Investigation of Melioidosis Outbreak in Pig Farms in Southern Thailand
Abstract
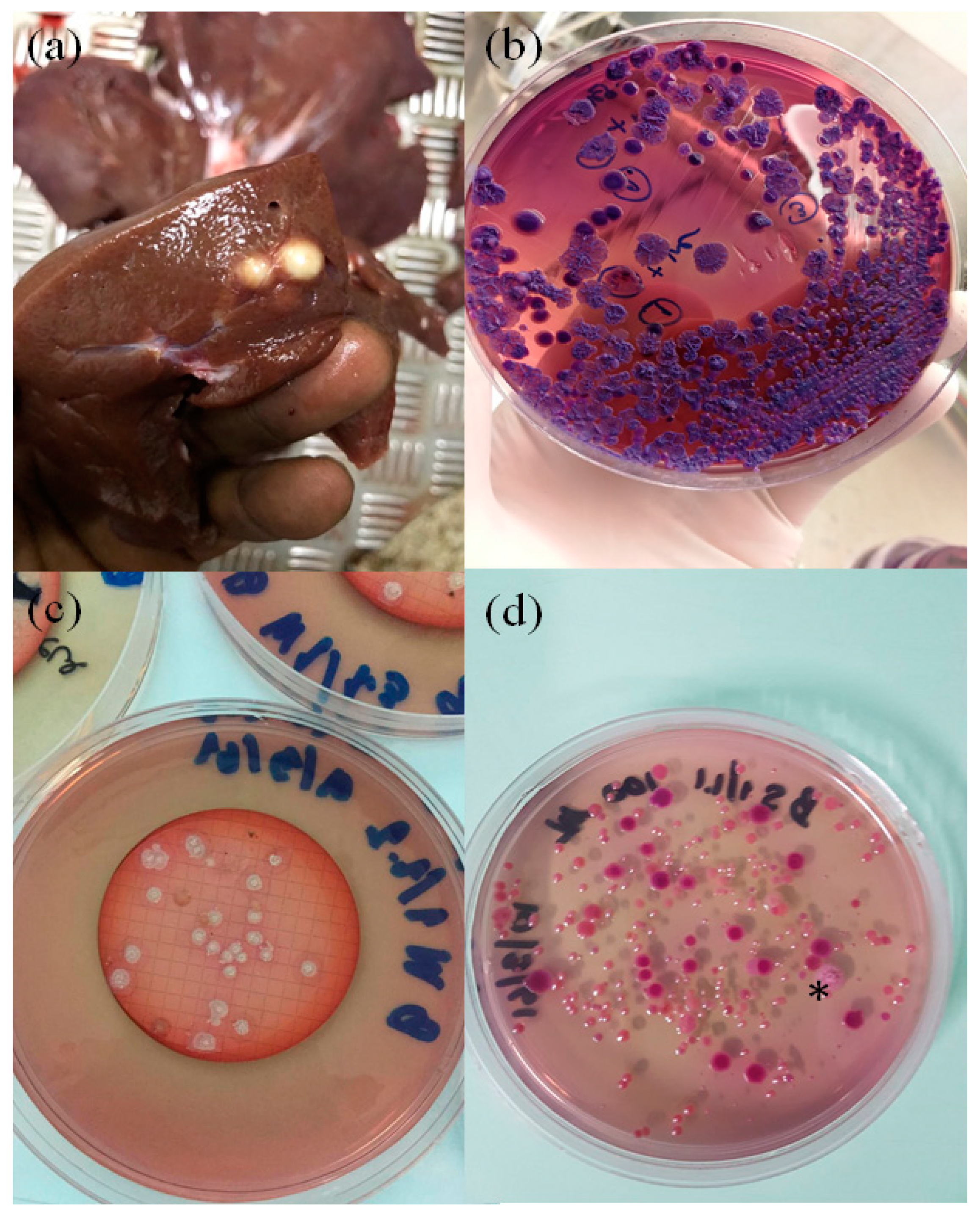
1. Swine Melioidosis
2. Environmental Source of the Infection and Additional Cases
3. Serological Surveys
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Limmathurotsakul, D.; Wongsuvan, G.; Aanensen, D.; Ngamwilai, S.; Saiprom, N.; Rongkard, P.; Thaipadungpanit, J.; Kanoksil, M.; Chantratita, N.; Day, N.P.; et al. Melioidosis caused by Burkholderia pseudomallei in drinking water, Thailand, 2012. Emerg. Infect. Dis. 2014, 20, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Choy, J.L.; Mayo, M.; Janmaat, A.; Currie, B.J. Animal melioidosis in Australia. Acta Trop. 2000, 74, 153–158. [Google Scholar] [CrossRef]
- Limmathurotsakul, D.; Thammasart, S.; Warrasuth, N.; Thapanagulsak, P.; Jatapai, A.; Pengreungrojanachai, V.; Anun, S.; Joraka, W.; Thongkamkoon, P.; Saiyen, P.; et al. Melioidosis in animals, Thailand, 2006-2010. Emerg. Infect. Dis. 2012, 18, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Novak, R.T.; Glass, M.B.; Gee, J.E.; Gal, D.; Mayo, M.J.; Currie, B.J.; Wilkins, P.P. Development and evaluation of a real-time PCR assay targeting the type III secretion system of Burkholderia pseudomallei. J. Clin. Microbiol. 2006, 44, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Limmathurotsakul, D.; Dance, D.A.; Wuthiekanun, V.; Kaestli, M.; Mayo, M.; Warner, J.; Wagner, D.M.; Tuanyok, A.; Wertheim, H.; Yoke Cheng, T.; et al. Systematic review and consensus guidelines for environmental sampling of Burkholderia pseudomallei. PLoS Negl. Trop. Dis. 2013, 7, e2105. [Google Scholar] [CrossRef] [PubMed]
- Godoy, D.; Randle, G.; Simpson, A.J.; Aanensen, D.M.; Pitt, T.L.; Kinoshita, R.; Spratt, B.G. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. J. Clin. Microbiol. 2003, 41, 2068–2079. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R.A.; Atthasampunna, P.; Chulasamaya, M. Pseudomonas (Burkholderia) pseudomallei in Thailand, 1964–1967: Geographic distribution of the organism, attempts to identify cases of active infection, and presence of antibody in representative sera. Am. J. Trop. Med. Hyg. 2000, 62, 232–239. [Google Scholar] [CrossRef] [PubMed]
- McCombie, R.L.; Finkelstein, R.A.; Woods, D.E. Multilocus sequence typing of historical Burkholderia pseudomallei isolates collected in Southeast Asia from 1964 to 1967 provides insight into the epidemiology of melioidosis. J. Clin. Microbiol. 2006, 44, 2951–2962. [Google Scholar] [CrossRef] [PubMed]
- Sprague, L.D.; Neubauer, H. Melioidosis in animals: A review on epizootiology, diagnosis and clinical presentation. J. Vet. Med. B Infect. Dis. Vet. Public Health 2004, 51, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.R. Observation on porcine melioidosis in Malaya. Br. Vet. J. 1962, 118, 421–429. [Google Scholar] [CrossRef]
