Training of Primary Chicken Monocytes Results in Enhanced Pro-Inflammatory Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethical Statement
2.2. Preparation of Microparticulate β-Glucan Suspension
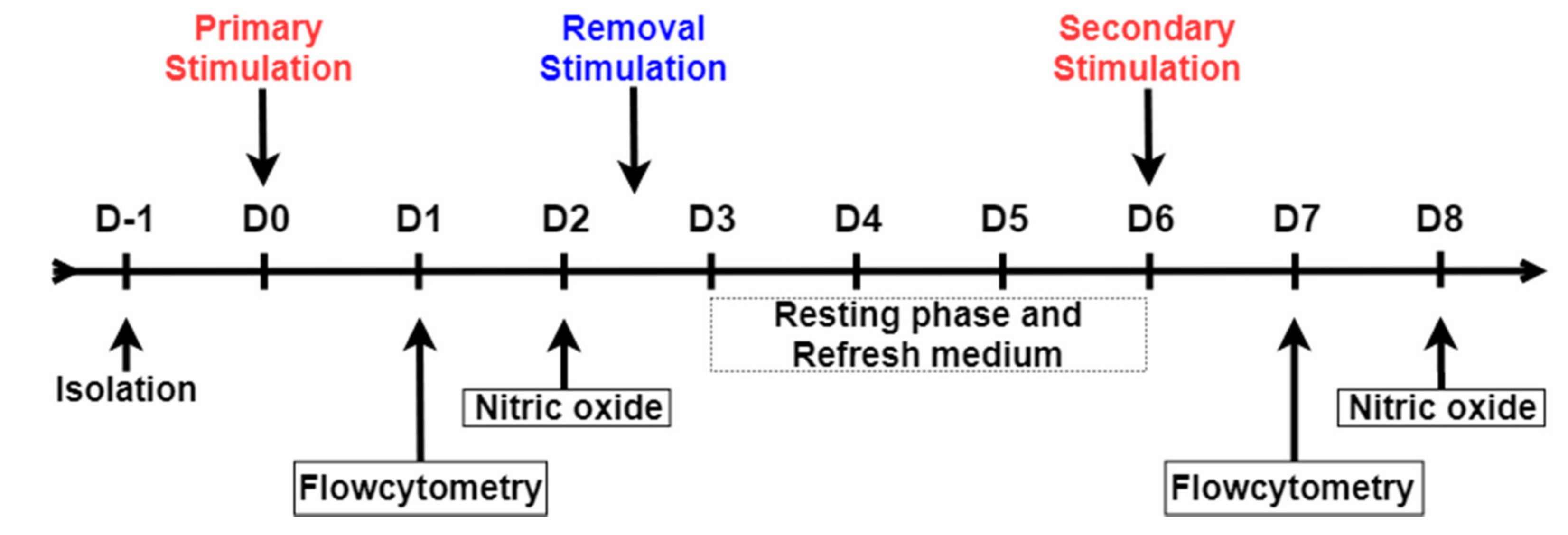
2.3. Stimulation of Primary Monocytes
2.4. Nitric Oxide (NO) Production Assay
2.5. Flow Cytometry
2.6. Statistical Analysis
3. Results
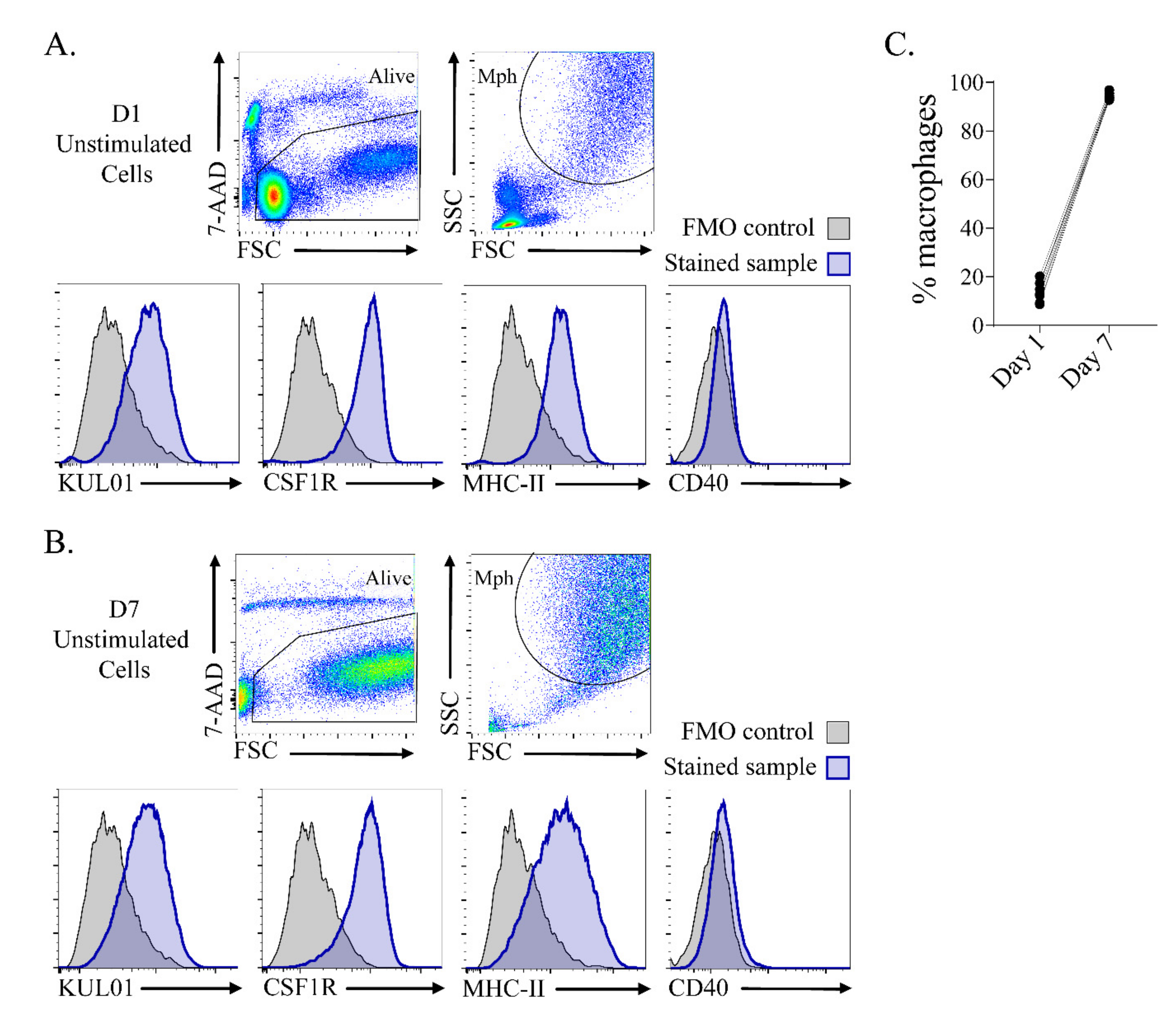
3.1. In Vitro Culture Resulted in a Highly Homogeneous Macrophage Population after 7 Days of Culture
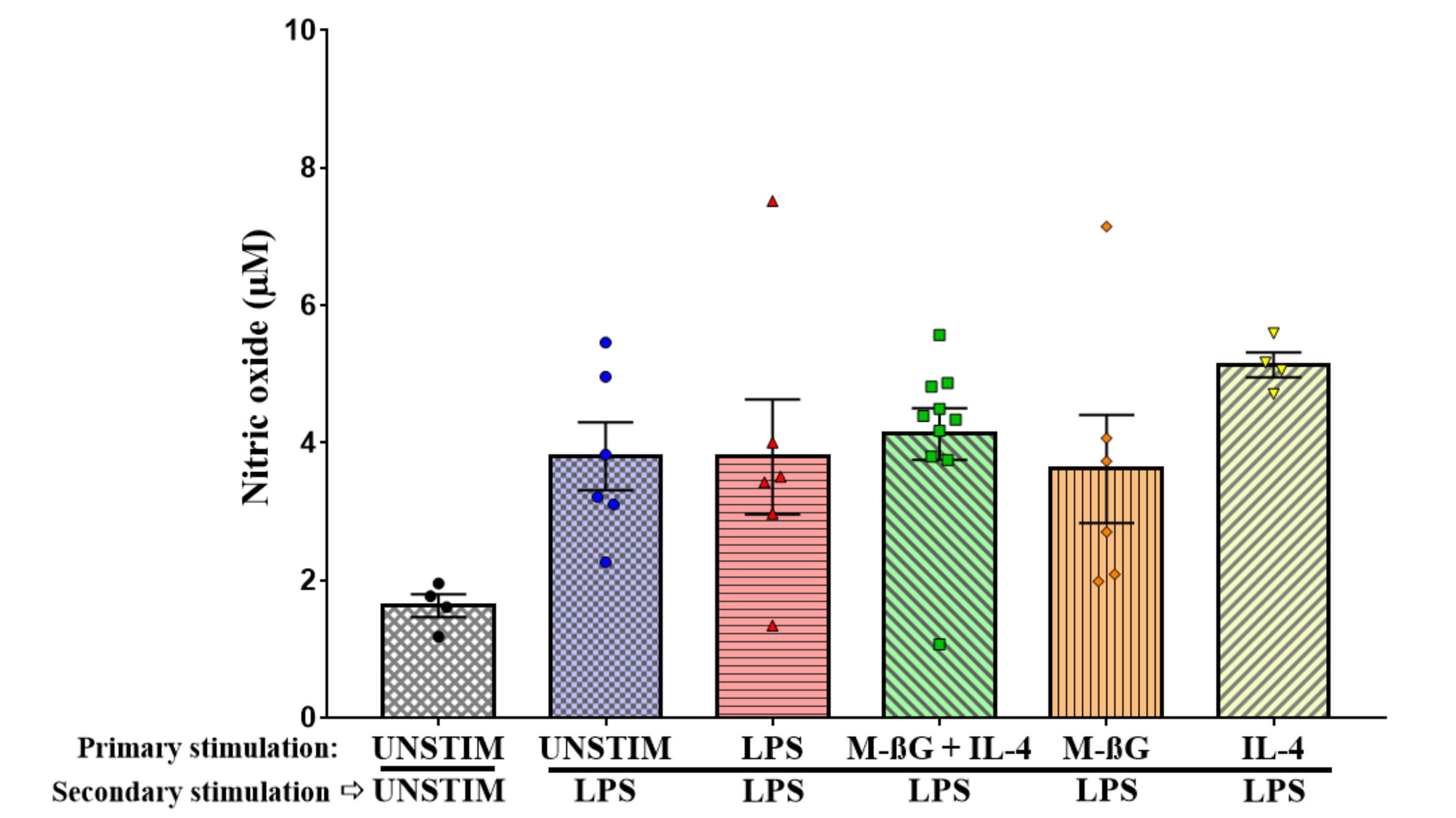
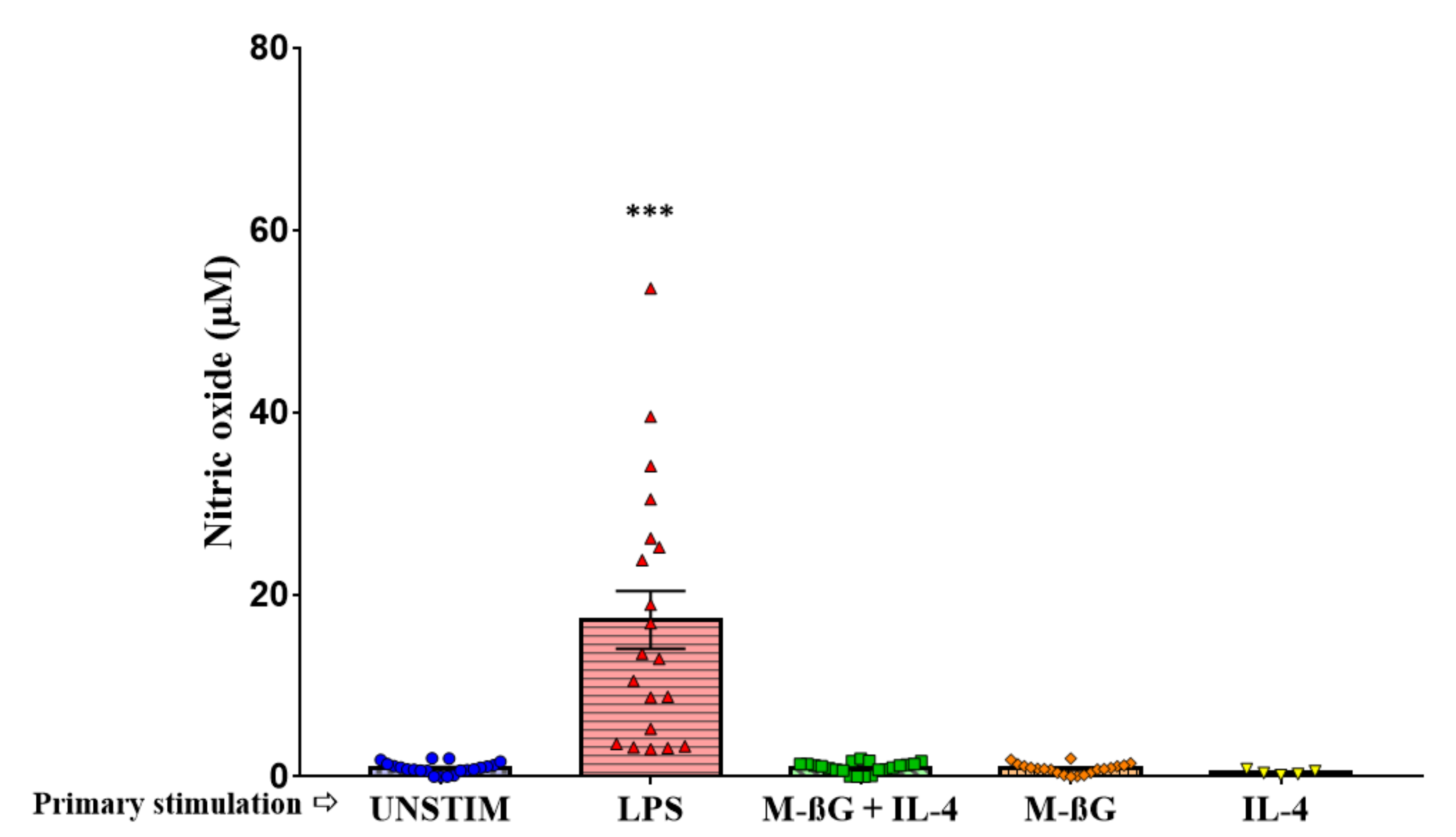
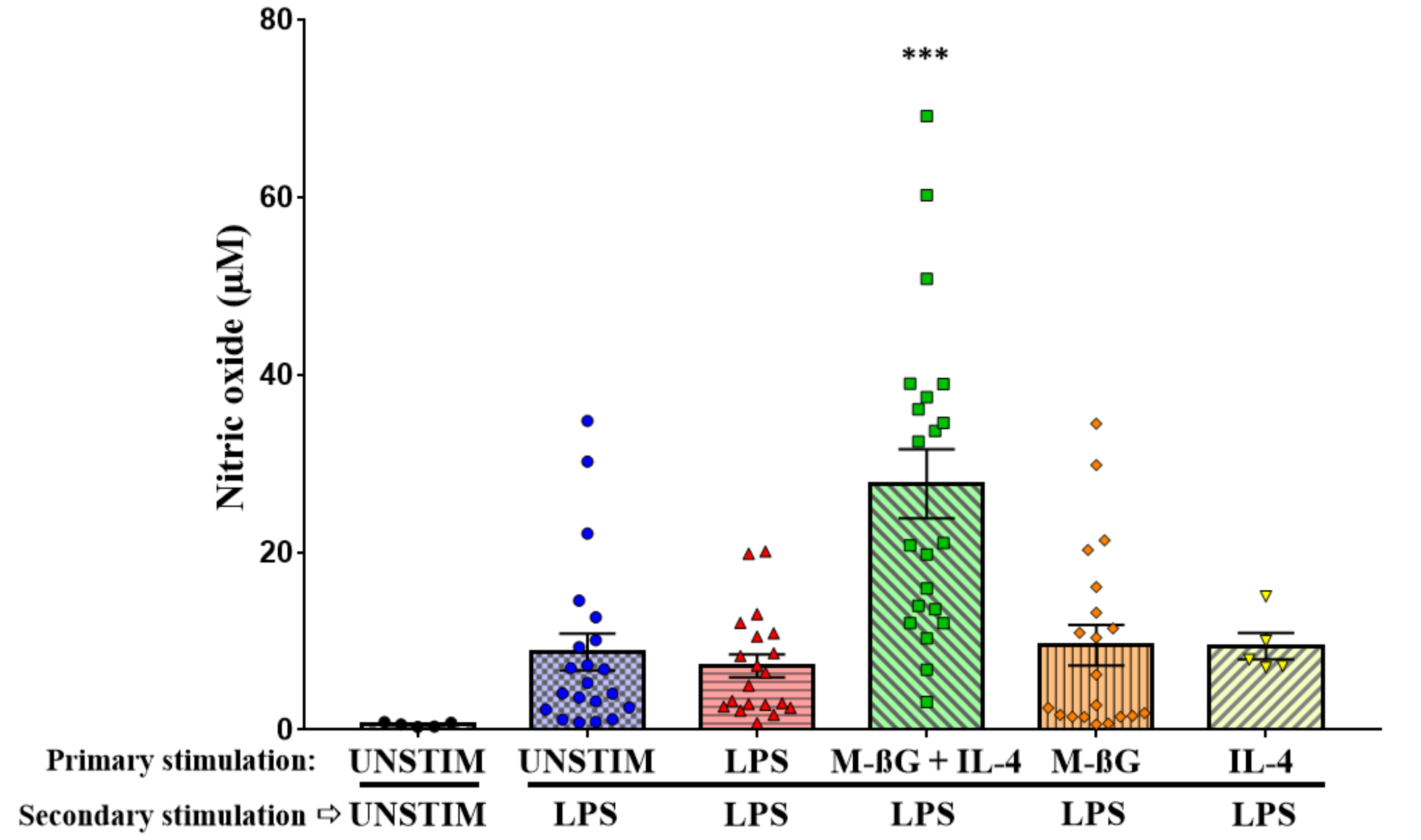
3.2. Primary Stimulation with β-Glucan Microparticulates and IL-4 Enhanced NO Production after Secondary Stimulation with LPS
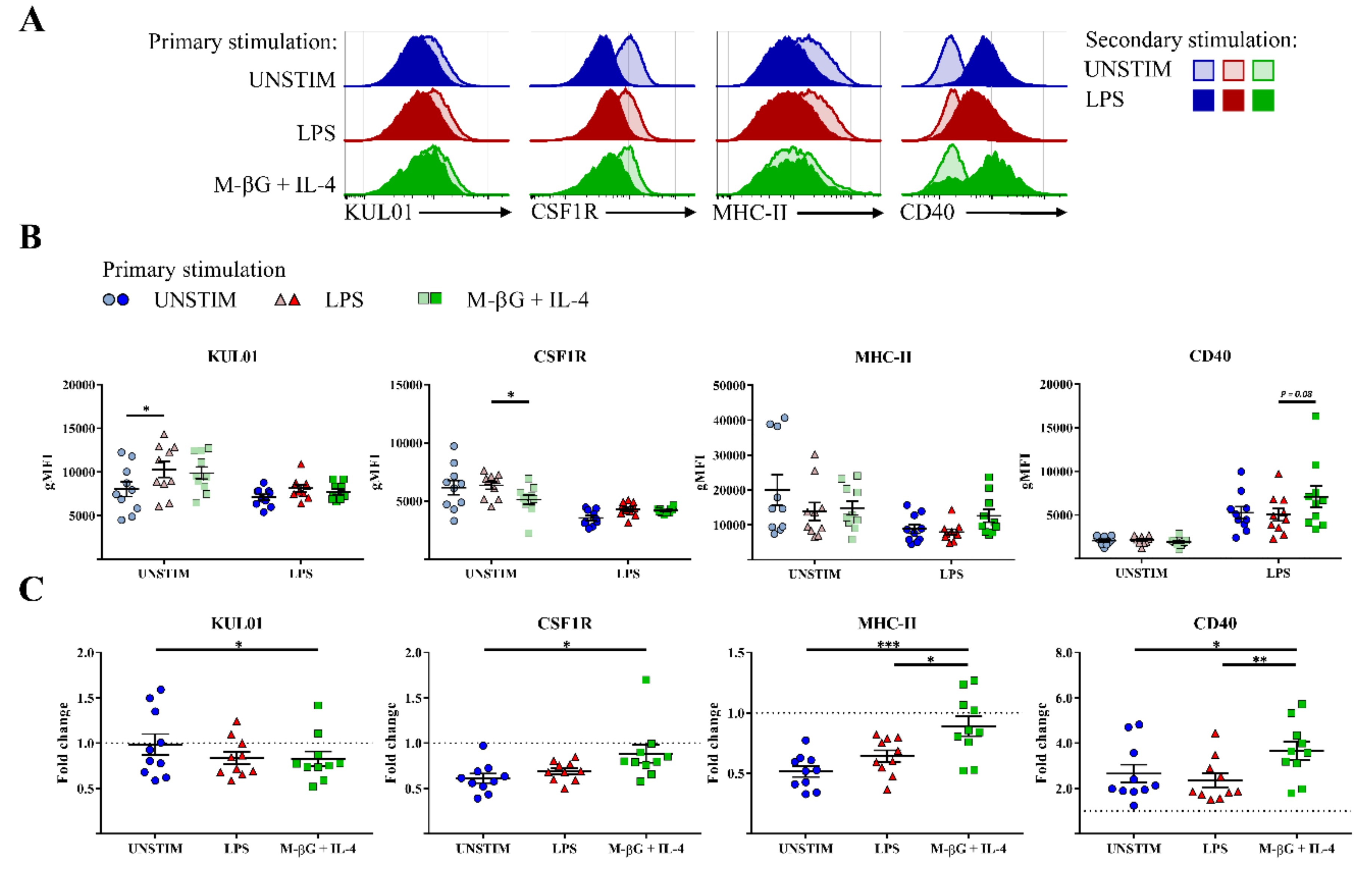
3.3. Primary Stimulation with β-Glucan Microparticulates and IL-4 Influenced CD40, MHC-II and CSF1R Surface Expression after Secondary Stimulation with LPS
3.4. No Evidence of Training in Chicken Bone Marrow-Derived Adherent Cells.
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Microparticulate β-Glucan Suspension
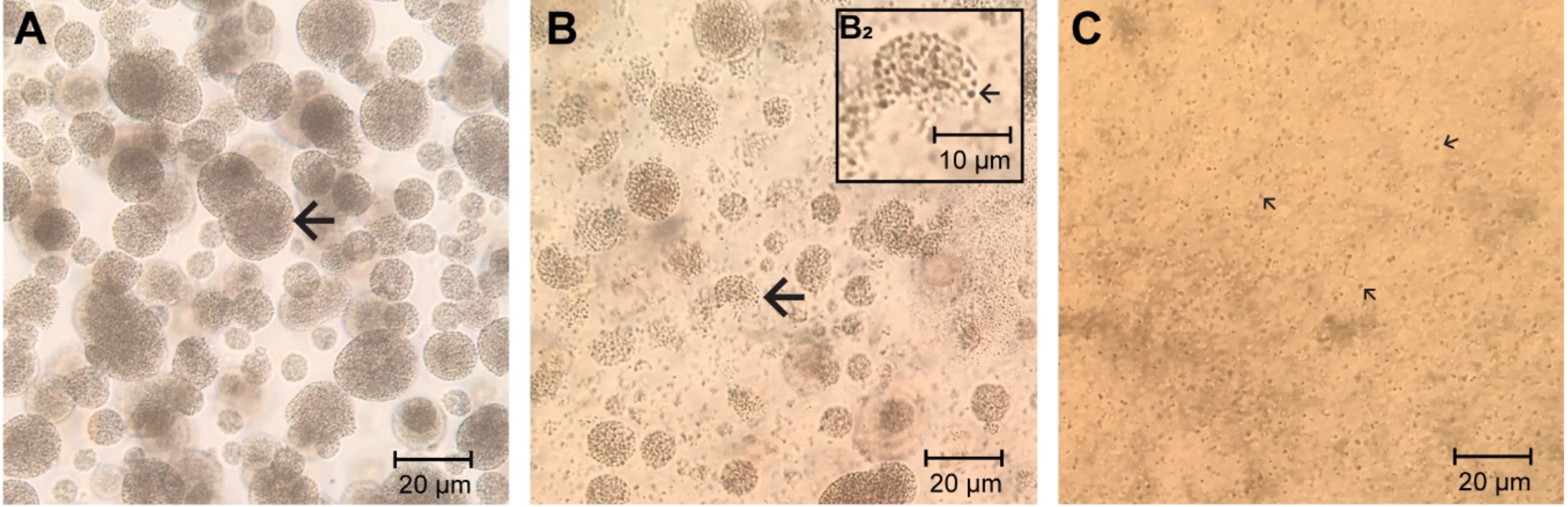
Appendix B. NO Production of Bone Marrow-Derived Adherent Cells
Materials and Methods
Results

References
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.J.; Pozo, M.J.; Ton, J.; van Dam, N.M.; Conrath, U. Recognizing Plant Defense Priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Lanz-Mendoza, H.; Garduño, J.C. Insect Innate Immune Memory. In Advances in Comparative Immunology; Cooper, E.L., Ed.; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Quintin, J.; Saeed, S.; Martens, J.H.A.