R0 Estimation for the African Swine Fever Epidemics in Wild Boar of Czech Republic and Belgium
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sets
2.2. R0 Estimation Method
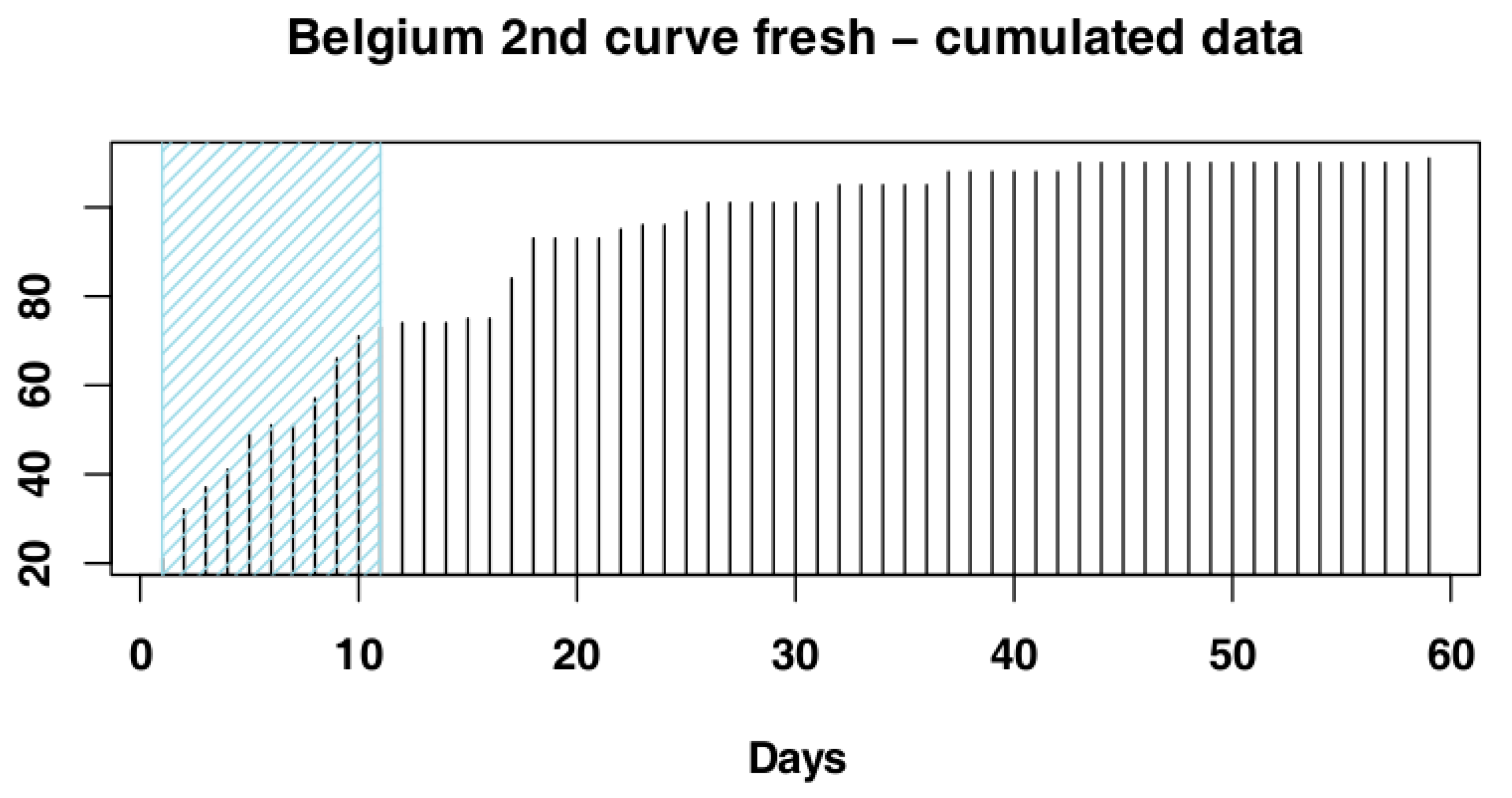
2.3. Data Processing
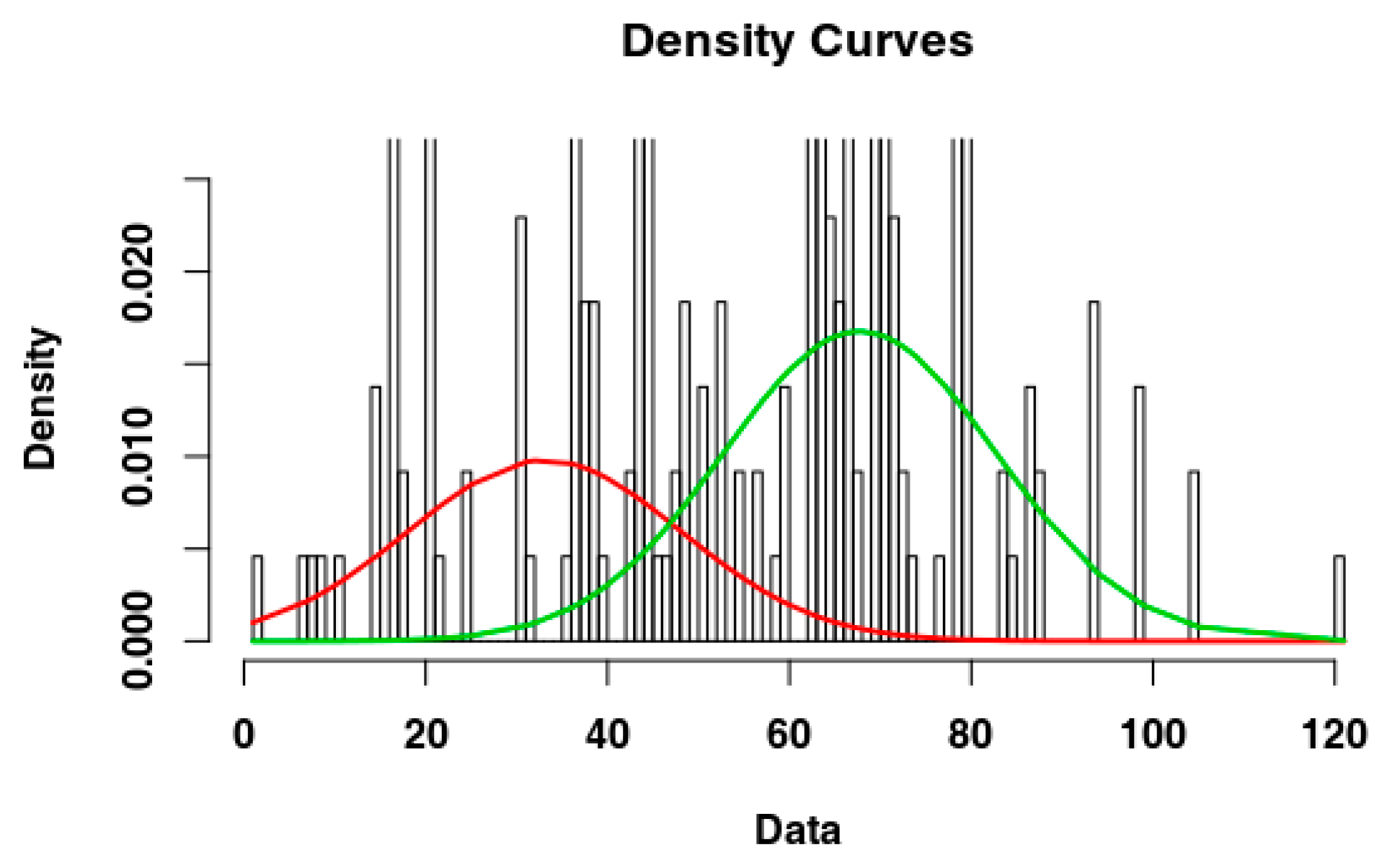
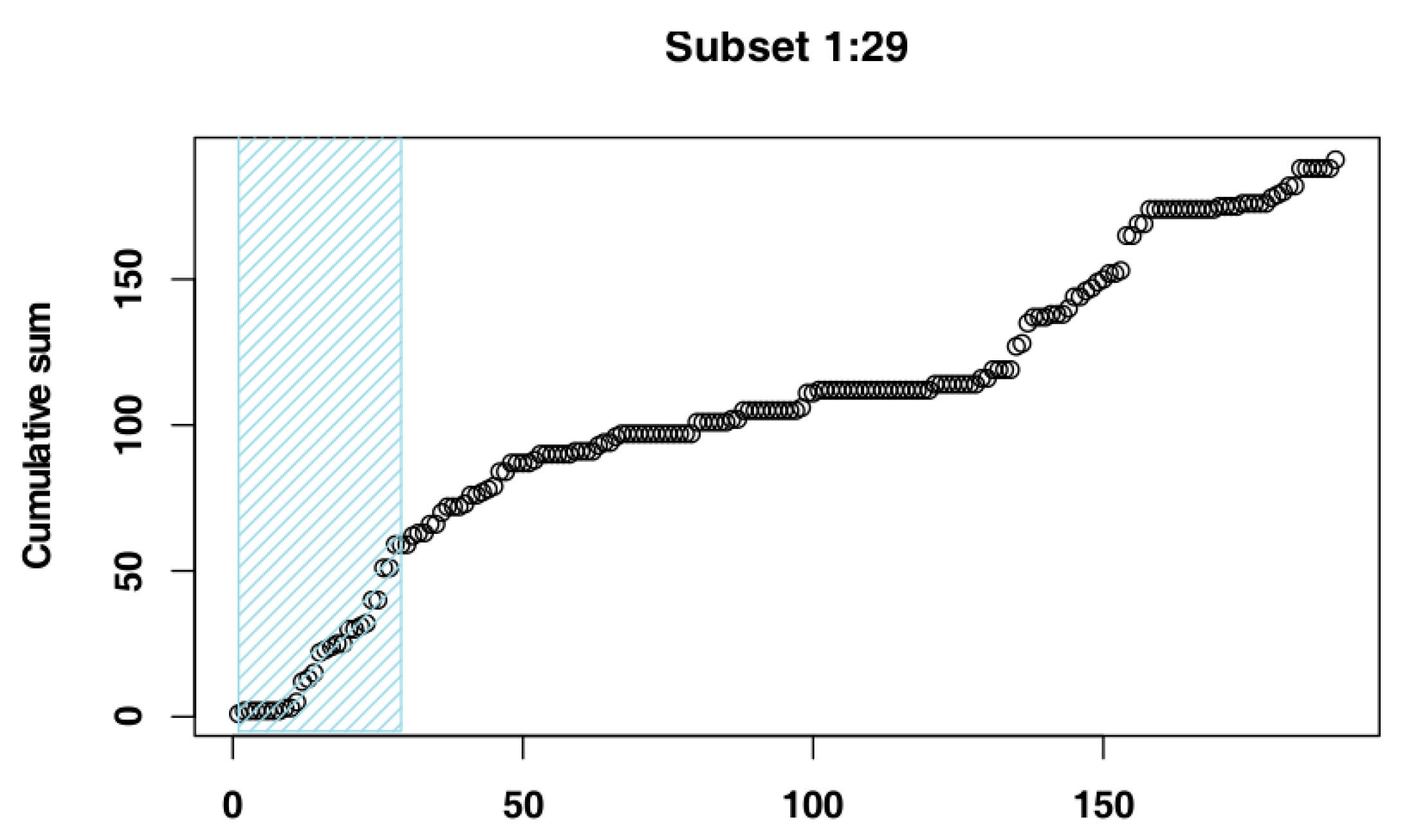
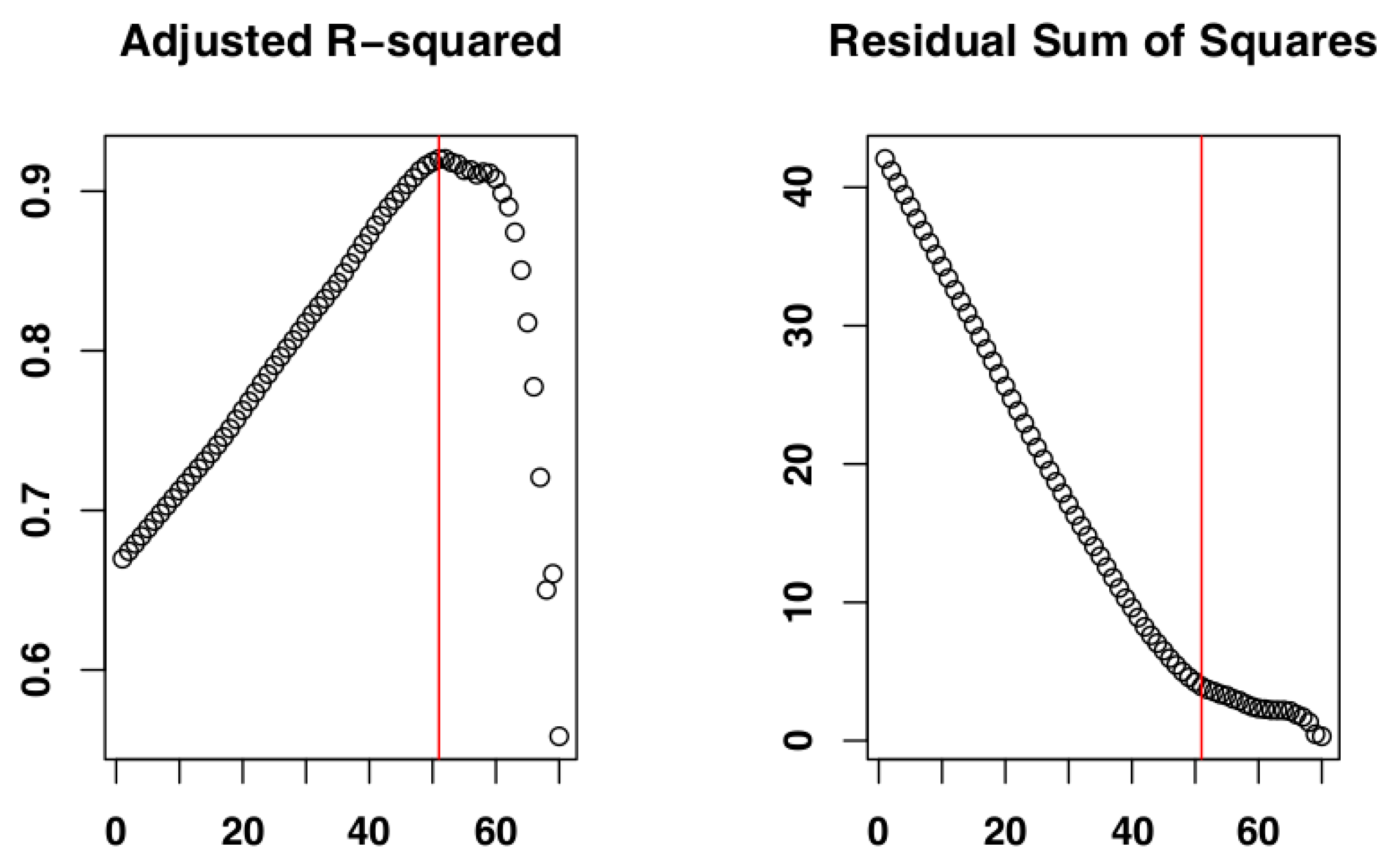
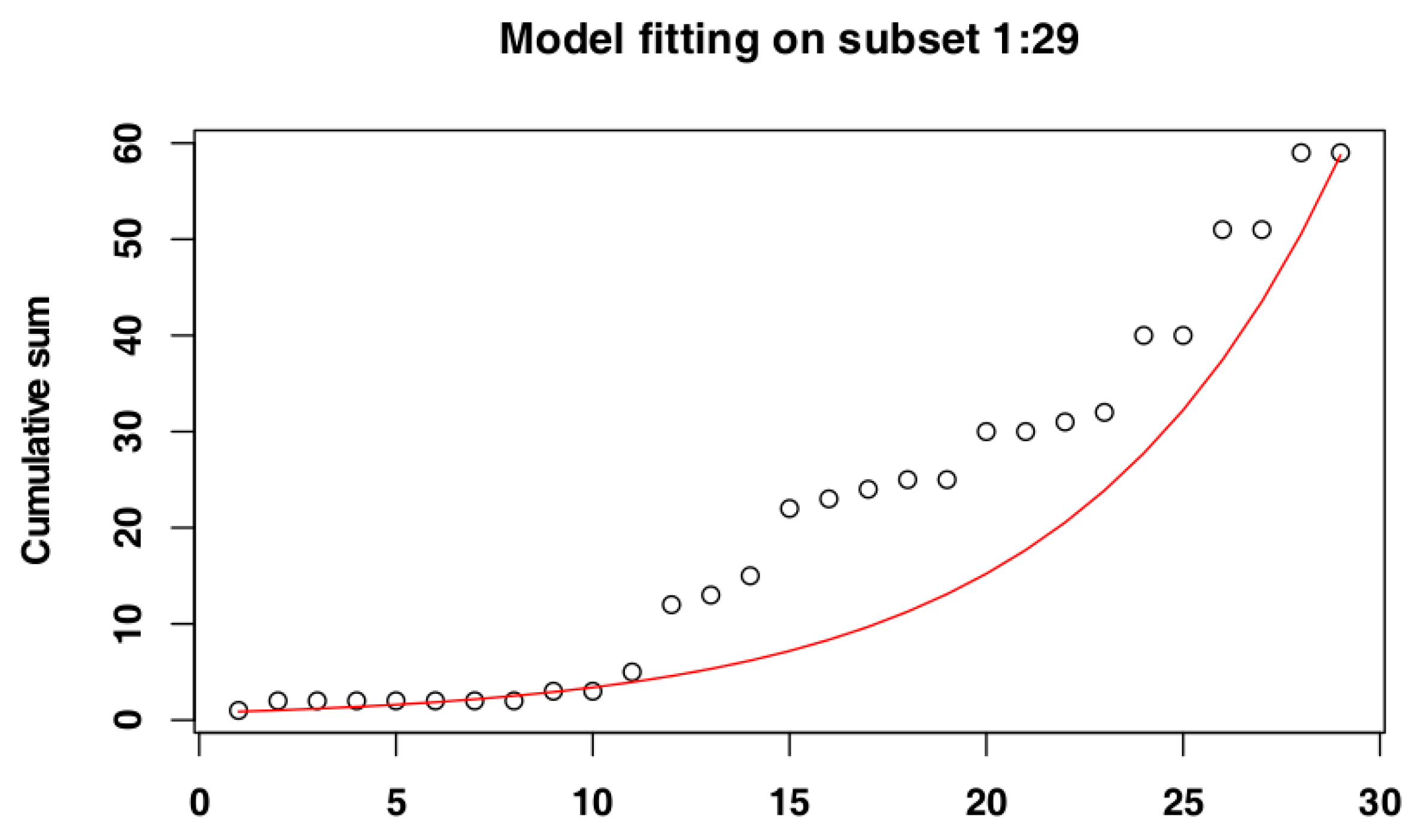
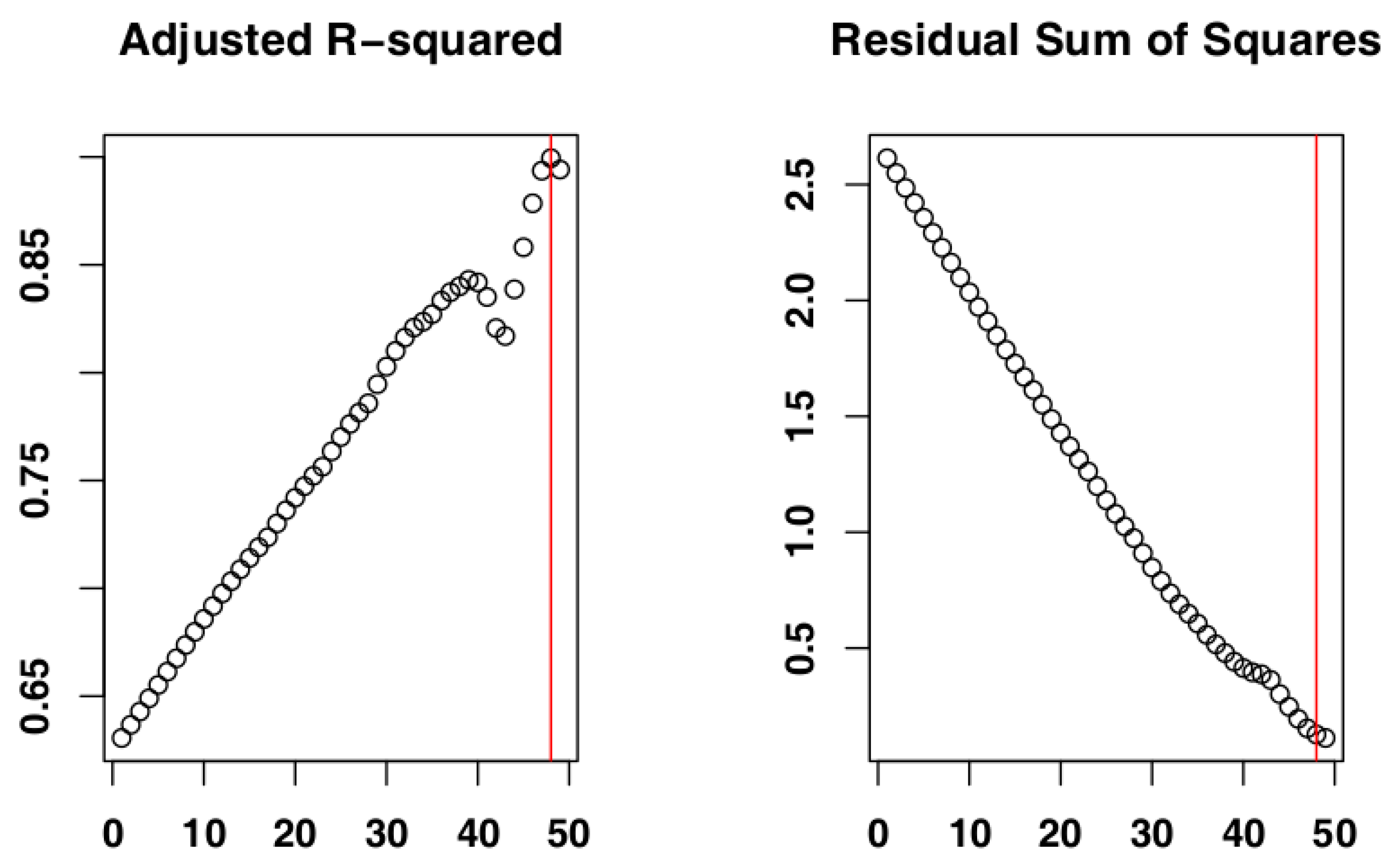
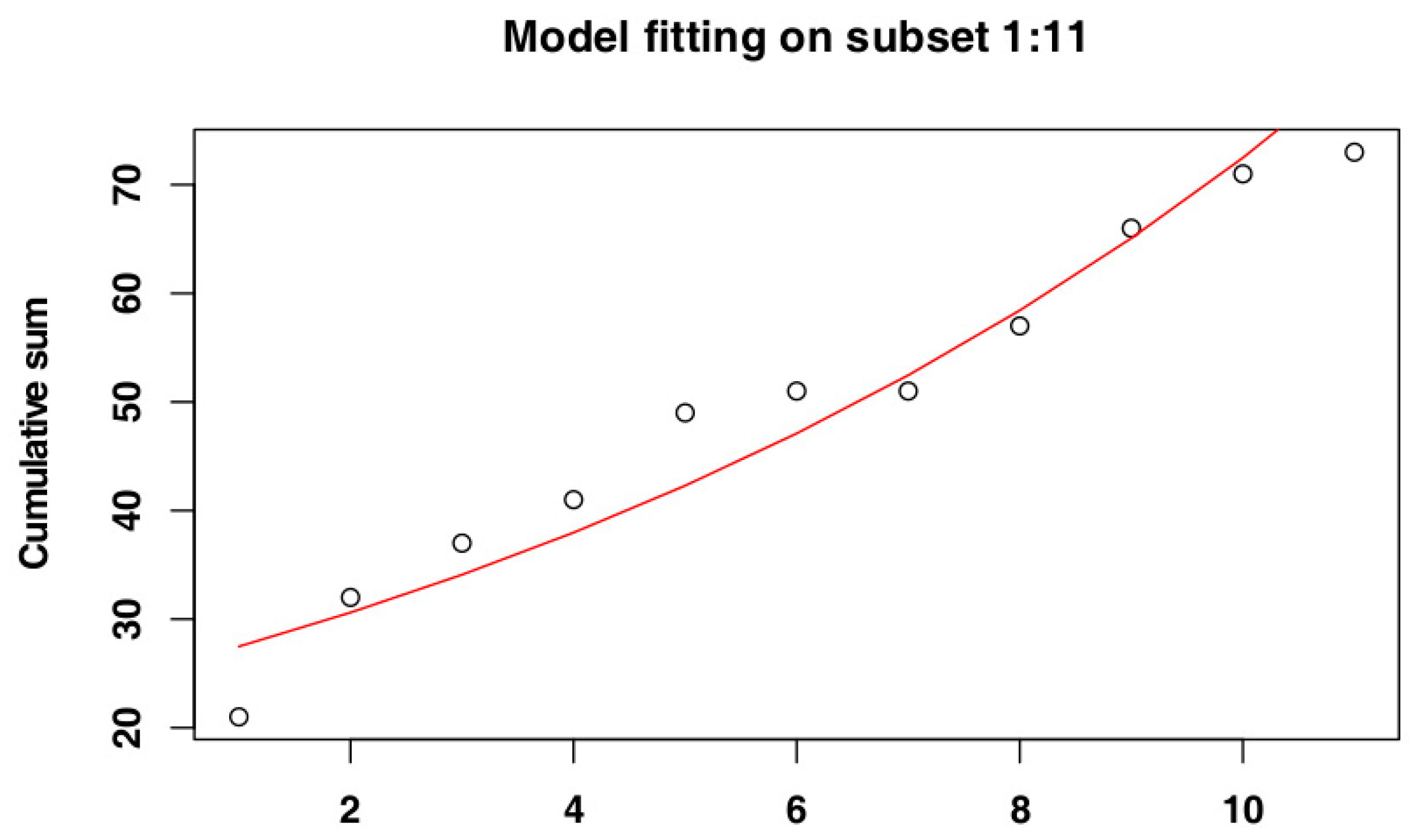
2.4. Identify the Most Suitable Subset of Data
2.5. Doubling Time Calculation
2.6. R0 Calculation
3. Results
3.1. Identify the Most Suitable Subset of Data
