Lactobacillus johnsonii L531 Ameliorates Escherichia coli-Induced Cell Damage via Inhibiting NLRP3 Inflammasome Activity and Promoting ATG5/ATG16L1-Mediated Autophagy in Porcine Mammary Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Porcine Mammary Epithelial Cell Culture
2.3. Bacterial Strains and Growth Conditions
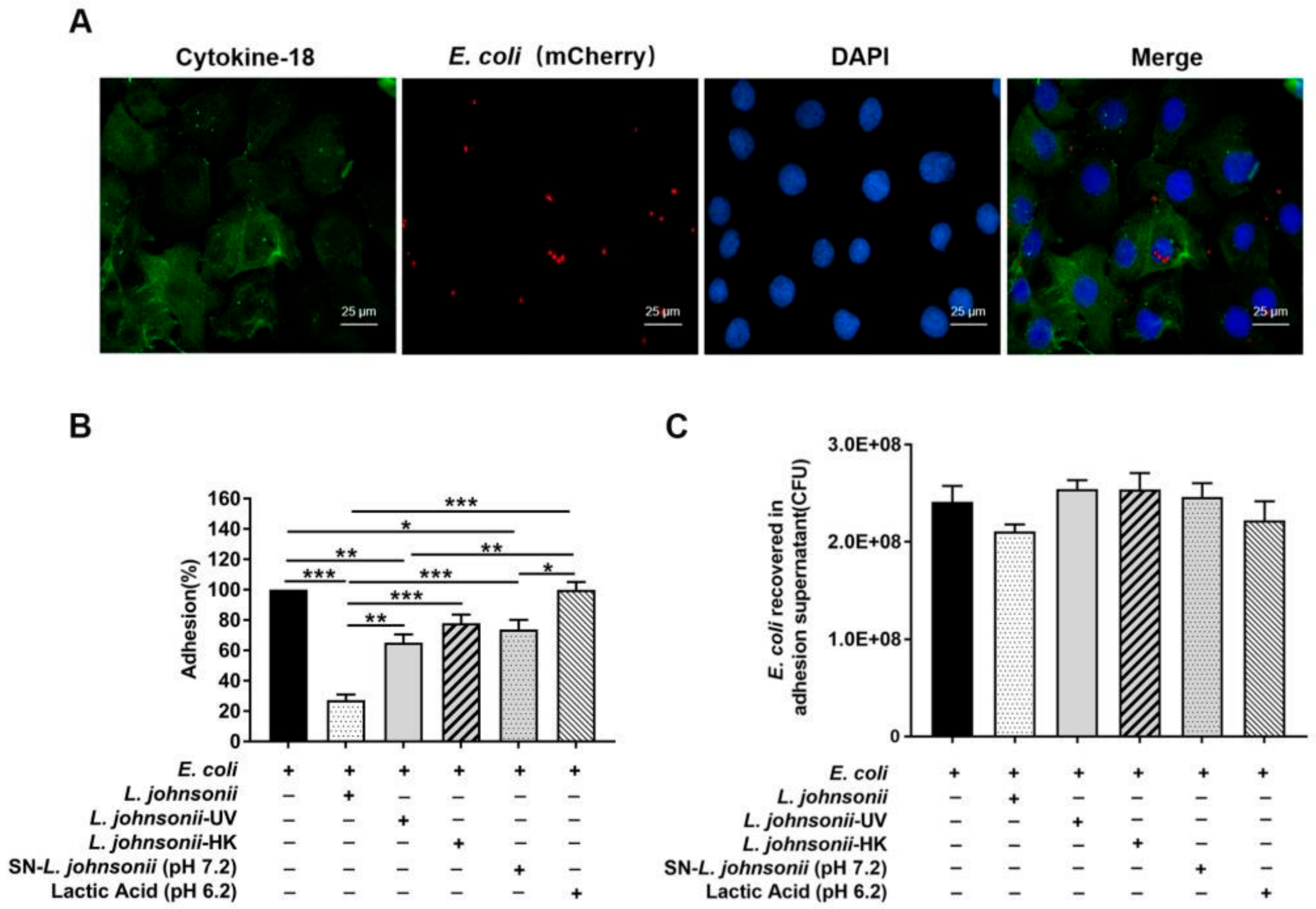
2.4. Immunofluorescence
2.5. Adhesion Assay
2.6. Internalization Assay
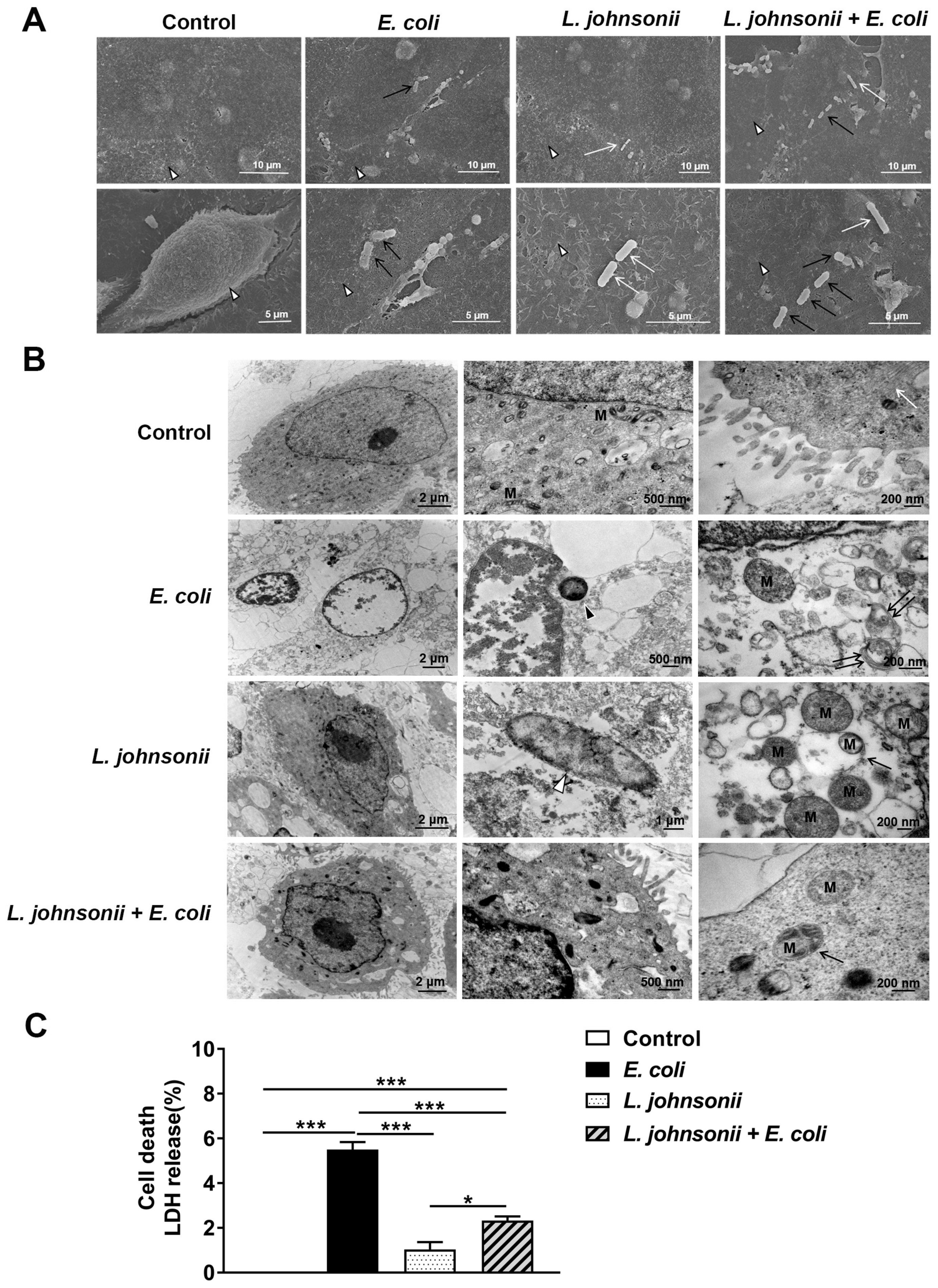
2.7. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
2.8. Cell Death Assay
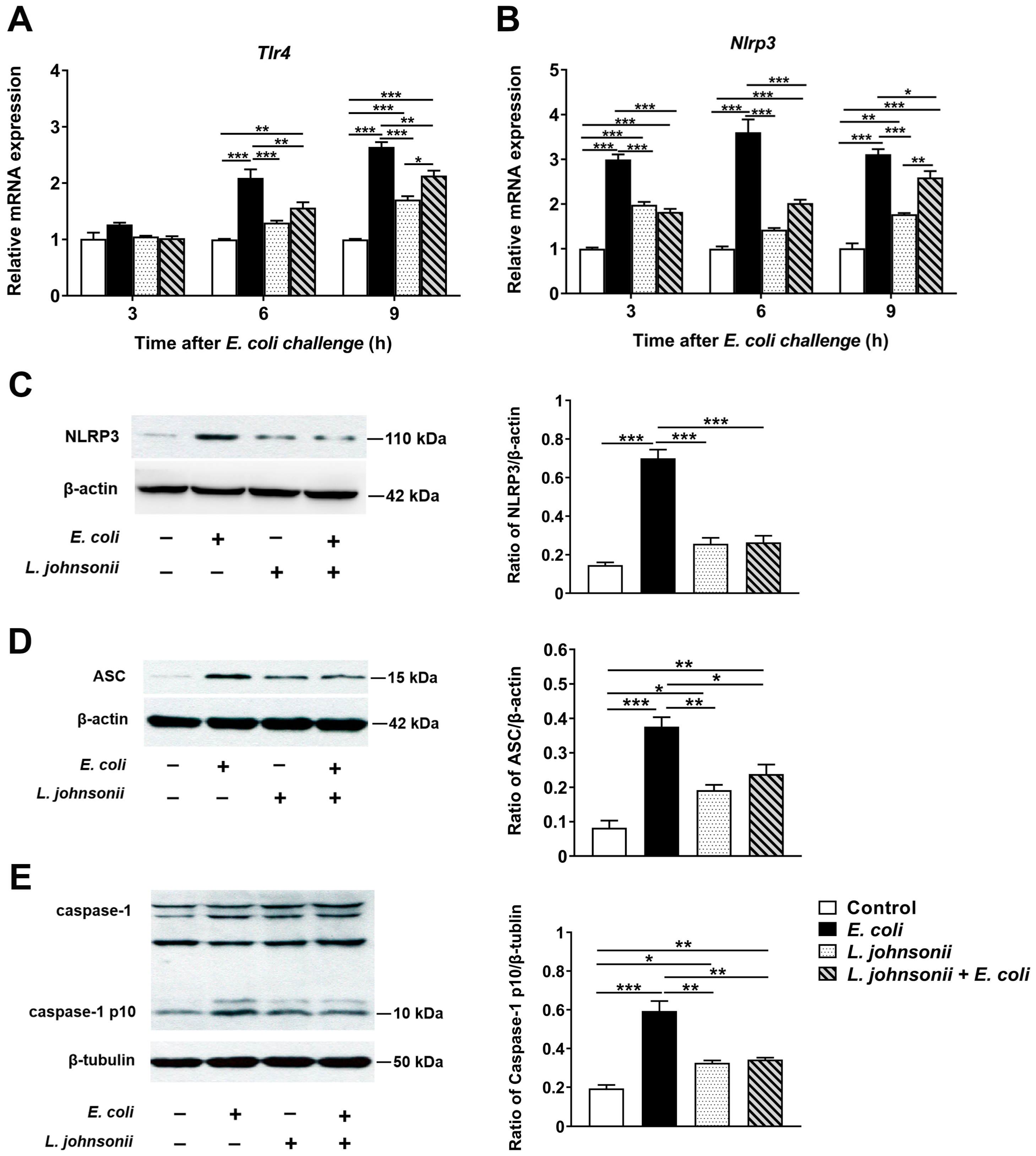
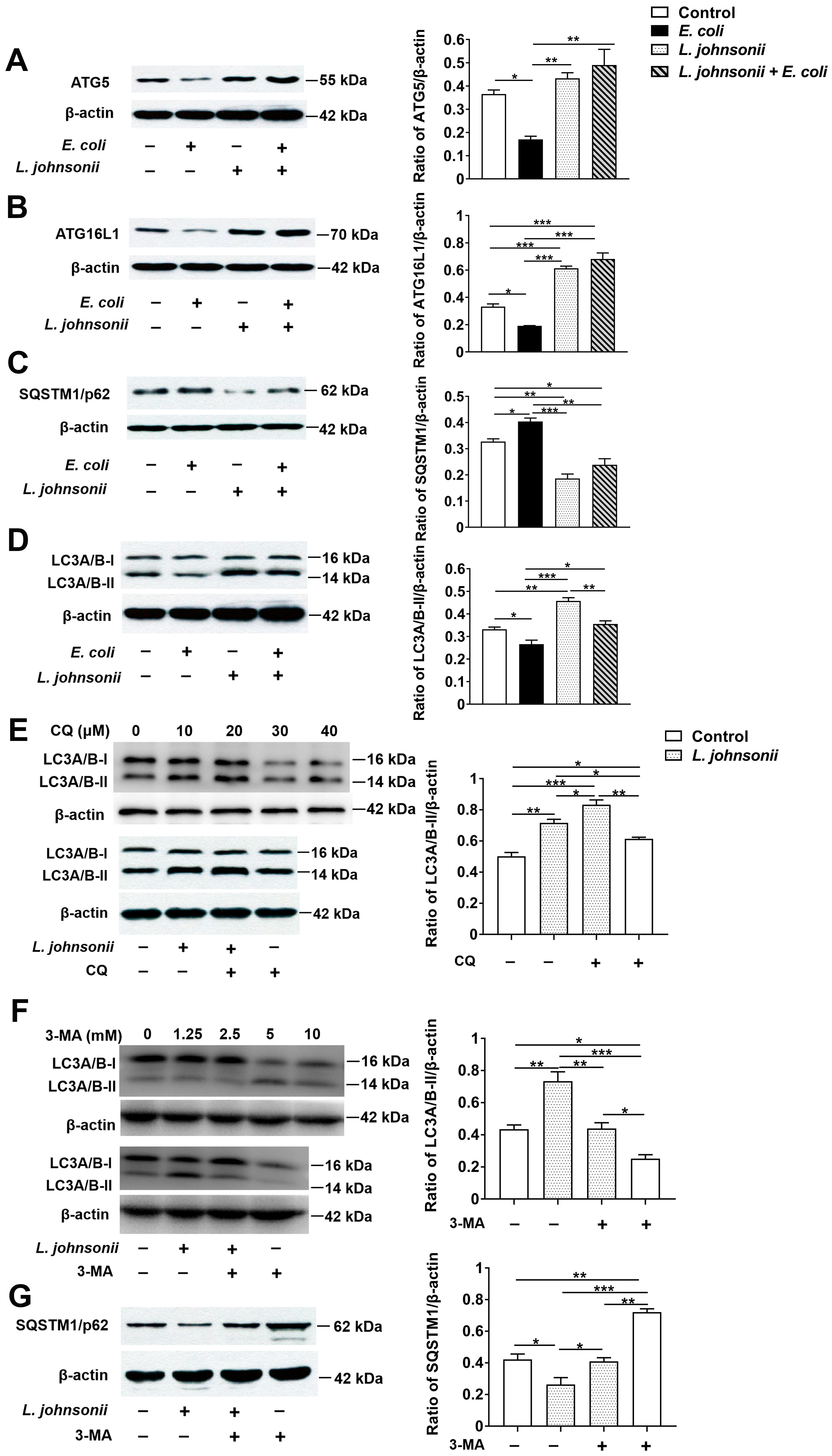
2.9. Real-Time Quantitative PCR
2.10. Western Blotting
2.11. Statistical Analysis
3. Results
3.1. L. johnsonii L531 Pretreatment Reduces the Adhesion of E. coli to PMECs
3.2. L. johnsonii L531 Pretreatment Reduces PMEC Damage Induced by E. coli
3.3. L. johnsonii L531 Pretreatment Ameliorates Disruption of PMEC Ultrastructure and Decreased Cell Death Induced by E. coli
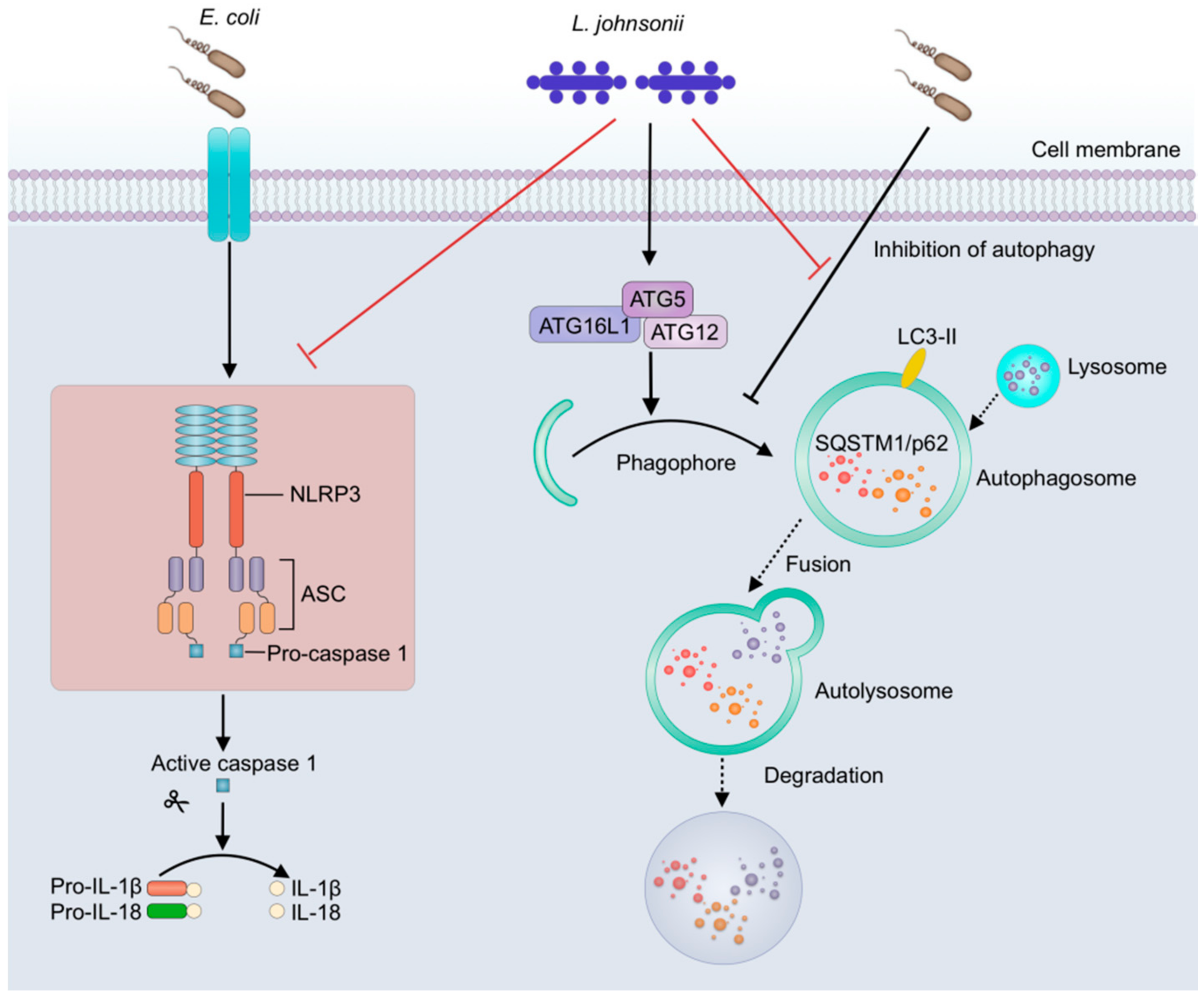
3.4. L. johnsonii L531 Pretreatment Ameliorates E. coli-Induced Activation of NLRP3 Inflammasome
3.5. L. johnsonii L531 Pretreatment Suppresses E. coli-Induced Cytokine and Chemokine mRNA Expression in PMECs
3.6. L. johnsonii L531 Pretreatment Reverses the Inhibitory Effect of E. coli on Autophagy in PMECs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gerjets, I.; Kemper, N. Coliform mastitis in sows: A review. J. Swine Health Prod. 2009, 17, 97–105. [Google Scholar]
- Gerjets, I.; Traulsen, I.; Reiners, K.; Kemper, N. Comparison of virulence gene profiles of Escherichia coli isolates from sows with coliform mastitis and healthy sows. Vet. Microbiol. 2011, 152, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Kemper, N.; Bardehle, D.; Lehmann, J.; Gerjets, I.; Preissler, R. The role of bacterial pathogens in coliform mastitis in sows. Berl. Munch. Tierarztl. Wochenschr. 2013, 126, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Persson, A.; Moerner, A.P.; Kuhl, W. A long term study on the health status and performance of sows on different feed allowances during late pregnancy II. The total cell content and its percentage of polymorphonuclear leucocytes in pathogen-free colostrum and milk collected from clinically healthy sows. Acta Vet. Scand. 1996, 37, 279. [Google Scholar] [CrossRef]
