High Prevalence of Multiple Antibiotic-Resistant, Extended-Spectrum β-Lactamase (ESBL)-Producing Escherichia coli in Fresh Seafood Sold in Retail Markets of Mumbai, India
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, Isolation and Identification of Escherichia coli From Seafood
2.2. Antibiotic Susceptibility Tests
2.3. Detection of ESBL and MBL Phenotypes
2.4. Detection of Antibiotic Resistance Genes
3. Results and Discussion
3.1. Distribution of ESBL+ E. coli in Seafood
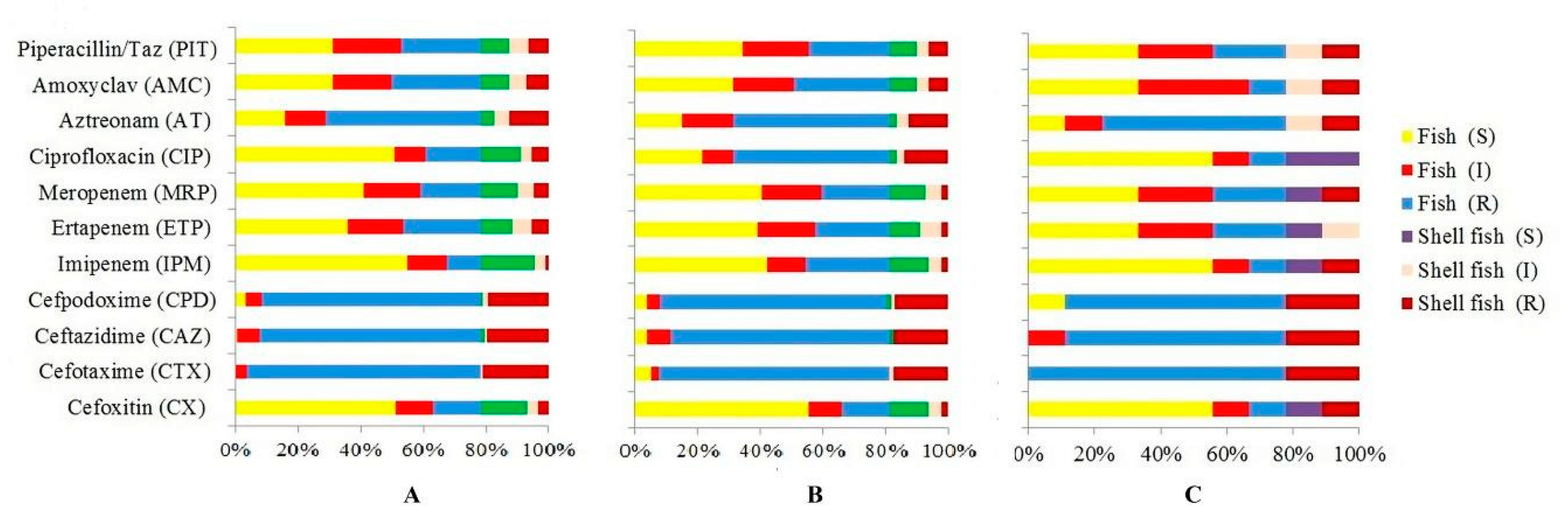
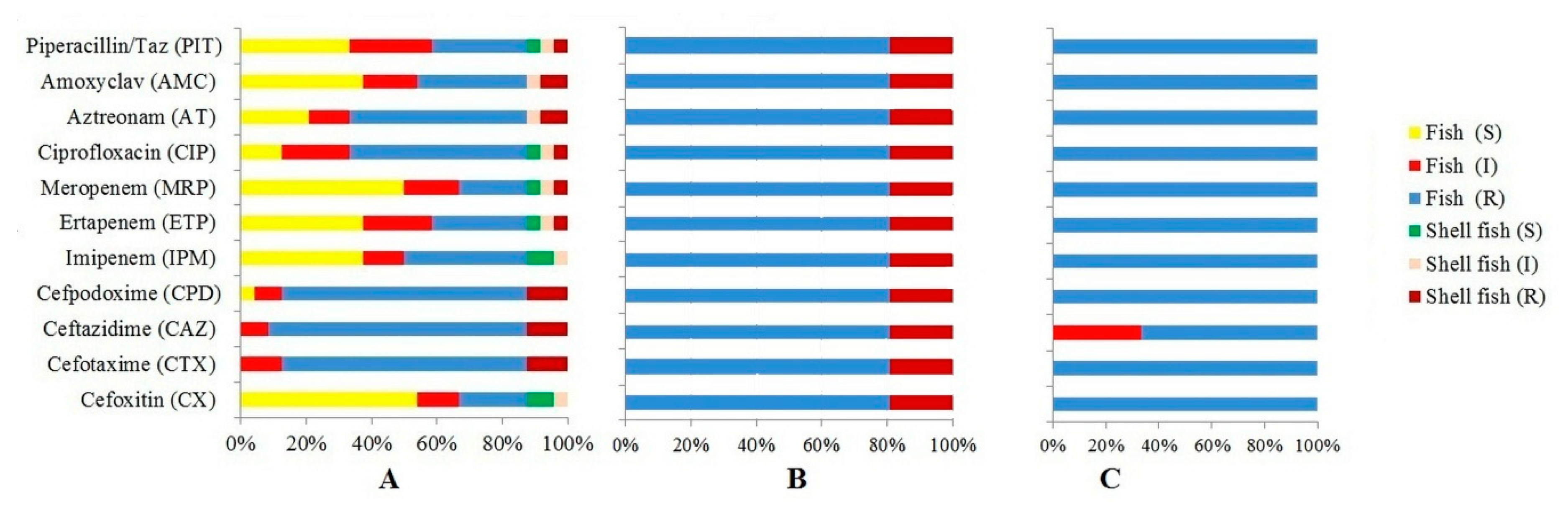
3.2. Antibiotic Resistance Profiles of E. coli
3.3. Molecular Characterization of ESBL+ E. coli
3.4. Detection of Carbapenemase-Encoding Genes
3.5. Multidrug Resistance in Seafood E. coli
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jang, J.; Hur, H.-G.; Sadowsky, M.J.; Byappanahalli, M.N.; Yan, T.; Ishii, S. Environmental Escherichia coli: Ecology and public health implications-a review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef]
- Kumar, H.S.; Karunasagar, I.; Karunasagar, I.; Teizou, T.; Shima, K.; Yamasaki, S. Characterisation of Shiga toxin-producing Escherichia coli (STEC) isolated from seafood and beef. FEMS Microbiol. Lett. 2004, 233, 173–178. [Google Scholar] [CrossRef][Green Version]
- Prakasan, S.; Prabhakar, P.; Lekshmi, M.; Kumar, S.; Nayak, B.B. Isolation of Shiga toxin-producing Escherichia coli harboring variant Shiga toxin genes from seafood. Vet. World 2018, 11, 379–385. [Google Scholar] [CrossRef]
- Gomes, T.A.T.; Elias, W.P.; Scaletsky, I.C.A.; Guth, B.E.C.; Rodrigues, J.F.; Piazza, R.M.F.; Ferreira, L.C.S.; Martinez, M.B. Diarrheagenic Escherichia coli. Braz. J. Microbiol. 2016, 47 (Suppl. 1), 3–30. [Google Scholar] [CrossRef]
- Lekshmi, M.; Ammini, P.; Kumar, S.; Varela, M.F. The food production environment and the development of antimicrobial resistance in human pathogens of animal origin. Microorganisms 2017, 5, 11. [Google Scholar] [CrossRef]
- Pitout, J.D.D. Multiresistant Enterobacteriaceae: New threat of an old problem. Expert Rev. Anti-Infect. Ther. 2008, 6, 657–669. [Google Scholar] [CrossRef]
- Sheng, W.-H.; Badal, R.E.; Hsueh, P.-R. SMART Program Distribution of extended-spectrum β-lactamases, AmpC β-lactamases, and carbapenemases among Enterobacteriaceae isolates causing intra-abdominal infections in the Asia-Pacific region: Results of the study for Monitoring Antimicrobial Resistance Trends (SMART). Antimicrob. Agents Chemother. 2013, 57, 2981–2988. [Google Scholar]
- Hawkey, P.M. Prevalence and clonality of extended-spectrum beta-lactamases in Asia. Clin. Microbiol. Infect. 2008, 14 (Suppl. 1), 159–165. [Google Scholar] [CrossRef]