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.J.; Wijmenga, C.; et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 2012, 12, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G. Training innate immunity: The changing concept of immunological memory in innate host defence. Eur. J. Clin. Investig. 2013, 43, 881–884. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, J.W.M.; Joosten, L.A.B.; Riksen, N.; Netea, M.G. Trained immunity: A smart way to enhance innate immune defence. Mol. Immunol. 2015, 68, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Jacobs, C.; Xavier, R.J.; van der Meer, J.W.; van Crevel, R.; Netea, M.G. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin. Immunol. (Orlando Fla.) 2014, 155, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Benn, C.S.; Joosten, L.A.; Jacobs, C.; van Loenhout, J.; Xavier, R.J.; Aaby, P.; van der Meer, J.W.; et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 2014, 6, 152–158. [Google Scholar] [CrossRef]
- Kjaer, J.B.; Sorensen, P.; Su, G. Divergent selection on feather pecking behaviour in laying hens (Gallus gallus domesticus). Appl. Anim. Behav. Sci. 2001, 71, 229–239. [Google Scholar] [CrossRef]
- Van der Eijk, J.A.J.; Verwoolde, M.B.; de Vries Reilingh, G.; Jansen, C.A.; Rodenburg, T.B.; Lammers, A. Chicken lines divergently selected on feather pecking differ in immune characteristics. Physiol. Behav. 2019, 212, 112680. [Google Scholar] [CrossRef]
- Hunter, K.W., Jr.; Gault, R.A.; Berner, M.D. Preparation of microparticulate beta-glucan from Saccharomyces cerevisiae for use in immune potentiation. Lett. Appl. Microbiol. 2002, 35, 267–271. [Google Scholar] [CrossRef]