- Frola, I.D.; Pellegrino, M.S.; Espeche, M.C.; Giraudo, J.A.; Nader-Macias, M.E.; Bogni, C.I. Effects of intramammary inoculation of Lactobacillus perolens CRL1724 in lactating cows’ udders. J. Dairy Res. 2012, 79, 84–92. [Google Scholar] [CrossRef]
- Moal, V.L.; Servin, A.L. Anti-infective activities of lactobacillus strains in the human intestinal microbiota: From probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clin. Microbiol. Rev. 2014, 27, 167–199. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, M.C.; Yang, J.; Wang, J.F.; Zhu, Y.H. Lactobacillus rhamnosus GR-1 ameliorates Escherichia coli–induced inflammation and cell damage via attenuation of ASC-independent NLRP3 inflammasome activation. Appl. Environ. Microbiol. 2015, 82, 1173–1182. [Google Scholar] [CrossRef]
- Bereswill, S.; Ekmekciu, I.; Escher, U.; Fiebiger, U.; Stingl, K.; Heimesaat, M.M. Lactobacillus johnsonii ameliorates intestinal, extra-intestinal and systemic pro-inflammatory immune responses following murine Campylobacter jejuni infection. Sci. Rep. 2017, 7, 2138. [Google Scholar] [CrossRef]
- Zeighamy, A.S.; Bita, B.; Sara, S.; Coy, A.I. The anti-apoptotic and anti-inflammatory effect of Lactobacillus acidophilus on Shigella sonnei and Vibrio cholerae interaction with intestinal epithelial cells: A comparison between invasive and non-invasive bacteria. PLoS ONE 2018, 13, e0196941. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, A.Y.; Jiang, X.; Zhang, H.; Mehmood, K.; Zhang, L.H.; Jiang, J.H.; Waqas, M.H.; Iqbal, M.; Li, J.K. Probiotic potential of Leuconostoc pseudomesenteroides and Lactobacillus strains isolated from Yaks. Front. Microbiol. 2018, 9, 2987. [Google Scholar] [CrossRef]
- He, T.; Zhu, Y.H.; Yu, J.; Xia, B.; Liu, X.; Yang, G.Y.; Su, J.H.; Guo, L.; Wang, M.L.; Wang, J.F. Lactobacillus johnsonii L531 reduces pathogen load and helps maintain short-chain fatty acid levels in the intestines of pigs challenged with Salmonella enterica Infantis. Vet. Microbiol. 2019, 230, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Yu, J.; He, T.; Liu, X.; Su, J.H.; Wang, M.J.; Wang, J.F.; Zhu, Y.H. Lactobacillus johnsonii L531 ameliorates enteritis via elimination of damaged mitochondria and suppression of SQSTM1-dependent mitophagy in a Salmonella infantis model of piglet diarrhea. FASEB J. 2019, 34, 2821–2839. [Google Scholar] [CrossRef] [PubMed]