- McDanel, J.; Schweizer, M.; Crabb, V.; Nelson, R.; Samore, M.; Khader, K.; Blevins, A.E.; Diekema, D.; Chiang, H.-Y.; Nair, R.; et al. Incidence of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella Infections in the United States: A systematic literature review. Infect. Control Hosp. Epidemiol. 2017, 38, 1209–1215. [Google Scholar] [CrossRef]
- Paterson, D.L.; Bonomo, R.A. Extended-spectrum beta-lactamases: A clinical update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef]
- Drieux, L.; Brossier, F.; Sougakoff, W.; Jarlier, V. Phenotypic detection of extended-spectrum beta-lactamase production in Enterobacteriaceae: Review and bench guide. Clin. Microbiol. Infect. 2008, 14 (Suppl. 1), 90–103. [Google Scholar] [CrossRef]
- Bradford, P.A. Extended-spectrum β-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 2001, 14, 933–951. [Google Scholar] [CrossRef]
- Bush, K. Past and Present Perspectives on β-Lactamases. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Nordmann, P.; Dortet, L.; Poirel, L. Carbapenem resistance in Enterobacteriaceae: Here is the storm! Trends Mol. Med. 2012, 18, 263–272. [Google Scholar] [CrossRef]
- Tzouvelekis, L.S.; Tzelepi, E.; Tassios, P.T.; Legakis, N.J. CTX-M-type beta-lactamases: An emerging group of extended-spectrum enzymes. Int. J. Antimicrob. Agents 2000, 14, 137–142. [Google Scholar] [CrossRef]
- Lutgring, J.D.; Limbago, B.M. The Problem of Carbapenemase-Producing-Carbapenem-Resistant-Enterobacteriaceae Detection. J. Clin. Microbiol. 2016, 54, 529–534. [Google Scholar] [CrossRef]
- Sanath Kumar, H.; Otta, S.K.; Karunasagar, I.; Karunasagar, I. Detection of Shiga-toxigenic Escherichia coli (STEC) in fresh seafood and meat marketed in Mangalore, India by PCR. Lett. Appl. Microbiol. 2001, 33, 334–338. [Google Scholar] [CrossRef]
- Thampuran, N.; Surendraraj, A.; Surendran, P.K. Prevalence and characterization of typical and atypical Escherichia coli from fish sold at retail in Cochin, India. J. Food Prot. 2005, 68, 2208–2211. [Google Scholar] [CrossRef]
- Sehgal, R.; Kumar, Y.; Kumar, S. Prevalence and geographical distribution of Escherichia coli O157 in India: A 10-year survey. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 380–383. [Google Scholar] [CrossRef]
- Kumar, H.S.; Parvathi, A.; Karunasagar, I.; Karunasagar, I. Prevalence and antibiotic resistance of Escherichia coli in tropical seafood. World J. Microbiol. Biotechnol. 2005, 21, 619–623. [Google Scholar] [CrossRef]
- Singh, A.S.; Lekshmi, M.; Nayak, B.B.; Kumar, S.H. Isolation of Escherichia coli harboring blaNDM-5 from fresh fish in India. J. Microbiol. Immunol. Infect. 2016, 49, 822–823. [Google Scholar] [CrossRef]