- Williams, D.L.; McNamee, R.B.; Jones, E.L.; Pretus, H.A.; Ensley, H.E.; Browder, I.W.; Di Luzio, N.R. A method for the solubilization of a (1–3)-beta-d-glucan isolated from Saccharomyces cerevisiae. Carbohydr. Res. 1991, 219, 203–213. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Parmentier, H.K.; Vries Reilingh, G.d.; Freke, P.; Koopmanschap, R.E.; Lammers, A. Immunological and Physiological Differences Between Layer- and Broiler Chickens after Concurrent Intratracheal Administration of Lipopolysaccharide and Human Serum Albumin. Int. J. Poult. Sci. 2010, 9, 574–583. [Google Scholar] [CrossRef]
- Mast, J.; Goddeeris, B.M.; Peeters, K.; Vandesande, F.; Berghman, L.R. Characterisation of chicken monocytes, macrophages and interdigitating cells by the monoclonal antibody KUL01. Vet. Immunol. Immunopathol. 1998, 61, 343–357. [Google Scholar] [CrossRef]
- Balic, A.; Garcia-Morales, C.; Vervelde, L.; Gilhooley, H.; Sherman, A.; Garceau, V.; Gutowska, M.W.; Burt, D.W.; Kaiser, P.; Hume, D.A.; et al. Visualisation of chicken macrophages using transgenic reporter genes: Insights into the development of the avian macrophage lineage. Development 2014, 141, 3255–3265. [Google Scholar] [CrossRef] [PubMed]
- Willment, J.A.; Lin, H.H.; Reid, D.M.; Taylor, P.R.; Williams, D.L.; Wong, S.Y.; Gordon, S.; Brown, G.D. Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J. Immunol. (Baltim. Md. 1950) 2003, 171, 4569–4573. [Google Scholar] [CrossRef]
- Jana, M.; Liu, X.; Koka, S.; Ghosh, S.; Petro, T.M.; Pahan, K. Ligation of CD40 stimulates the induction of nitric-oxide synthase in microglial cells. J. Biol. Chem. 2001, 276, 44527–44533. [Google Scholar] [CrossRef]