- Rainard, P.; Riollet, C. Innate immunity of the bovine mammary gland. Vet. Res. 2006, 37, 369–400. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Liu, P.Q.; Weng, X.G.; Zhuge, Z.Y.; Zhang, R.; Ma, J.L.; Qiu, X.Q.; Zhang, X.L.; Wang, J.F. Short communication: Pheromonicin-SA affects mRNA expression of toll-like receptors, cytokines, and lactoferrin by Staphylococcus aureus-infected bovine mammary epithelial cells. J. Dairy Sci. 2012, 95, 759–764. [Google Scholar] [CrossRef]
- Skeldon, A.M.; Faraj, M.; Saleh, M. Caspases and inflammasomes in metabolic inflammation. Immunol. Cell Biol. 2014, 92, 304–313. [Google Scholar] [CrossRef]
- Tack, C.J.; Stienstra, R.; Joosten, L.A.; Netea, M.G. Inflammation links excess fat to insulin resistance: The role of the interleukin-1 family. Immunol. Rev. 2012, 249, 239–252. [Google Scholar] [CrossRef]
- Man, S.M.; Kanneganti, T.D. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat. Rev. Immunol. 2016, 16, 7–21. [Google Scholar] [CrossRef]
- Hirota, S.A.; Ng, J.; Lueng, A.; Khajah, M.; Parhar, K.; Li, Y.; Lam, V.; Potentier, M.S.; Ng, K.; Bawa, M.; et al. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm. Bowel Dis. 2011, 17, 1359–1372. [Google Scholar] [CrossRef]
- Sharma, D.; Kanneganti, T.D. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J. Cell Biol. 2016, 213, 617–629. [Google Scholar] [CrossRef]
- Mangan, M.S.J.; Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018, 17, 688. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V.; Saitoh, T.; Akira, S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 2013, 13, 722–737. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, D.H.A.; Khalil, H.; Cormet-Boyaka, E.; Amer, A.O. The cooperation between the autophagy machinery and the inflammasome to implement an appropriate innate immune response: Do they regulate each other? Immunol. Rev. 2015, 265, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V.; Levine, B. Autophagy balances inflammation in innate immunity. Autophagy 2018, 14, 243–251. [Google Scholar] [CrossRef]
- Xue, Y.; Du, M.; Sheng, H.; Hovde, C.J.; Zhu, M.J. Escherichia coli O157:H7 suppresses host autophagy and promotes epithelial adhesion via Tir-mediated and cAMP-independent activation of protein kinase A. Cell Death Discov. 2017, 3, 17055. [Google Scholar] [CrossRef][Green Version]
- Takahama, M.; Akira, S.; Saitoh, T. Autophagy limits activation of the inflammasomes. Immunol. Rev. 2018, 281, 62–73. [Google Scholar] [CrossRef]
- Nakahira, K.; Haspel, J.A.; Rathinam, V.A.; Lee, S.J.; Dolinay, T.; Lam, H.C.; Englert, J.A.; Rabinovitch, M.; Cernadas, M.; Kim, H.P.; et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 2011, 12, 222–230. [Google Scholar] [CrossRef]