- Sanjit Singh, A.; Lekshmi, M.; Prakasan, S.; Nayak, B.B.; Kumar, S. Multiple antibiotic-resistant, extended spectrum-β-lactamase (ESBL)-producing enterobacteria in fresh seafood. Microorganisms 2017, 5, 53. [Google Scholar] [CrossRef]
- MacFaddin, J.F. Biochemical Tests for Identification of Medical Bacteria, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Krumperman, P.H. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol. 1983, 46, 165–170. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement Performance Standards for Antimicrobial Susceptibility Testing. Document M100-24; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2014. [Google Scholar]
- Nordmann, P.; Poirel, L.; Carrër, A.; Toleman, M.A.; Walsh, T.R. How to detect NDM-1 producers. J. Clin. Microbiol. 2011, 49, 718–721. [Google Scholar] [CrossRef]
- Bonnet, R. Growing group of extended-spectrum beta-lactamases: The CTX-M enzymes. Antimicrob. Agents Chemother. 2004, 48, 1–14. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, M.; Ray, P. Detection of TEM & SHV genes in Escherichia coli & Klebsiella pneumoniae isolates in a tertiary care hospital from India. Indian J. Med. Res. 2010, 132, 332–336. [Google Scholar]
- Hornsey, M.; Phee, L.; Wareham, D.W. A novel variant, NDM-5, of the New Delhi metallo-β-lactamase in a multidrug-resistant Escherichia coli ST648 isolate recovered from a patient in the United Kingdom. Antimicrob. Agents Chemother. 2011, 55, 5952–5954. [Google Scholar] [CrossRef]
- Mushtaq, S.; Irfan, S.; Sarma, J.B.; Doumith, M.; Pike, R.; Pitout, J.; Livermore, D.M.; Woodford, N. Phylogenetic diversity of Escherichia coli strains producing NDM-type carbapenemases. J. Antimicrob. Chemother. 2011, 66, 2002–2005. [Google Scholar] [CrossRef]
- Siu, L.K.; Lo, J.Y.; Yuen, K.Y.; Chau, P.Y.; Ng, M.H.; Ho, P.L. beta-lactamases in Shigella flexneri isolates from Hong Kong and Shanghai and a novel OXA-1-like beta-lactamase, OXA-30. Antimicrob. Agents Chemother. 2000, 44, 2034–2038. [Google Scholar] [CrossRef]
- Shirani, K.; Ataei, B.; Roshandel, F. Antibiotic resistance pattern and evaluation of metallo-beta lactamase genes (VIM and IMP) in Pseudomonas aeruginosa strains producing MBL enzyme, isolated from patients with secondary immunodeficiency. Adv. Biomed. Res. 2016, 5. [Google Scholar] [CrossRef]