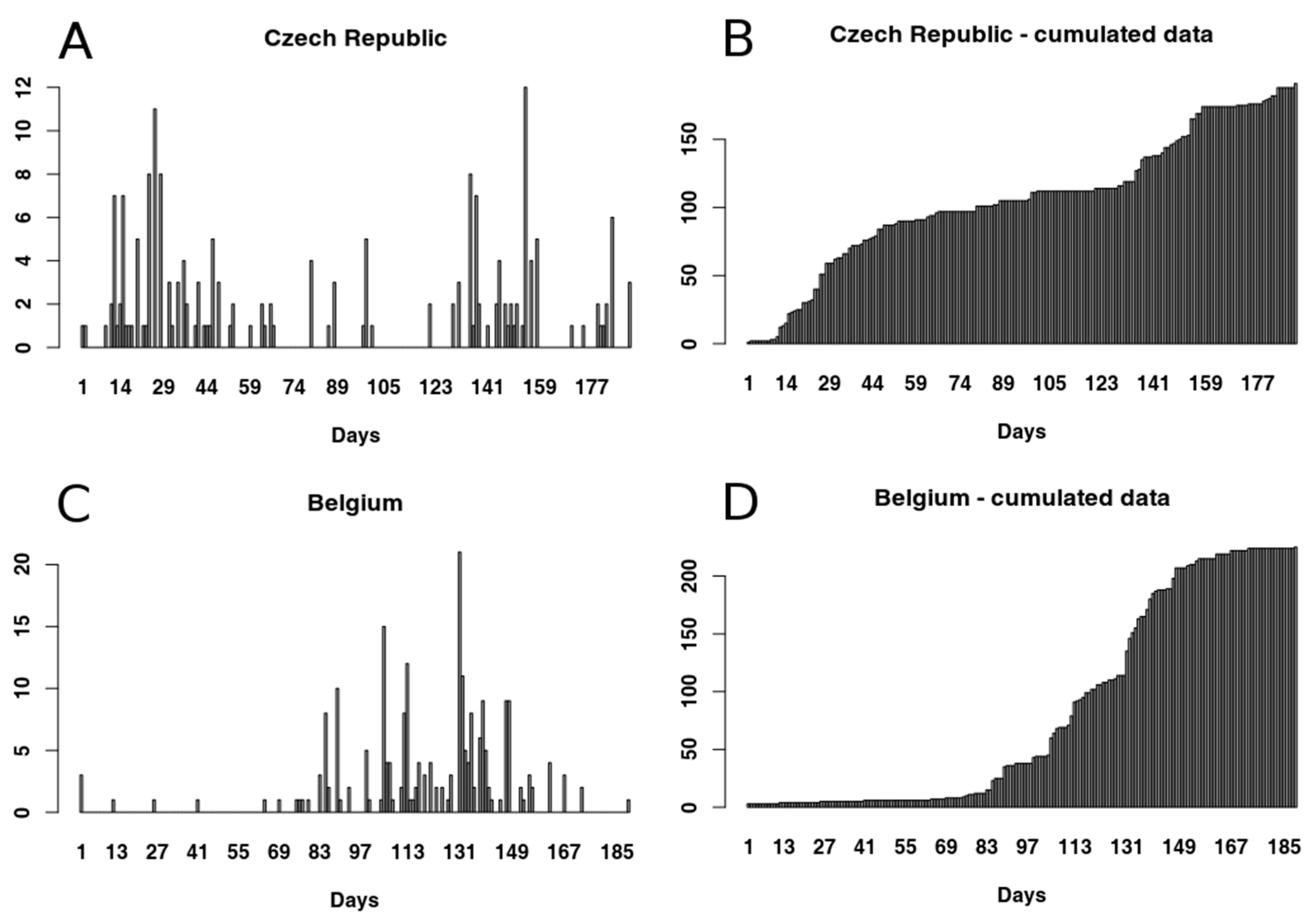
3.1.1. Czech Republic
3.1.2. Belgium
3.2. Doubling Time and R0 Estimation
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Rowlands, R.J.; Michaud, V.; Heath, L.; Hutchings, G.; Oura, C.; Vosloo, W.; Dwarka, R.; Onashvili, T.; Albina, E.; Dixon, L.K. African Swine Fever Virus Isolate, Georgia, 2007. Emerg. Infect. Dis. 2008, 14, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vizcaíno, J.M.; Mur, L.; Martínez-López, B. African swine fever (ASF): Five years around Europe. Vet. Microbiol. 