- Saitoh, T.; Fujita, N.; Jang, M.H.; Uematsu, S.; Yang, B.G.; Satoh, T.; Omori, H.; Noda, T.; Yamamoto, N.; Komatsu, M.; et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 2008, 456, 264–268. [Google Scholar] [CrossRef]
- Shi, C.S.; Shenderov, K.; Huang, N.N.; Kabat, J.; Abu-Asab, M.; Fitzgerald, K.A.; Sher, A.; Kehrl, J.H. Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 2012, 13, 255–263. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.H.; Yang, G.Y.; Liu, X.; Xia, B.; Hu, X.; Su, J.H.; Wang, J.F. Lactobacillus rhamnosus GG affects microbiota and suppresses autophagy in the intestines of pigs challenged with Salmonella Infantis. Front. Microbiol. 2018, 8, 2705. [Google Scholar] [CrossRef]
- Dahanayaka, S.; Rezaei, R.; Porter, W.W.; Johnson, G.A.; Burghardt, R.C.; Bazer, F.W.; Hou, Y.Q.; Wu, Z.L.; Wu, G. Technical note: Isolation and characterization of porcine mammary epithelial cells. J. Anim. Sci. 2015, 93, 5186–5193. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Cárdenas, N.; Arroyo, R.; Manzano, S.; Rodríguez, J.M. Prevention of infectious mastitis by oral administration of Lactobacillus salivarius PS2 during late pregnancy. Clin. Infect. Dis. 2015, 62, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, M.; Berardo, N.; Giraudo, J.; Nader-Macías, M.E.F.; Bogni, C. Bovine mastitis prevention: Humoral and cellular response of dairy cows inoculated with lactic acid bacteria at the dry-off period. Benef. Microbes 2017, 8, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.R.; Wang, F.; Qiu, X.; McFarland, L.V.; Chen, P.F.; Zhou, R.; Liu, J.; Zhao, Q.; Li, J. Efficacy and safety of probiotic-supplemented triple therapy for eradication of Helicobacter pylori in children: A systematic review and network meta-analysis. Eur. J. Clin. Pharmacol. 2017, 73, 1199–1208. [Google Scholar] [CrossRef]
- Kleta, S.; Nordhoff, M.; Tedin, K.; Wieler, L.H.; Kolenda, R.; Oswald, S.; Oelschlaeger, T.A.; Bleiss, W.; Schierack, P. Role of F1C Fimbriae, Flagella, and secreted bacterial components in the inhibitory effect of probiotic Escherichia coli nissle 1917 on atypical enteropathogenic E. coli infection. Infect. Immun. 2014, 82, 1801–1812. [Google Scholar] [CrossRef]