- Adesiji, Y.O.; Deekshit, V.K.; Karunasagar, I. Antimicrobial-resistant genes associated with Salmonella spp. isolated from human, poultry, and seafood sources. Food Sci. Nutr. 2014, 2, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Deekshit, V.K.; Kumar, B.K.; Rai, P.; Karunasagar, I.; Karunasagar, I. Differential expression of virulence genes and role of gyrA mutations in quinolone resistant and susceptible strains of Salmonella Weltevreden and Newport isolated from seafood. J. Appl. Microbiol. 2015, 119, 970–980. [Google Scholar] [CrossRef] [PubMed]
- Deekshit, V.K.; Kumar, B.K.; Rai, P.; Srikumar, S.; Karunasagar, I.; Karunasagar, I. Detection of class 1 integrons in Salmonella Weltevreden and silent antibiotic resistance genes in some seafood-associated nontyphoidal isolates of Salmonella in south-west coast of India. J. Appl. Microbiol. 2012, 112, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N.; Singh, A.S.; Lekshmi, M.; Nayak, B.B.; Kumar, S. Characterization of blaNDM-harboring, multidrug-resistant Enterobacteriaceae isolated from seafood. Environ. Sci. Pollut. Res. Int. 2019, 26, 2455–2463. [Google Scholar] [CrossRef]
- Divyashree, M.; Kumar, D.V.; Ballamoole, K.K.; Shetty, V.; Chakraborty, A.; Karunasagar, I. Occurrence of antibiotic resistance among gram-negative bacteria isolated from effluents of fish processing plants in and around Mangalore. Int. J. Environ. Health Res. 2019, 1–8. [Google Scholar] [CrossRef]
- Vu, T.T.T.; Alter, T.; Roesler, U.; Roschanski, N.; Huehn, S. Investigation of extended-spectrum and AmpC β-lactamase-producing Enterobacteriaceae from retail seafood in Berlin, Germany. J. Food Prot. 2018, 81, 1079–1086. [Google Scholar] [CrossRef]
- Sellera, F.P.; Fernandes, M.R.; Moura, Q.; Carvalho, M.P.N.; Lincopan, N. Extended-spectrum-β-lactamase (CTX-M)-producing Escherichia coli in wild fishes from a polluted area in the Atlantic Coast of South America. Mar. Pollut. Bull. 2018, 135, 183–186. [Google Scholar] [CrossRef]
- Nadimpalli, M.; Vuthy, Y.; de Lauzanne, A.; Fabre, L.; Criscuolo, A.; Gouali, M.; Huynh, B.-T.; Naas, T.; Phe, T.; Borand, L.; et al. Meat and fish as sources of extended-spectrum β-lactamase-producing Escherichia coli, Cambodia. Emerg. Infect. Dis. 2019, 25. [Google Scholar] [CrossRef]
- Baraniak, A.; Fiett, J.; Hryniewicz, W.; Nordmann, P.; Gniadkowski, M. Ceftazidime-hydrolysing CTX-M-15 extended-spectrum β-lactamase (ESBL) in Poland. J. Antimicrob. Chemother. 2002, 50, 393–396. [Google Scholar] [CrossRef]
- Naas, T.; Oxacelay, C.; Nordmann, P. Identification of CTX-M-type extended-spectrum-β-lactamase genes using real-time PCR and pyrosequencing. Antimicrob. Agents Chemother. 2007, 51, 223–230. [Google Scholar] [CrossRef]
- Pitout, J.D.D.; Nordmann, P.; Laupland, K.B.; Poirel, L. Emergence of Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBLs) in the community. J. Antimicrob. Chemother. 2005, 56, 52–59. [Google Scholar] [CrossRef]