2013, 165, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Stahl, K.; Jori, F.; Vial, L.; Pfeiffer, D.U. African Swine Fever Epidemiology and Control. Annu. Rev. Anim. Biosci. 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Halasa, T.; Bøtner, A.; Mortensen, S.; Christensen, H.; Toft, N.; Boklund, A. Simulating the epidemiological and economic effects of an African swine fever epidemic in industrialized swine populations. Vet. Microbiol. 2016, 193, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Chenais, E.; Depner, K.; Guberti, V.; Dietze, K.; Viltrop, A.; Ståhl, K. Epidemiological considerations on African swine fever in Europe 2014–2018. Porc. Health Manag. 2019, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- EFSA; Boklund, A.; Cay, B.; Depner, K.; Földi, Z.; Guberti, V.; Masiulis, M.; Miteva, A.; More, S.; Olsevskis, E.; et al. Epidemiological analyses of African swine fever in the European Union (November 2017 until November 2018). EFSA J. 2018, 16, e05494. [Google Scholar]
- Guberti, V.; Khomenko, S.; Masiulis, M.; Kerba, S. African Swine Fever in Wild Boar Ecology and Biosecurity; FAO Animal Production and Health Manual; FAO; OIE; EC: Rome, Italy, 2019. [Google Scholar]
- European Commission. European Commission Working Document—Strategic Approach to the Management of African Swine Fever for the EU—SANTE/7113/2015—Rev 