- Johnson, H.K.; Hagen, K.E.; Gordonpour, M.; Tompkins, T.A.; Sherman, P.M. Surface-layer protein extracts from Lactobacillus helveticus inhibit enterohaemorrhagic Escherichia coli O157:H7 adhesion to epithelial cells. Cell Microbiol. 2007, 9, 356–367. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, S.; Kim, S.H. Released exopolysaccharide (r-EPS) produced from probiotic bacteria reduce biofilm formation of enterohemorrhagic Escherichia coli O157:H7. Biochem. Biophys. Res. Commun. 2009, 379, 324–329. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Z.M.; Tian, F.W.; Zhao, J.X.; Zhang, H.; Zhai, Q.X.; Chen, W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb. Cell Fact. 2020, 19, 23. [Google Scholar] [CrossRef]
- Rekha, R.S.; Rao Muvva, S.S.; Wan, M.; Raqib, R.; Bergman, P.; Brighenti, S.; Gudmundsson, G.H.; Agerberth, B. Phenylbutyrate induces LL-37-dependent autophagy and intracellular killing of Mycobacterium tuberculosis in human macrophages. Autophagy 2015, 11, 1688–1699. [Google Scholar] [CrossRef]
- Gabriela, P.; Castillo, N.A.; Alejandra, L.B. Oral administration of a probiotic Lactobacillus modulates cytokine production and TLR expression improving the immune response against Salmonella enterica serovar Typhimurium infection in mice. BMC Microbiol. 2011, 11, 177. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.H.; Yang, J.C.; Yang, G.Y.; Zhou, D.; Wang, J.F. A selected Lactobacillus rhamnosus strain promotes EGFR-independent Akt activation in an enterotoxigenic Escherichia coli K88-infected IPEC-J2 cell model. PLoS ONE 2015, 10, e0125717. [Google Scholar] [CrossRef] [PubMed]
- Finamore, A.; Roselli, M.; Imbinto, A.; Seeboth, J.; Oswald, I.P.; Mengheri, E. Lactobacillus amylovorus inhibits the TLR4 inflammatory signaling triggered by enterotoxigenic Escherichia coli via modulation of the negative regulators and involvement of TLR2 in intestinal Caco-2 cells and pig explants. PLoS ONE 2014, 9, e94891. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, W.; Wang, T.; Jiang, H.C.; Zhang, Z.C.; Fu, Y.H.; Yang, Z.T.; Cao, Y.G.; Zhang, N.S. Geniposide plays an anti-inflammatory role via regulating TLR4 and downstream signaling pathways in lipopolysaccharide-induced mastitis in mice. Inflammation 2014, 37, 1588–1598. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, F.T.; Elsasser, T.H.; Kerr, D.E. Variation in fibroblast expression of TLR4 and lipopolysaccharide-induced cytokine production between animals predicts control of bacterial growth but not severity of E. coli mastitis. J. Dairy Sci. 2018, 101, 10098–10115. [Google Scholar] [CrossRef]