- Lepeule, R.; Ruppé, E.; Le, P.; Massias, L.; Chau, F.; Nucci, A.; Lefort, A.; Fantin, B. Cefoxitin as an alternative to carbapenems in a murine model of urinary tract infection due to Escherichia coli harboring CTX-M-15-type extended-spectrum β-lactamase. Antimicrob. Agents Chemother. 2012, 56, 1376–1381. [Google Scholar] [CrossRef]
- Ananthan, S.; Subha, A. Cefoxitin resistance mediated by loss of a porin in clinical strains of Klebsiella pneumoniae and Escherichia coli. Indian J. Med. Microbiol. 2005, 23, 20–23. [Google Scholar] [CrossRef]
- Mesa, R.J.; Blanc, V.; Blanch, A.R.; Cortés, P.; González, J.J.; Lavilla, S.; Miró, E.; Muniesa, M.; Saco, M.; Tórtola, M.T.; et al. Extended-spectrum beta-lactamase-producing Enterobacteriaceae in different environments (humans, food, animal farms and sewage). J. Antimicrob. Chemother. 2006, 58, 211–215. [Google Scholar] [CrossRef]
- Cantón, R.; González-Alba, J.M.; Galán, J.C. CTX-M enzymes: Origin and diffusion. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef]
- Lauretti, L.; Riccio, M.L.; Mazzariol, A.; Cornaglia, G.; Amicosante, G.; Fontana, R.; Rossolini, G.M. Cloning and characterization of blaVIM, a new integron-borne metallo-beta-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 1999, 43, 1584–1590. [Google Scholar] [CrossRef]
- Walsh, T.R. The emergence and implications of metallo-beta-lactamases in Gram-negative bacteria. Clin. Microbiol. Infect. 2005, 11 (Suppl. 6), 2–9. [Google Scholar] [CrossRef]
- Cornaglia, G.; Giamarellou, H.; Rossolini, G.M. Metallo-β-lactamases: A last frontier for β-lactams? Lancet Infect. Dis. 2011, 11, 381–393. [Google Scholar] [CrossRef]
- Castanheira, M.; Bell, J.M.; Turnidge, J.D.; Mathai, D.; Jones, R.N. Carbapenem resistance among Pseudomonas aeruginosa strains from India: Evidence for nationwide endemicity of multiple metallo-β-lactamase clones (VIM-2, -5, -6, and -11 and the newly characterized VIM-18). Antimicrob. Agents Chemother. 2009, 53, 1225–1227. [Google Scholar] [CrossRef]
- Subramaniyan, J.S.; Sundaram, J.M. Occurrence of bla genes encoding carbapenem-resistant Pseudomonas aeruginosa and Acinetobacter baumannii from intensive care unit in a tertiary care hospital. J. Lab. Physicians 2018, 10, 208. [Google Scholar] [CrossRef]
- Nagaraj, S.; Chandran, S.P.; Shamanna, P.; Macaden, R. Carbapenem resistance among Escherichia coli and Klebsiella pneumoniae in a tertiary care hospital in south India. Indian J. Med. Microbiol. 2012, 30, 93. [Google Scholar] [PubMed]