- Miller, M.M.; Taylor, R.L., Jr. Brief review of the chicken Major Histocompatibility Complex: The genes, their distribution on chromosome 16, and their contributions to disease resistance. Poult. Sci. 2016, 95, 375–392. [Google Scholar] [CrossRef]
- Kalaiyarasu, S.; Bhatia, S.; Mishra, N.; Sood, R.; Kumar, M.; SenthilKumar, D.; Bhat, S.; Dass Prakash, M. Elevated level of pro inflammatory cytokine and chemokine expression in chicken bone marrow and monocyte derived dendritic cells following LPS induced maturation. Cytokine 2016, 85, 140–147. [Google Scholar] [CrossRef]
- de Geus, E.D.; Jansen, C.A.; Vervelde, L. Uptake of Particulate Antigens in a Nonmammalian Lung: Phenotypic and Functional Characterization of Avian Respiratory Phagocytes Using Bacterial or Viral Antigens. J. Immunol. 2012, 188, 4516–4526. [Google Scholar] [CrossRef]
- Lin, H.K.; Bloom, S.E.; Dietert, R.R. Macrophage anti-tumor functions in a chicken MHC chromosome dosage model. Anim. Biotechnol. 1993, 4, 121–141. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.B.; Latz, E.; Mills, K.H.G.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.J.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [PubMed]
- Santecchia, I.; Vernel-Pauillac, F.; Rasid, O.; Quintin, J.; Gomes-Solecki, M.; Boneca, I.G.; Werts, C. Innate immune memory through TLR2 and NOD2 contributes to the control of Leptospira interrogans infection. PLOS Pathog. 2019, 15, e1007811. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, B.; Habibi, E.; Wang, S.Y.; Arts, R.J.W.; Davar, R.; Megchelenbrink, W.; Kim, B.; Kuznetsova, T.; Kox, M.; Zwaag, J.; et al. Beta-Glucan Reverses the Epigenetic State of LPS-Induced Immunological Tolerance. Cell 2016, 167, 1354–1368.e14. [Google Scholar] [CrossRef] [PubMed]