11 2019; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Morelle, K.; Jezek, M.; Licoppe, A.; Podgorski, T. Deathbed choice by ASF-infected wild boar can help find carcasses. Transbound. Emerg. Dis. 2019, 66, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Halloran, M.E. Concepts of transmission and dynamics. In Epidemiologic Methods for the Study of Infectious Diseases; Oxford University Press: New York, NY, USA, 2001; pp. 63–64. [Google Scholar]
- Guinat, C.; Porphyre, T.; Gogin, A.; Dixon, L.; Pfeiffer, D.U.; Gubbins, S. Inferring within-herd transmission parameters for African swine fever virus using mortality data from outbreaks in the Russian Federation. Transbound. Emerg. Dis. 2018, 65, e264–e271. [Google Scholar] [CrossRef]
- Gulenkin, V.M.; Korennoy, F.I.; Karaulov, A.K.; Dudnikov, S.A. Cartographical analysis of African swine fever outbreaks in the territory of the Russian Federation and computer modeling of the basic reproduction ratio. Prev. Vet. Med. 2011, 102, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Bett, B.; Henning, J.; Abdu, P.; Okike, I.; Poole, J.; Young, J.; Randolph, T.F.; Perry, B.D. Transmission Rate and Reproductive Number of the H5N1 Highly Pathogenic Avian Influenza Virus During the December 2005–July 