- Zheng, Z.; Ye, L.; Chan, E.W.-C.; Chen, S. Identification and characterization of a conjugative blaVIM-1-bearing plasmid in Vibrio alginolyticus of food origin. J. Antimicrob. Chemother. 2019, 74, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Baño, J.; Gutiérrez-Gutiérrez, B.; Machuca, I.; Pascual, A. Treatment of Infections Caused by Extended-Spectrum-Beta-Lactamase-, AmpC-, and Carbapenemase-Producing Enterobacteriaceae. Clin. Microbiol. Rev. 2018, 31. [Google Scholar] [CrossRef]
| Sl. No. | Samples | No. Analysed | No. of E. coli Isolated | No. (%) ESBL+ E. coli |
|---|---|---|---|---|
| Finfish | ||||
| 1. | Sardinella longiceps | 5 | 32 | 24 (75) |
| 2. | Terapon jarbua | 5 | 29 | 29 (100) |
| 3. | Otolithes cuvieri | 3 | 19 | 19 (100) |
| 4. | Epinephelus diacanthus | 2 | 15 | 15 (100) |
| 5. | Nemipterus randalli | 2 | 22 | 14 (64.28) |
| 6. | Mene maculata | 2 | 24 | 13 (53.84) |
| 7. | Coilia dussumieri | 3 | 45 | 32 (71.87) |
| 8. | Harpadon nehereus | 4 | 42 | 35 (82.85) |
| 9. | Trichiurus lepturus | 2 | 21 | 12 (58.33) |
| 10. | Priacanthus hamrur | 2 | 19 | 13 (69.23) |
| 11. | Megalaspis cordyla | 2 | 25 | 25 (100) |
| 12. | Anodontostoma chacunda | 3 | 13 | 13 (100) |
| 13. | Pampus argenteus | 2 | 19 | 17 (88.23) |
| Shellfish | ||||
| 14. | Acetes indicus | 5 | 56 | 27 (48.21) |
| 15. | Metapenaeus dobsoni | 3 | 41 | 19 (73.68) |
| 16. | Meretrix meretrix | 3 | 26 | 18 (44.44) |
| 17. | Loligo duvauceli | 2 | 27 | 15 (26.66) |
| Total | 50 | 475 | 340 (71.58) |
| Antibiotic Tested | No. (%) Resistant | No. (%) Intermediate Resistant | No. (%) Susceptible |
|---|---|---|---|
| Cefoxitin (CX) 30 mcg | 66 (19.41%) | 47 (13.82%) | 227 (66.76%) |
| Cefotaxime (CTX) 30 mcg | 323 (95%) | 12 (3.53%) | 5 (1.47%) |
| Ceftazidime (CAZ) 30mcg | 307 (90.29%) | 26 (7.65%) | 7 (2.05%) |
| Cefpodoxime (CPD) 10 mcg | 309 (90.88%) | 17 (5.0%) | 14 (4.11%) |
| Imipenem (IPM) 10mcg | 31 (9.11%) | 56 (16.47%) | 253 (74.41%) |
| Ertapenem (ETP) 10 mcg | 107 (31.47%) | 81 (23.82%) | 152 (44.71%) |
| Meropenem (MRP) 10 mcg | 87 (25.58%) | 79 (23.24%) | 174 (51.18%) |
| Ciprofloxacin (CIP) 5mcg | 152 (44.71%) | 43 (12.65%) | 145 (42.65%) |
| Aztreonam (AT) 30 mcg | 219 (64.41%) | 57 (16.76%) | 64 (18.82%) |
| Amoxyclav (AMC) 30 mcg | 127 (37.35%) | 83 (24.41%) | 130 (38.24%) |
| Piperacillin/Taz 100/10mcg | 114 (33.53%) | 95 (27.94%) | 131 (38.53%) |
| Samples Analysed (No.) | No. of E. coli Isolated | No. (%) ESBL+ | No. (%) CTX-M+ | No. (%) SHV+ | No. (%) TEM+ | No. (%) OXA+ | No. (%) VIM+ | No. (%) NDM+ |
|---|---|---|---|---|---|---|---|---|
| Sardinella longiceps (5) | 32 | 24 (75) | 22 (91.66) | 3 (12.5) | 3 (12.5) | 3 (12.5) | 1 (3.13) | 3 (9.38) |