- Wiegertjes, G. Macrophage polarization in the immune response to parasites. Bull. Eur. Assoc. Fish Pathol. 2004, 24, 5–10. [Google Scholar]
- Tümer, C.; Bilgin, H.M.; Obay, B.D.; Diken, H.; Atmaca, M.; Kelle, M. Effect of nitric oxide on phagocytic activity of lipopolysaccharide-induced macrophages: Possible role of exogenous l-arginine. Cell Biol. Int. 2007, 31, 565–569. [Google Scholar] [CrossRef]
- Juste, R.A.; Alonso-Hearn, M.; Garrido, J.M.; Abendaño, N.; Sevilla, I.A.; Gortazar, C.; de la Fuente, J.; Dominguez, L. Increased Lytic Efficiency of Bovine Macrophages Trained with Killed Mycobacteria. PLoS ONE 2016, 11, e0165607. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Kalupahana, R.S.; Mastroeni, P.; Maskell, D.; Blacklaws, B.A. Activation of murine dendritic cells and macrophages induced by Salmonella enterica serovar Typhimurium. Immunology 2005, 115, 462–472. [Google Scholar] [CrossRef]
- Morris, D.L.; Cho, K.W.; Delproposto, J.L.; Oatmen, K.E.; Geletka, L.M.; Martinez-Santibanez, G.; Singer, K.; Lumeng, C.N. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes 2013, 62, 2762–2772. [Google Scholar] [CrossRef]
- Brown, G.D.; Herre, J.; Williams, D.L.; Willment, J.A.; Marshall, A.S.J.; Gordon, S. Dectin-1 Mediates the Biological Effects of β-Glucans. J. Exp. Med. 2003, 197, 1119–1124. [Google Scholar] [CrossRef]
