0.101 | 0.075 | |
| Ho | 0.034 | 0.000 | |
| p | 0.0175 | 0.0196 | |
| VD114 | N | 28 | 33 |
| Na | 1 | 2 | |
| He | 0.000 | 0.060 | |
| Ho | 0.000 | 0.000 | |
| p | NA | 0.0154 | |
| VD016 | N | 29 | 30 |
| Na | 1 | 1 | |
| He | 0.000 | 0.000 | |
| Ho | 0.000 | 0.000 | |
| p | NA | NA | |
| VJ295 | N | 28 | 28 |
| Na | 4 | 3 | |
| He | 0.546 | 0.450 | |
| Ho | 0.500 | 0.500 | |
| p | 0.4031 | 0.0618 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza, Y.; Tomasco, I.H.; Antúnez, K.; Castelli, L.; Branchiccela, B.; Santos, E.; Invernizzi, C. Unraveling Honey Bee–Varroa destructor Interaction: Multiple Factors Involved in Differential Resistance between Two Uruguayan Populations. Vet. Sci. 2020, 7, 116. https://doi.org/10.3390/vetsci7030116
Mendoza Y, Tomasco IH, Antúnez K, Castelli L, Branchiccela B, Santos E, Invernizzi C. Unraveling Honey Bee–Varroa destructor Interaction: Multiple Factors Involved in Differential Resistance between Two Uruguayan Populations. Veterinary Sciences. 2020; 7(3):116. https://doi.org/10.3390/vetsci7030116
Chicago/Turabian StyleMendoza, Yamandú, Ivanna H. Tomasco, Karina Antúnez, Loreley Castelli, Belén Branchiccela, Estela Santos, and Ciro Invernizzi. 2020. "Unraveling Honey Bee–Varroa destructor Interaction: Multiple Factors Involved in Differential Resistance between Two Uruguayan Populations" Veterinary Sciences 7, no. 3: 116. https://doi.org/10.3390/vetsci7030116
APA StyleMendoza, Y., Tomasco, I. H., Antúnez, K., Castelli, L., Branchiccela, B., Santos, E., & Invernizzi, C. (2020). Unraveling Honey Bee–Varroa destructor Interaction: Multiple Factors Involved in Differential Resistance between Two Uruguayan Populations. Veterinary Sciences, 7(3), 116. https://doi.org/10.3390/vetsci7030116





