Comparative Morphological Effects of Cold-Blade, Electrosurgical, and Plasma Scalpels on Dog Skin
Abstract
1. Introduction
2. Materials and Methods
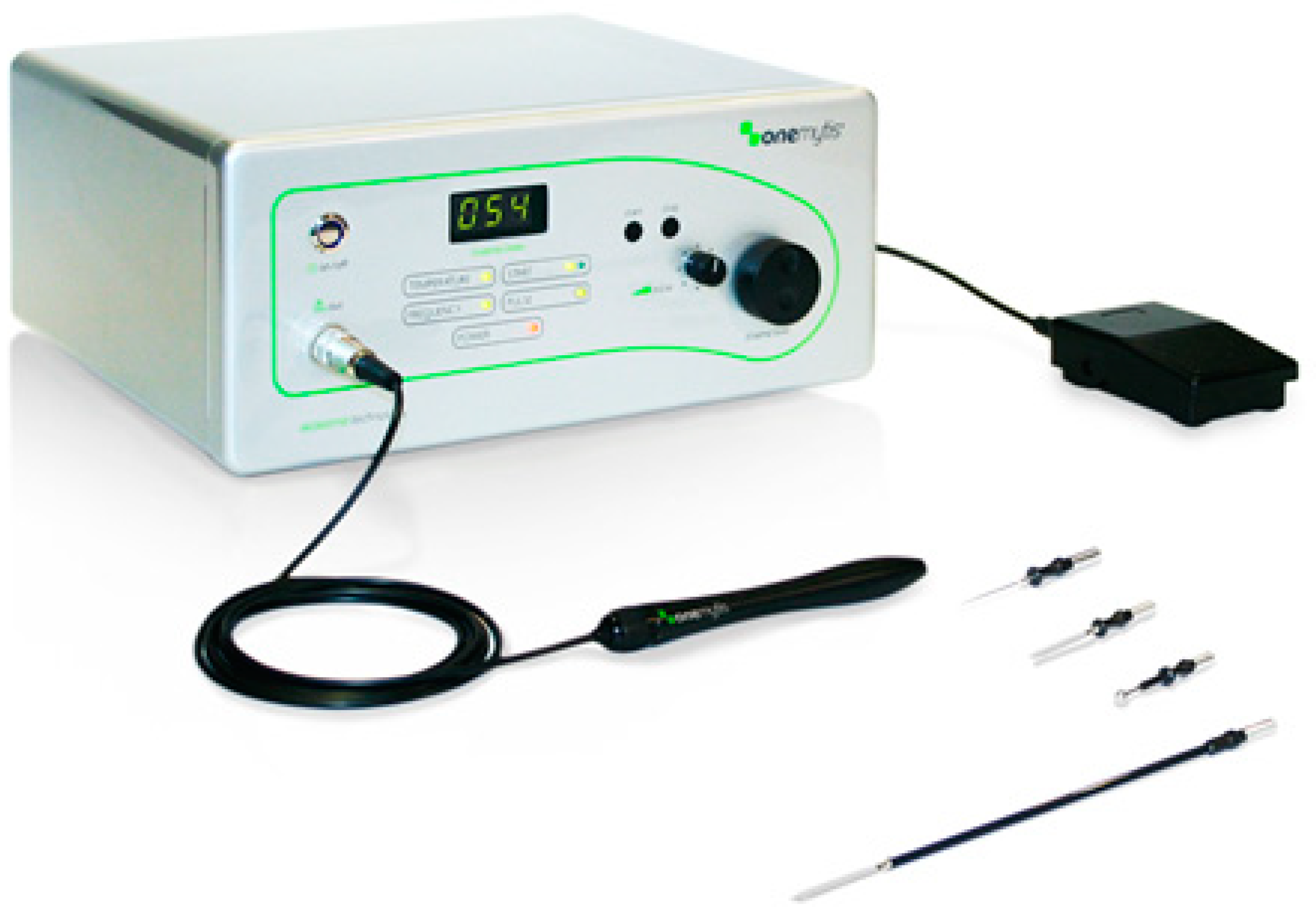
2.1. Devices
2.2. Surgical Technique
2.3. Histology Processing
2.4. Scanning Electron Microscopy
2.5. Histomorphometric Analysis
2.6. Statistical Analysis
3. Results
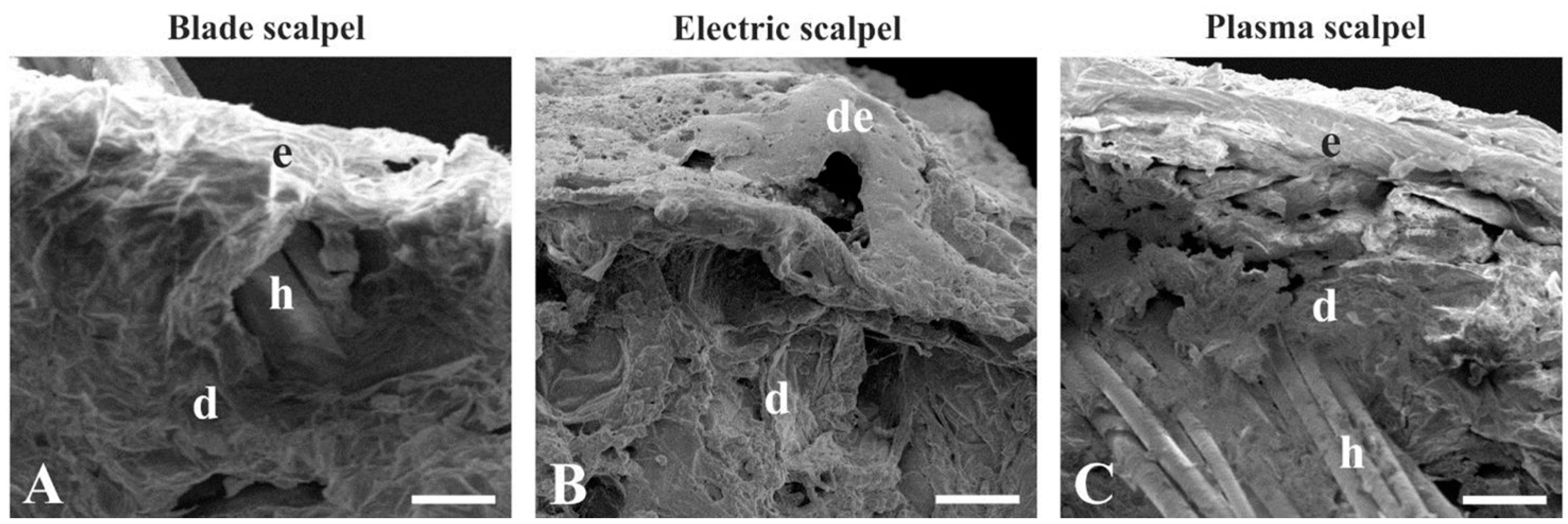
3.1. Scanning Electron Microscopy
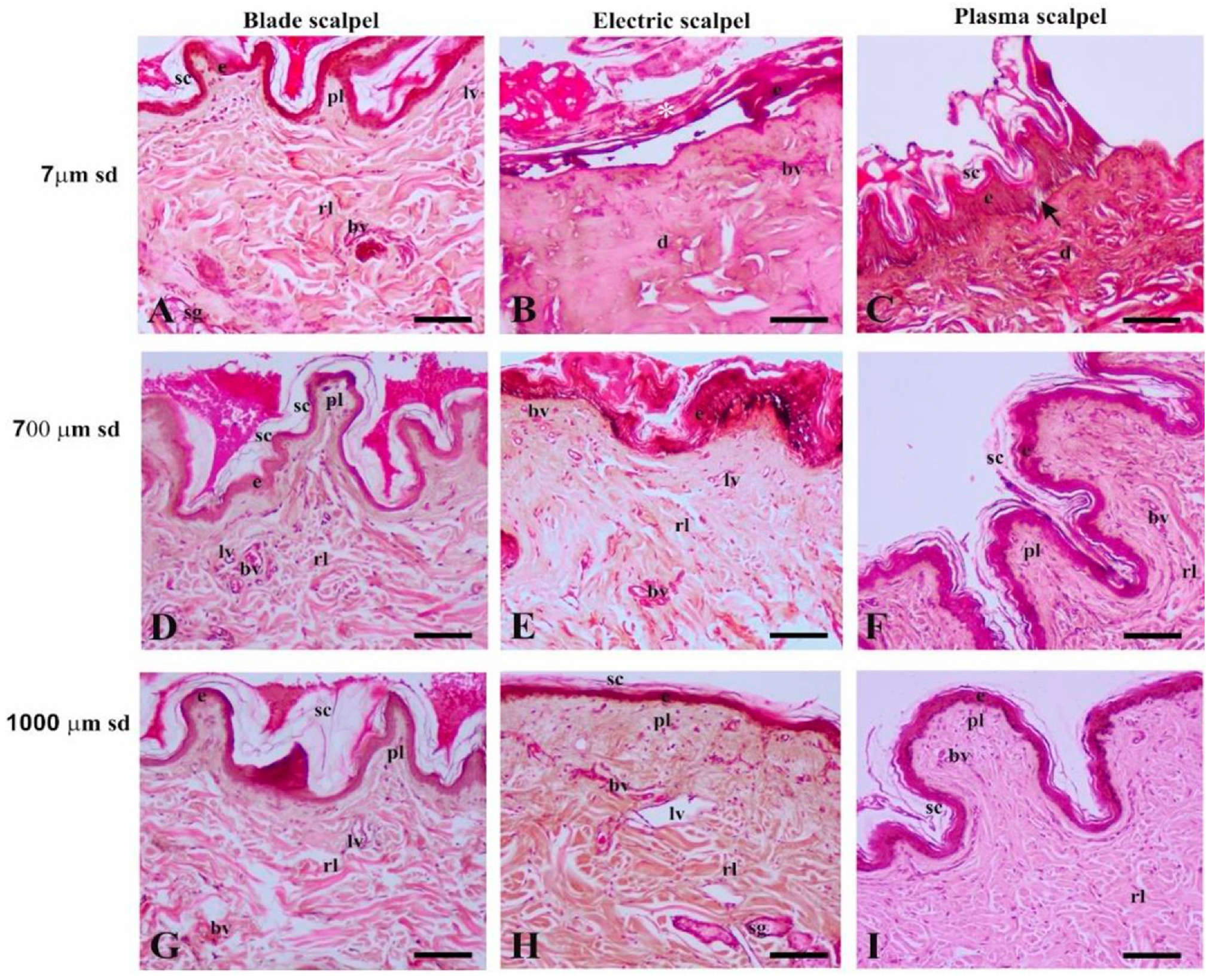
3.2. Light Microscopy
3.2.1. Distance of 7 µm from the Surgical Cutting Surface
3.2.2. Distance of 700 µm from the Surgical Cutting Surface
3.2.3. Distance of 1000 µm from the Surgical Cutting Surface
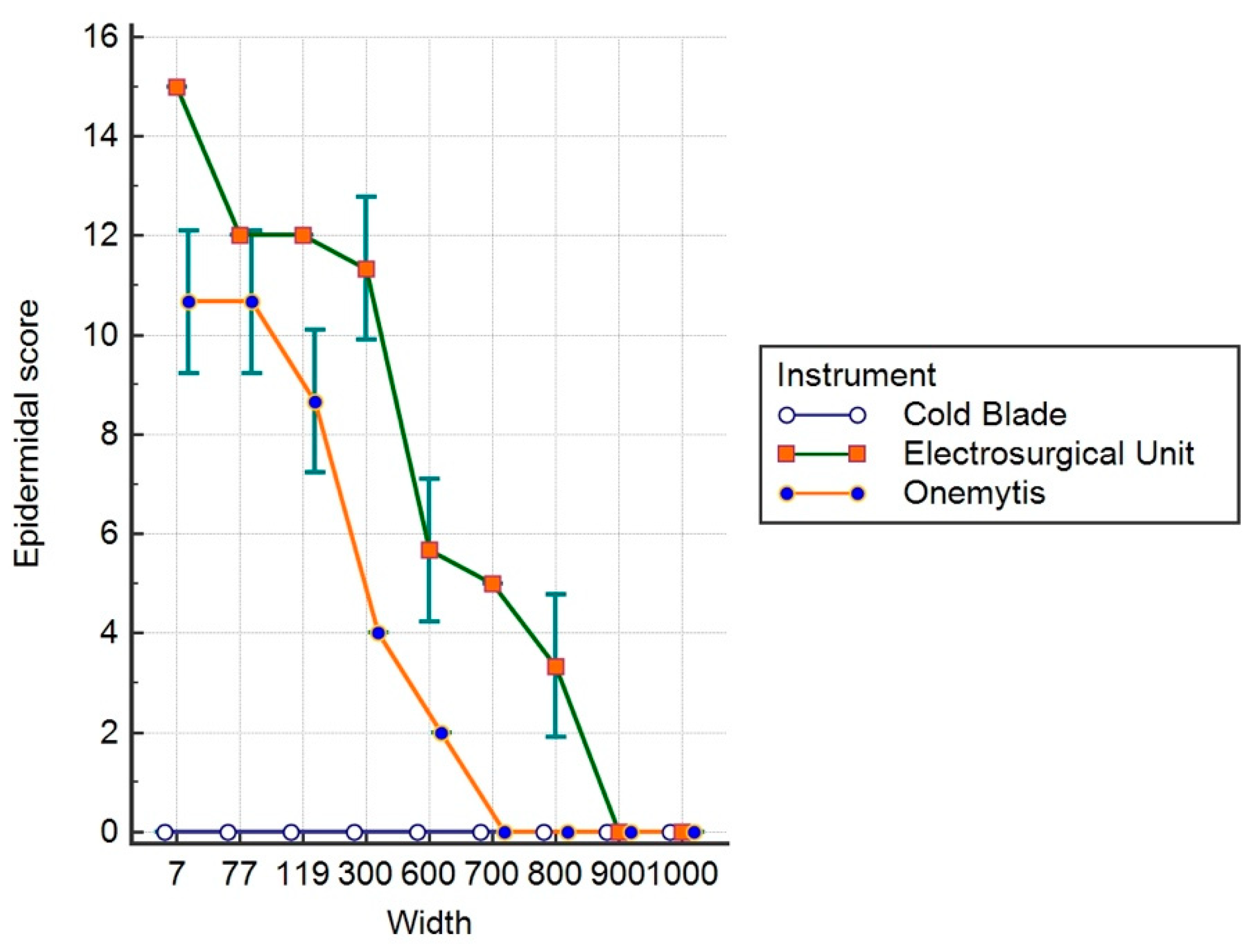
3.3. Histomorphological Damage Quantification
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ekin, M.; Dagdeviren, H.; Caypinar, S.S.; Erdogan, B.; Ayag, M.E.; Cengiz, H.; Yasar, L.; Helvacioglu, C. Comparative