- Nerren, J.R.; Kogut, M.H. The selective Dectin-1 agonist, curdlan, induces an oxidative burst response in chicken heterophils and peripheral blood mononuclear cells. Vet. Immunol. Immunopathol. 2009, 127, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Gantner, B.N.; Simmons, R.M.; Canavera, S.J.; Akira, S.; Underhill, D.M. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 2003, 197, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Ozinsky, A.; Underhill, D.M.; Fontenot, J.D.; Hajjar, A.M.; Smith, K.D.; Wilson, C.B.; Schroeder, L.; Aderem, A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl. Acad. Sci. USA 2000, 97, 13766–13771. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Rathinam, V.A.K.; Bossaller, L.; Army, K.; Kaiser, W.J.; Mocarski, E.S.; Dillon, C.P.; Green, D.R.; Mayadas, T.N.; Levitz, S.M.; et al. Caspase-8 Modulates Dectin-1 and Complement Receptor 3–Driven IL-1β Production in Response to β-Glucans and the Fungal Pathogen, Candida albicans. J. Immunol. 2014, 193, 2519–2530. [Google Scholar] [CrossRef] [PubMed]
- Batbayar, S.; Lee, D.H.; Kim, H.W. Immunomodulation of Fungal β-Glucan in Host Defense Signaling by Dectin-1. Biomol. Ther. (Seoul) 2012, 20, 433–445. [Google Scholar] [CrossRef]
- Jablonski, K.A.; Amici, S.A.; Webb, L.M.; Ruiz-Rosado, J.d.D.; Popovich, P.G.; Partida-Sanchez, S.; Guerau-de-Arellano, M. Novel Markers to Delineate Murine M1 and M2 Macrophages. PLoS ONE 2015, 10, e0145342. [Google Scholar] [CrossRef]
- Wideman, R.F.; Bowen, O.T.; Erf, G.F. Broiler pulmonary hypertensive responses during lipopolysaccharide-induced tolerance and cyclooxygenase inhibition. Poult. Sci. 2009, 88, 72–85. [Google Scholar] [CrossRef]
- Marais, M.; Maloney, S.K.; Gray, D.A. The development of endotoxin tolerance, and the role of hypothalamo-pituitary-adrenal function and glucocorticoids in Pekin ducks. J. Exp. Biol. 2011, 214, 3378. [Google Scholar] [CrossRef]