- Cao, D.; Luo, J.; Chen, D.K.; Xu, H.F.; Shi, H.P.; Jing, C.Q.; Zang, W.J. CD36 regulates lipopolysaccharide-induced signaling pathways and mediates the internalization of Escherichia coli in cooperation with TLR4 in goat mammary gland epithelial cells. Sci. Rep. 2019, 9, 6457. [Google Scholar] [CrossRef]
- Sharif, H.; Wang, L.; Wang, W.L.; Magupalli, V.G.; Andreeva, L.; Qiao, Q.; Hauenstein, A.V.; Wu, Z.; Núñez, G.; Mao, Y.; et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 2019, 570, 338–343. [Google Scholar] [CrossRef]
- Ramakrishnan, S.K.; Zhang, H.; Ma, X.; Jung, I.; Schwartz, A.J.; Triner, D.; Devenport, S.N.; Das, N.K.; Xue, X.; Zeng, M.Y.; et al. Intestinal non-canonical NFkappaB signaling shapes the local and systemic immune response. Nat. Commun. 2019, 10, 660. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Fossum, C.; Berg, M.; Magnusson, U. Morphometric analysis of proinflammatory cytokines in mammary glands of sows suggests an association between clinical mastitis and local production of IL-1beta, IL-6 and TNF-alpha. Vet. Res. 2007, 38, 871–882. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Berg, M.; Fossum, C.; Magnusson, U. Proinflammatory cytokine mRNA expression in mammary tissue of sows following intramammary inoculation with Escherichia coli. Vet. Immunol. Immunopathol. 2007, 116, 98–103. [Google Scholar] [CrossRef]
- Jaeger, A.; Bardehle, D.; Oster, M.; Günther, J.; Muráni, E.; Ponsuksili, S.; Wimmers, K.; Kemper, N. Gene expression profiling of porcine mammary epithelial cells after challenge with Escherichia coli and Staphylococcus aureus in vitro. Vet. Res. 2015, 46, 50. [Google Scholar] [CrossRef]
- Jaeger, A.; Hadlich, F.; Kemper, N.; Lübke-Becker, A.; Muráni, E.; Wimmers, K.; Ponsuksili, S. MicroRNA expression profiling of porcine mammary epithelial cells after challenge with Escherichia coli in vitro. BMC Genom. 2017, 18, 660. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, S.T.; Saitoh, T.; Nowag, H.; Munz, C.; Yoshimori, T. Autophagy and autophagy-related proteins in the immune system. Nat. Immunol. 2015, 16, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Conway, K.L.; Kuballa, P.; Song, J.H.; Patel, K.K.; Castoreno, A.B.; Yilmaz, O.H.; Jijon, H.B.; Zhang, M.; Aldrich, L.N.; Villablanca, E.J.; et al. Atg16l1 is required for autophagy in intestinal epithelial cells and protection of mice from Salmonella infection. Gastroenterology 2013, 145, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Khandia, R.; Dadar, M.; Munjal, A. A comprehensive review of autophagy and its various roles in infectious, non-infectious, and lifestyle diseases: Current knowledge and prospects for disease prevention, novel drug design, and therapy. Cells 2019, 8, e674. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Zou, H.; Wang, B.; Sun, Q.; Fu, A.; Wang, Y.; Wang, Y.; Xu, X.; Li, W. Probiotic Bacillus amyloliquefaciens SC06 Induces Autophagy to Protect against Pathogens in Macrophages. Front. Microbiol. 2017, 9, 2987. [Google Scholar] [CrossRef]
- Benjamin, J.L.; Sumpter, R., Jr.; Levine, B.; Hooper, L.V. Intestinal epithelial autophagy is essential for host defense against invasive bacteria. Cell Host Microbe 2013, 13, 723–734. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Koike, M.; Sou, Y.S.; Ueno, T.; Hara, T.; Mizushima, N.; Iwata, J.; Ezaki, J.; Murata, S.; et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 2007, 131, 1149–1163. [Google Scholar] [CrossRef]
- Jo, E.K.; Kim, J.K.; Shin, D.M.; Sasakawa, C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol. Immunol. 2016, 13, 148–159. [Google Scholar] [CrossRef]
- Van der Burgh, R.; Nijhuis, L.; Pervolaraki, K.; Compeer, E.B.; Jongeneel, L.H.; van Gijn, M.; Coffer, P.J.; Murphy, M.P.; Mastroberardino, P.G.; Frenkel, J.; et al. Defects in mitochondrial clearance predispose human monocytes to interleukin-1β hypersecretion. J. Biol. Chem. 2014, 289, 5000–5012. [Google Scholar] [CrossRef]
- Harris, J.; Hartman, M.; Roche, C.; Zeng, S.G.; O’Shea, A.; Sharp, F.A.; Lambe, E.M.; Creagh, E.M.; Golenbock, D.T.; Tschopp, J.; et al. Autophagy controls IL-1β secretion by targeting pro-IL-1β for degradation. J. Biol. Chem. 2011, 286, 9587–9597. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Yang, C.H.; Chou, C.C.; Chiang, Y.P.; Chuang, T.H.; Hsu, L.C. TLR-induced PAI-2 expression suppresses IL-1β processing via increasing autophagy and NLRP3 degradation. Proc. Natl. Acad. Sci. USA 2013, 110, 16079–16084. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Umemura, A.; Sanchez-Lopez, E.; Liang, S.; Shalapour, S.; Wong, J.; He, F.; Boassa, D.; Perkins, G.; Ali, S.R.; et al. NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell 2016, 164, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Santeford, A.; Wiley, L.A.; Park, S.; Bamba, S.; Nakamura, R.; Gdoura, A.; Ferguson, T.A.; Rao, P.K.; Guan, J.L.; Saitoh, T.; et al. Impaired autophagy in macrophages promotes inflammatory eye disease. Autophagy 2016, 12, 1876–1885. [Google Scholar] [CrossRef] [PubMed]