cosmetic outcome of surgical incisions created by the PEAK Plasma Blade and a scalpel after cesarean section by Patient and Observer Assessment Scale (POSAS): A randomized double blind study. Taiwan J. Obstet. Gynecol. 2018, 57, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Palanker, D.V.; Miller, J.M.; Marmor, M.F.; Sanislo, S.R.; Huie, P.; Blumenkranz, M.S. Pulsed electron avalanche knife (PEAK) for intraocular surgery. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2673–2678. [Google Scholar]
- Palanker, D.V.; Vankov, A.; Huie, P. Electrosurgery with cellular precision. IEEE Trans. Biomed. Eng. 2008, 55, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Link, W.J.; Incropera, F.P.; Glover, J.L. A plasma scalpel: Comparison of tissue damage and wound healing with electrosurgical and steel scalpels. Arch. Surg. 1976, 111, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Glover, J.L.; Bendick, P.J.; Link, W.J. The use of thermal knives in surgery: Electrosurgery, lasers, plasma scalpel. Curr. Probl. Surg. 1978, 15, 1–78. [Google Scholar] [CrossRef]
- Glover, J.L.; Bendick, P.J.; Link, W.J.; Plunkett, R.J. The plasma scalpel: A new thermal knife. Lasers Surg. Med. 1982, 2, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Vezzoni, A.; De Lorenzi, M.; Vezzoni, L. Utilizzo clinico di un nuovo strumento di elettrocoagulazione al plasma. Veterinaria 2016, 30, 227–234. [Google Scholar]
- Loh, S.A.; Carlson, G.A.; Chang, E.I.; Huang, E.; Palanker, D.; Gurtner, G.C. Comparative healing of surgical incisions created by the PEAK PlasmaBlade, conventional electrosurgery, and a scalpel. Plast. Reconstr. Surg. 2009, 124, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Ruidiaz, M.E.; Messmer, D.; Atmodjo, D.Y.; Vose, J.G.; Huang, E.J.; Kummel, A.C.; Rosenberg, H.L.; Gurtner, G.C. Comparative