- Wang, W.; Wideman, R.F., Jr.; Chapman, M.E.; Bersi, T.K.; Erf, G.F. Effect of intravenous endotoxin on blood cell profiles of broilers housed in cages and floor litter environments. Poult. Sci. 2003, 82, 1886–1897. [Google Scholar] [CrossRef]
- Seeley, J.J.; Ghosh, S. Molecular mechanisms of innate memory and tolerance to LPS. J. Leukoc. Biol. 2017, 101, 107–119. [Google Scholar] [CrossRef]
- Wu, Z.; Rothwell, L.; Young, J.R.; Kaufman, J.; Butter, C.; Kaiser, P. Generation and characterization of chicken bone marrow-derived dendritic cells. Immunology 2010, 129, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Sutton, K.M.C.; Hu, T.; Wu, Z.; Siklodi, B.; Vervelde, L.; Kaiser, P. The functions of the avian receptor activator of NF-κB ligand (RANKL) and its receptors, RANK and osteoprotegerin, are evolutionarily conserved. Dev. Comp. Immunol. 2015, 51, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Quintin, J.; Kerstens, H.H.D.; Rao, N.A.; Aghajanirefah, A.; Matarese, F.; Cheng, S.-C.; Ratter, J.; Berentsen, K.; van der Ent, M.A.; et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014, 345. [Google Scholar] [CrossRef] [PubMed]
- Bar-Shira, E.; Sklan, D.; Friedman, A. Establishment of immune competence in the avian GALT during the immediate post-hatch period. Dev. Comp. Immunol. 2003, 27, 147–157. [Google Scholar] [CrossRef]
- Lammers, A.; Wieland, W.H.; Kruijt, L.; Jansma, A.; Straetemans, T.; Schots, A.; den Hartog, G.; Parmentier, H.K. Successive immunoglobulin and cytokine expression in the small intestine of juvenile chicken. Dev. Comp. Immunol. 2010, 34, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Den Hartog, G.; De Vries-Reilingh, G.; Wehrmaker, A.M.; Savelkoul, H.F.; Parmentier, H.K.; Lammers, A. Intestinal immune maturation is accompanied by temporal changes in the composition of the microbiota. Benef. Microbes 2016, 7, 677–685. [Google Scholar] [CrossRef]