| Primers Name | Direction a | Sequence (5′→3′) | Accession Number |
|---|---|---|---|
| Gapdh | F | CCAGAACATCATCCCTGCTT | NM_001206359 |
| R | GTCCTCAGTGTAGCCCAGGA | ||
| Il-1β | F | GGCCGCCAAGATATAACTGA | NM_214055 |
| R | GGACCTCTGGGTATGGCTTTC | ||
| Il-18 | F | GCTGCTGAACCGGAAGACAA | NM_213997.1 |
| R | AAACACGGCTTGATGTCCCT | ||
| Il-6 | F | GGGAAATGTCGAGGCTGTG | NM_214399 |
| R | AGGGGTGGTGGCTTTGTCT | ||
| Il-8 | F | TCCTGCTTTCTGCAGCTCTC | NM_213867 |
| R | GGGTGGAAAGGTGTGGAATG | ||
| Tnf-α | F | GCCCACGTTGTAGCCAATGTCAAA | NM_214022 |
| R | GTTGTCTTTCAGCTTCACGCCGTT | ||
| Cxcl2 | F | GGAAGTTTGTCTCAACCCCGC | NM_001001861 |
| R | AGCCAGTAAGTTTCCTCCATCTC | ||
| Tlr4 | F | GCCATCGCTGCTAACATCATC | NM_001113039 |
| R | CTCATACTCAAAGATACACCATCGG | ||
| Nlrp3 | F | GAGCCTAGGAACTCGGAGGA | NM_001256770.1 |
| R | GCTCATCAAAGGCACCTTGC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Y.-J.; Xu, J.-J.; Wang, X.; Zhu, Y.-H.; Wu, Q.; Wang, J.-F. Lactobacillus johnsonii L531 Ameliorates Escherichia coli-Induced Cell Damage via Inhibiting NLRP3 Inflammasome Activity and Promoting ATG5/ATG16L1-Mediated Autophagy in Porcine Mammary Epithelial Cells. Vet. Sci. 2020, 7, 112. https://doi.org/10.3390/vetsci7030112
Zou Y-J, Xu J-J, Wang X, Zhu Y-H, Wu Q, Wang J-F. Lactobacillus johnsonii L531 Ameliorates Escherichia coli-Induced Cell Damage via Inhibiting NLRP3 Inflammasome Activity and Promoting ATG5/ATG16L1-Mediated Autophagy in Porcine Mammary Epithelial Cells. Veterinary Sciences. 2020; 7(3):112. https://doi.org/10.3390/vetsci7030112
Chicago/Turabian StyleZou, Yun-Jing, Jia-Jia Xu, Xue Wang, Yao-Hong Zhu, Qiong Wu, and Jiu-Feng Wang. 2020. "Lactobacillus johnsonii L531 Ameliorates Escherichia coli-Induced Cell Damage via Inhibiting NLRP3 Inflammasome Activity and Promoting ATG5/ATG16L1-Mediated Autophagy in Porcine Mammary Epithelial Cells" Veterinary Sciences 7, no. 3: 112. https://doi.org/10.3390/vetsci7030112
APA StyleZou, Y.-J., Xu, J.-J., Wang, X., Zhu, Y.-H., Wu, Q., & Wang, J.-F. (2020). Lactobacillus johnsonii L531 Ameliorates Escherichia coli-Induced Cell Damage via Inhibiting NLRP3 Inflammasome Activity and Promoting ATG5/ATG16L1-Mediated Autophagy in Porcine Mammary Epithelial Cells. Veterinary Sciences, 7(3), 112. https://doi.org/10.3390/vetsci7030112