healing of human cutaneous surgical incisions created by the PEAK PlasmaBlade, conventional electrosurgery, and a standard scalpel. Plast. Reconstr. Surg. 2011, 128, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Marangi, G.F.; Pallara, T.; Lamberti, D.; Perrella, E.; Stilo, F.; De Caridi, G.; Onetti Muda, A.; Persichetti, P. An electrical plasma dissection tool for surgical treatment of chronic ulcers: Results of a prospective randomised trial. Int. Wound J. 2018, 15, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Dubiel, B.; Shires, P.K.; Korvick, D.; Chekan, E.G. Electromagnetic energy sources in surgery. Vet. Surg. 2010, 39, 909–924. [Google Scholar] [CrossRef] [PubMed]
- Guild, G.N., 3rd; Runner, R.P.; Castilleja, G.M.; Smith, M.J.; Vu, C.L. Efficacy of Hybrid Plasma Scalpel in Reducing Blood Loss and Transfusions in Direct Anterior Total Hip Arthroplasty. J. Arthroplast. 2017, 32, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Sutton, C.; Abbott, J. History of power sources in endoscopic surgery. J. Minim. Invasive Gynecol. 2013, 20, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Dogan, L.; Gulcelik, M.A.; Yuksel, M.; Uyar, O.; Erdogan, O.; Reis, E. The effect of plasmakinetic cautery on wound healing and complications in mastectomy. J. Breast Cancer 2013, 16, 198–201. [Google Scholar] [CrossRef]
- Palanker, D.; Vankov, A.; Freyvert, Y.; Huie, P. Pulsed electrical stimulation for control of vasculature: Temporary vasoconstriction and permanent thrombosis. Bioelectromagnetics 2008, 29, 100–107. [Google Scholar] [CrossRef]
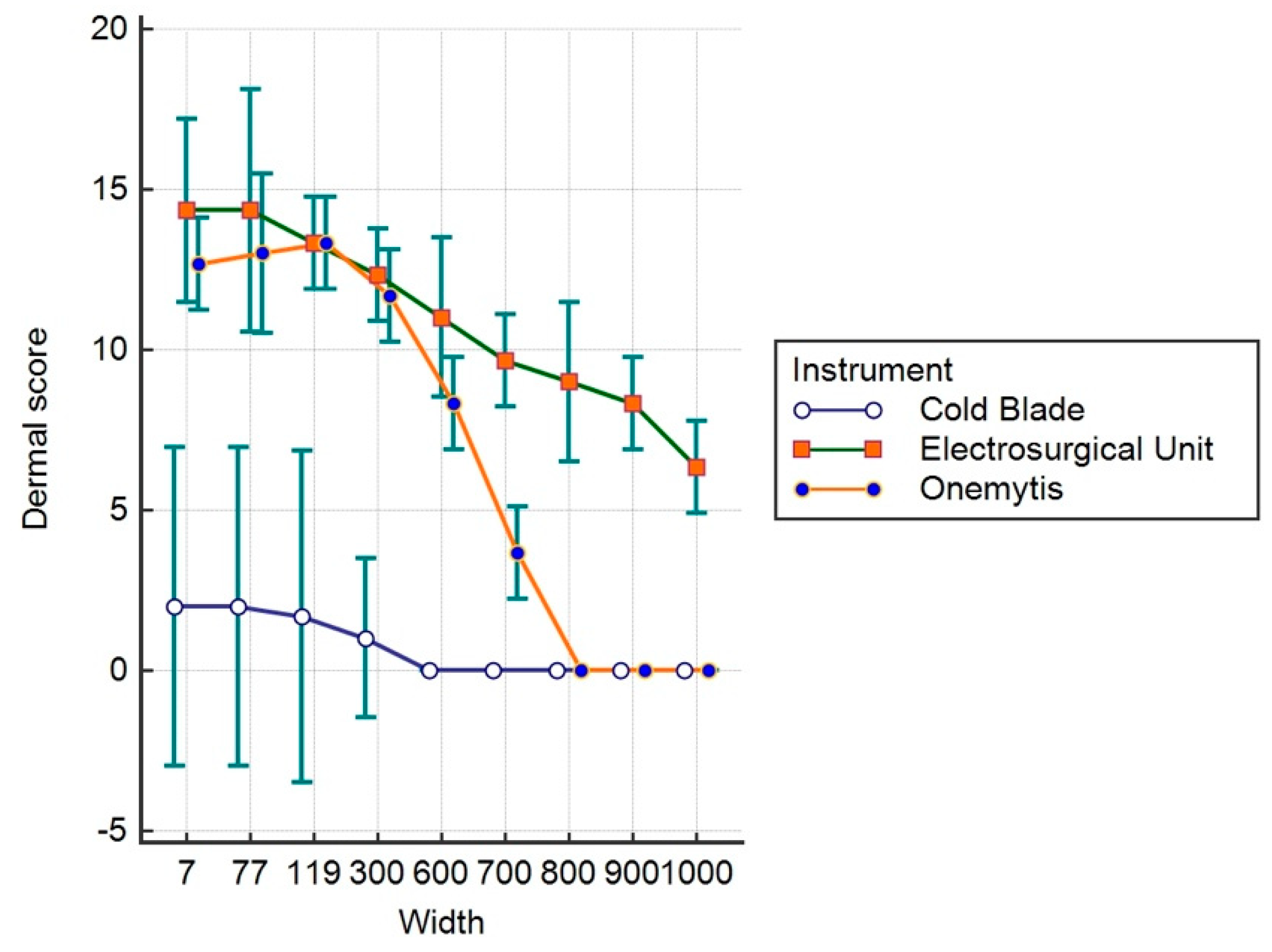
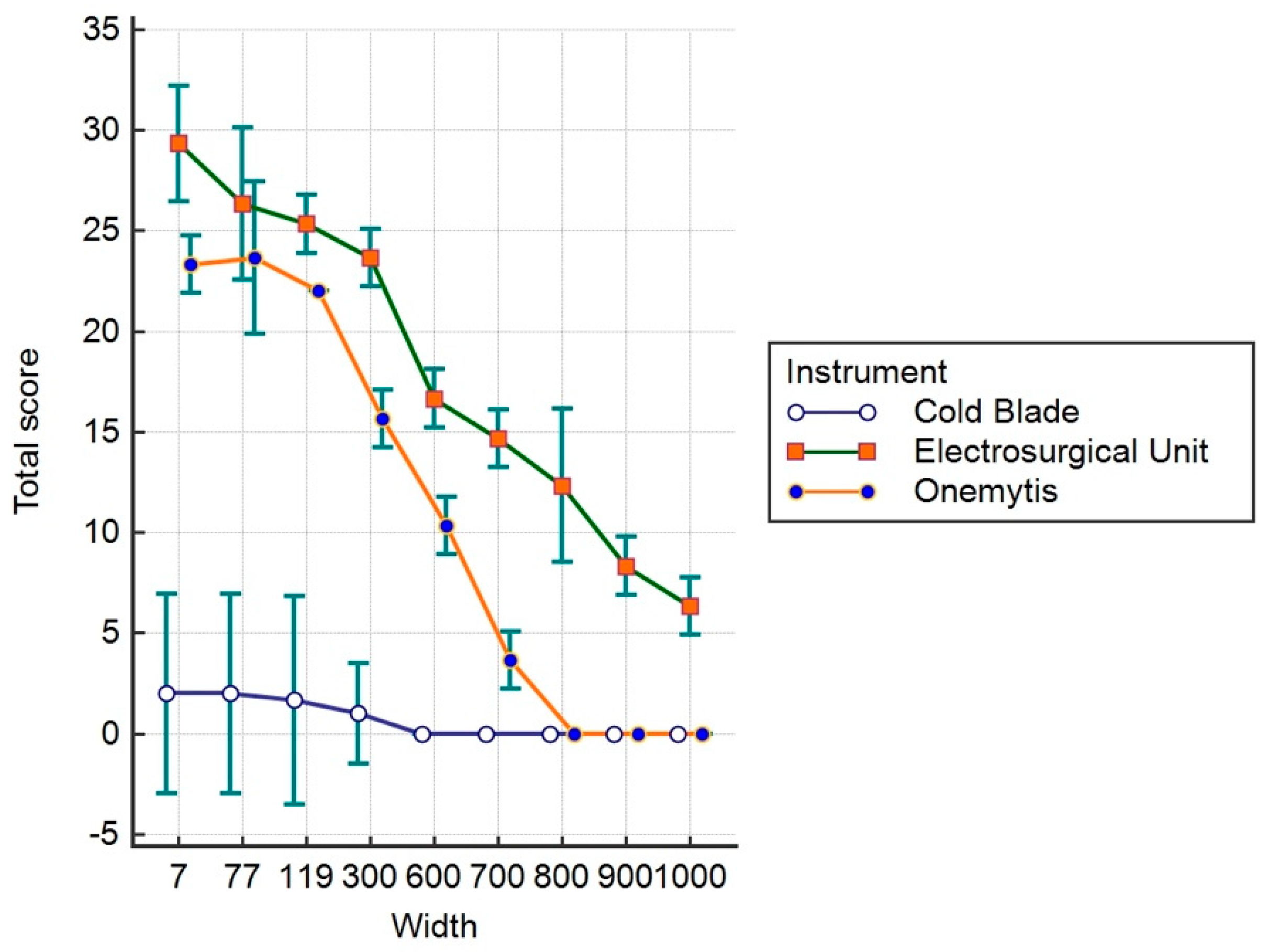
| Device | Mean | Std. Error | 95% Confidence Interval |
|---|---|---|---|