| Terapon jarbua (5) | 29 | 29 (100) | 18 (62.06) | 1 (3.45) | - | 1 (3.45) | 1 (3.45) | - |
| Otolithes cuvieri (3) | 19 | 19 (100) | 17 (89.47) | 3 (15.79) | 1(5.26) | 1(5.26) | 1 (5.26) | 3 (15.78) |
| Epinephelus diacanthus (2) | 15 | 15 (100) | 13 (86.66) | - | 1 (6.67) | 1(6.67) | - | 1 (6.66) |
| Nemipterus randalli (2) | 22 | 14 (64.28) | 5 (35.71) | 5 (35.71) | - | - | - | 2 (9.09) |
| Mene maculata (2) | 24 | 13 (53.84) | 5 (38.46) | 2 (15.38) | - | - | - | - |
| Coilia dussumieri (3) | 45 | 32 (71.87) | 25 (78.13) | 7 (21.86) | 1 (3.13) | 7 (21.88) | - | 3 (6.66) |
| Harpadon nehereus (4) | 42 | 35 (82.85) | 27 (77.14) | 7 (20) | 1 (2.86) | 4 (11.43) | - | 1 (2.38) |
| Trichiurus lepturus (2) | 21 | 12 (58.33) | 5 (41.67) | 9 (75) | - | - | - | 2 (9.52) |
| Priacanthus hamrur (2) | 19 | 13 (69.23) | 3 (23.08) | 7 (53.85) | - | - | - | - |
| Megalaspis cordyla (2) | 25 | 25 (100) | 9 (36) | 11 (44) | - | 1 (4) | - | 2 (8) |
| Anodontostoma chacunda (3) | 13 | 13 (100) | 4 (30.77) | - | - | 1 (7.69) | - | - |
| Pampus argenteus (2) | 19 | 17 (88.23) | 13 (76.47) | 9 (52.94) | - | 2 (11.76) | - | - |
| Acetes indicus (5) | 27 | 27 (100) | 23 (85.19) | 12 (44.44) | - | 1 (3.70) | - | 2 (7.4) |
| Metapenaeus dobsoni (3) | 26 | 19 (73.68) | 11 (57.89) | 2 (10.53) | 2 (10.53) | 1 (5.26) | - | - |
| Meretrix meretrix (3) | 41 | 18 (44.44) | 9 (50) | 1 (5.56) | - | 1 (5.56) | - | 2 (4.88) |
| Loligo duvauceli (2) | 56 | 15 (26.66) | 3 (20) | - | - | - | - | - |
| Total | 475 | 340 (71.58) | 212 (62.35) | 79 (21.35) | 9 (2.65) | 24 (7.06) | 3 (0.88) | 21 (4.42) |
| No. of Antibiotic | MAR Index | No. (%) of Isolates |
|---|---|---|
| 1 | 0.09 | 9 (2.64) |
| 2 | 0.18 | 7 (2.06) |
| 3 | 0.27 | 71 (20.88) |
| 4 | 0.36 | 11 (3.24) |
| 5 | 0.45 | 102 (30) |
| 6 | 0.55 | 13 (3.82) |
| 7 | 0.64 | 53 (15.59) |
| 9 | 0.82 | 41 (12.06) |
| 11 | 1.00 | 21 (6.18) |
| Isolate No. | Source | Resistance Genotype | Antibiotic Resistance Profile | Antibiotic to which Susceptible |
|---|---|---|---|---|
| EC21 | Sardinella longiceps | blaCTX-M, blaSHV, blaTEM, blaNDM, blaOXA, blaVIM | CX, CTX, CAZ, CPD, IPM, ETP, MRP, CIP, AT, AMC, PIT | CL |
| EC123 | Terapon jarbua | blaCTX-M, blaSHV, blaOXA,blaVIM | CX, CTX, CAZ, CPD, MRP, AT | IPM, CIP, ETP, AMC, PIT |
| EC31 | Otolithes cuvieri | blaCTX-M, blaSHV, blaTEM, blaNDM, blaOXA, blaVIM | CX, CTX, CAZ, CPD, IPM, ETP, MRP, CIP, AT, AMC, PIT | CL |
| EC91 | Epinephelus diacanthus | blaCTX-M, blaTEM, blaNDM, blaOXA | CX, CTX, CAZ, CPD, IPM, ETP, MRP, CIP, AT, AMC, PIT | CL |
| EC81 | Nemipterus randalli | blaCTX-M, blaSHV, blaNDM | CX, CTX, CAZ, CPD, IPM, ETP, MRP, CIP, AT, AMC, PIT | CL |