- Najdenski, H.; Kussovski, V.; Vesselinova, A. Experimental Burkholderia pseudomallei infection of pigs. J. Vet. Med. B Infect. Dis. Vet. Public Health 2004, 51, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Bhengsri, S.; Baggett, H.C.; Jorakate, P.; Kaewpan, A.; Prapasiri, P.; Naorat, S.; Thamthitiwat, S.; Tanwisaid, K.; Chantra, S.; Salika, P.; et al. Incidence of bacteremic melioidosis in eastern and northeastern Thailand. Am. J. Trop. Med. Hyg. 2011, 85, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.D.; Norton, J.H.; Forbes-Faulkner, J.C.; Woodland, G. Melioidosis in an intensive piggery. Aust. Vet. J. 1981, 57, 144–145. [Google Scholar] [CrossRef] [PubMed]
| Date of Incidence | Type of Specimens | No. of Culture Positive Samples/Total | Farm | ST | No. of B. pseudomallei Isolates Analyzed by MLST |
|---|---|---|---|---|---|
| 12/20/17 | Liver abscess | 2/2 * | Farm B | 392 | 5 |
| 3/15/18 | Soil | 1/17 | Farm A | 1721 | 5 |
| 3/15/18 | Soil | 1/17 | Farm B | 392 | 5 |
| 3/15/18 | Water | 0/3 | Farm A | N/A | N/A |
| 3/15/18 | Water | 3/3 | Farm B | 392 | 36 |
| 05/22/18 | Liver abscess | 0/5 * | Not specified | N/A | N/A |
| 05/26/18 | Lung abscess | 5/5 * | Not specified | 51 | 5 |
| 06/05/18 | Spleen abscess | 5/5 * | Farm B | 392 | 5 |
| 06/05/18 | Liver abscess | 5/5 * | Farm B | 392 | 5 |
| 06/15/18 | Liver abscess | 5/5 * | Not specified | 298 | 5 |
| 06/23/18 | Lung abscess | 5/5 * | Not specified | 51 | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwanhian, W.; Jiranantasak, T.; Kessler, A.T.; Tolchinsky, B.E.; Parker, S.; Songsri, J.; Wisessombat, S.; Pukanha, K.; Testamenti, V.A.; Khrongsee, P.; et al. Investigation of Melioidosis Outbreak in Pig Farms in Southern Thailand. Vet. Sci. 2020, 7, 9. https://doi.org/10.3390/vetsci7010009
Kwanhian W, Jiranantasak T, Kessler AT, Tolchinsky BE, Parker S, Songsri J, Wisessombat S, Pukanha K, Testamenti VA, Khrongsee P, et al. Investigation of Melioidosis Outbreak in Pig Farms in Southern Thailand. Veterinary Sciences. 2020; 7(1):9. https://doi.org/10.3390/vetsci7010009
Chicago/Turabian StyleKwanhian, Wiyada, Treenate Jiranantasak, Aleeza T. Kessler, Bryn E. Tolchinsky, Sarah Parker, Jirarat Songsri, Suebtrakool Wisessombat, Kawinsaya Pukanha, Vincentius A. Testamenti, Pacharapong Khrongsee, and et al. 2020. "Investigation of Melioidosis Outbreak in Pig Farms in Southern Thailand" Veterinary Sciences 7, no. 1: 9. https://doi.org/10.3390/vetsci7010009
APA StyleKwanhian, W., Jiranantasak, T., Kessler, A. T., Tolchinsky, B. E., Parker, S., Songsri, J., Wisessombat, S., Pukanha, K., Testamenti, V. A., Khrongsee, P., Sretrirutchai, S., Kaewrakmuk, J., Tangpong, J., & Tuanyok, A. (2020). Investigation of Melioidosis Outbreak in Pig Farms in Southern Thailand. Veterinary Sciences, 7(1), 9. https://doi.org/10.3390/vetsci7010009