| Cold Blade | 2.53 | 0.05238 | −0.1050 to 0.1050 |
| Electrosurgical Unit | 7.1481 | 0.05238 | 7.0431 to 7.2532 |
| Onemytis® | 4 | 0.05238 | 3.8950 to 4.1050 |
| Device | Mean | Std. Error | 95% Confidence Interval |
|---|---|---|---|
| Cold Blade | 0.7407 | 0.1789 | 0.3821 to 1.0994 |
| Electrosurgical Unit | 1.,963 | 0.1789 | 10.6043 to 11.3216 |
| Onemytis® | 6.963 | 0.1789 | 6.6043 to 7.3216 |
| Device | Mean | Std. Error | 95% Confidence 3nterval |
|---|---|---|---|
| Cold Blade | 0.7407 | 0.1852 | 0.3695 to 1.1120 |
| Electrosurgical Unit | 18.1111 | 0.1852 | 17.7398 to 18.4824 |
| Onemytis® | 10.963 | 0.1852 | 10.5917 to 11.3342 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacitignola, L.; Desantis, S.; Izzo, G.; Staffieri, F.; Rossi, R.; Resta, L.; Crovace, A. Comparative Morphological Effects of Cold-Blade, Electrosurgical, and Plasma Scalpels on Dog Skin. Vet. Sci. 2020, 7, 8. https://doi.org/10.3390/vetsci7010008
Lacitignola L, Desantis S, Izzo G, Staffieri F, Rossi R, Resta L, Crovace A. Comparative Morphological Effects of Cold-Blade, Electrosurgical, and Plasma Scalpels on Dog Skin. Veterinary Sciences. 2020; 7(1):8. https://doi.org/10.3390/vetsci7010008
Chicago/Turabian StyleLacitignola, Luca, Salvatore Desantis, Giovanni Izzo, Francesco Staffieri, Roberta Rossi, Leonardo Resta, and Antonio Crovace. 2020. "Comparative Morphological Effects of Cold-Blade, Electrosurgical, and Plasma Scalpels on Dog Skin" Veterinary Sciences 7, no. 1: 8. https://doi.org/10.3390/vetsci7010008
APA StyleLacitignola, L., Desantis, S., Izzo, G., Staffieri, F., Rossi, R., Resta, L., & Crovace, A. (2020). Comparative Morphological Effects of Cold-Blade, Electrosurgical, and Plasma Scalpels on Dog Skin. Veterinary Sciences, 7(1), 8. https://doi.org/10.3390/vetsci7010008