| EC13 | Mene maculata | blaCTX, blaSHV | CX, CTX, CAZ, CPD, CIP, AT | IPM, MRP, ETP, AMC, PIT |
| EC221 | Coilia dussumieri | blaCTX-M, blaSHV, blaTEM, blaNDM, blaOXA | CX, CTX, CAZ, CPD, IPM, ETP, MRP, CIP, AT, AMC, PIT | CL |
| EC201 | Harpadon nehereus | blaCTX-M, blaSHV, blaTEM, blaNDM, blaOXA | CX, CTX, CAZ, CPD, IPM, ETP, MRP, CIP, AT, AMC, PIT | CL |
| EC303 | Trichiurus lepturus | blaCTX-M, blaSHV, blaNDM | CX, CTX, CAZ, CPD, IPM, ETP, MRP, CIP, AT, AMC, PIT | CL |
| EC48 | Priacanthus hamrur | blaCTX, blaSHV | CX, CTX, CAZ, CPD, CIP, AT, AMC, PIT | IPM, ETP, MRP |
| EC51 | Megalaspis cordyla | blaCTX-M, blaSHV, blaNDM, blaOXA | CX, CTX, CAZ, CPD, IPM, ETP, MRP, CIP, AT, AMC, PIT | CL |
| EC253 | Anodontostoma chacunda | blaCTX, blaOXA | CX, CTX, CAZ, CPD, CIP, AT, ETP | IPM, MRP, AMC, PIT |
| EC271 | Pampus argenteus | blaCTX-M, blaSHV, blaOXA | CX, CTX, CAZ, CPD, CIP, AT, PIT | IPM, ETP, MRP, AMC |
| EC281 | Acetes indicus | blaCTX-M, blaSHV, blaNDM, blaOXA | CX, CTX, CAZ, CPD, IPM, ETP, MRP, CIP, AT, AMC, PIT | CL |
| EC253 | Metapenaeus dobsoni | blaCTX-M, blaSHV, blaTEM, blaOXA | CX, CTX, CAZ, CPD, IPM, CIP, AT, AMC, PIT | IPM, AMC, PIT |
| EC305 | Meretrix meretrix | blaCTX-M, blaSHV, blaNDM, blaOXA | CX, CTX, CAZ, CPD, IPM, ETP, MRP, CIP, AT, AMC, PIT | CL |
| EC131 | Loligo duvauceli | blaCTX-M | CX, CTX, CAZ, CPD, CIP | IPM, ETP, MRP, AMC, PIT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.S.; Nayak, B.B.; Kumar, S.H. High Prevalence of Multiple Antibiotic-Resistant, Extended-Spectrum β-Lactamase (ESBL)-Producing Escherichia coli in Fresh Seafood Sold in Retail Markets of Mumbai, India. Vet. Sci. 2020, 7, 46. https://doi.org/10.3390/vetsci7020046
Singh AS, Nayak BB, Kumar SH. High Prevalence of Multiple Antibiotic-Resistant, Extended-Spectrum β-Lactamase (ESBL)-Producing Escherichia coli in Fresh Seafood Sold in Retail Markets of Mumbai, India. Veterinary Sciences. 2020; 7(2):46. https://doi.org/10.3390/vetsci7020046
Chicago/Turabian StyleSingh, Asem Sanjit, Binaya Bhusan Nayak, and Sanath H. Kumar. 2020. "High Prevalence of Multiple Antibiotic-Resistant, Extended-Spectrum β-Lactamase (ESBL)-Producing Escherichia coli in Fresh Seafood Sold in Retail Markets of Mumbai, India" Veterinary Sciences 7, no. 2: 46. https://doi.org/10.3390/vetsci7020046
APA StyleSingh, A. S., Nayak, B. B., & Kumar, S. H. (2020). High Prevalence of Multiple Antibiotic-Resistant, Extended-Spectrum β-Lactamase (ESBL)-Producing Escherichia coli in Fresh Seafood Sold in Retail Markets of Mumbai, India. Veterinary Sciences, 7(2), 46. https://doi.org/10.3390/vetsci7020046