- Domínguez-Andrés, J.; Joosten, L.A.B.; Netea, M.G. Induction of innate immune memory: The role of cellular metabolism. Curr. Opin. Immunol. 2019, 56, 10–16. [Google Scholar] [CrossRef]
- Cheng, S.-C.; Joosten, L.A.B.; Netea, M.G. The interplay between central metabolism and innate immune responses. Cytokine Growth Factor Rev. 2014, 25, 707–713. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verwoolde, M.B.; van den Biggelaar, R.H.G.A.; van Baal, J.; Jansen, C.A.; Lammers, A. Training of Primary Chicken Monocytes Results in Enhanced Pro-Inflammatory Responses. Vet. Sci. 2020, 7, 115. https://doi.org/10.3390/vetsci7030115
Verwoolde MB, van den Biggelaar RHGA, van Baal J, Jansen CA, Lammers A. Training of Primary Chicken Monocytes Results in Enhanced Pro-Inflammatory Responses. Veterinary Sciences. 2020; 7(3):115. https://doi.org/10.3390/vetsci7030115
Chicago/Turabian StyleVerwoolde, Michel B., Robin H. G. A. van den Biggelaar, Jürgen van Baal, Christine A. Jansen, and Aart Lammers. 2020. "Training of Primary Chicken Monocytes Results in Enhanced Pro-Inflammatory Responses" Veterinary Sciences 7, no. 3: 115. https://doi.org/10.3390/vetsci7030115
APA StyleVerwoolde, M. B., van den Biggelaar, R. H. G. A., van Baal, J., Jansen, C. A., & Lammers, A. (2020). Training of Primary Chicken Monocytes Results in Enhanced Pro-Inflammatory Responses. Veterinary Sciences, 7(3), 115. https://doi.org/10.3390/vetsci7030115






_Lammers.jpeg)