2008 Epidemic in Nigeria. Transbound. Emerg. Dis. 2014, 61, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.P.; Maftei, D.; Apostu, C.; Suru, A. Estimation of the basic reproductive number (R0) for epidemic, highly pathogenic avian influenza subtype H5N1 spread. Epidemiol. Infect. 2009, 137, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Apollonio, M.; Andersen, R.; Putman, R. European Ungulates and Their Management in the 21st Century; Cambridge University Press: Cambridge, UK, 2010; ISBN 978-0-521-76061-4. [Google Scholar]
- Keuling, O.; Strauß, E.; Siebert, U. Regulating wild boar populations is “somebody else’s problem”!—Human dimension in wild boar management. Sci. Total Environ. 2016, 554–555, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; May, R.M. Infectious Diseases of Humans: Dynamics and Control; OUP Oxford: New York, NY, USA, 1992; ISBN 978-0-19-854040-3. [Google Scholar]
- Iglesias, I.; Muñoz, M.J.; Montes, F.; Perez, A.; Gogin, A.; Kolbasov, D.; de la Torre, A. Reproductive Ratio for the Local Spread of African Swine Fever in Wild Boars in the Russian Federation. Transbound. Emerg. Dis. 2016, 63, e237–e245. [Google Scholar] [CrossRef] [PubMed]
- Barongo, M.B.; Ståhl, K.; Bett, B.; Bishop, R.P.; Fèvre, E.M.; Aliro, T.; Okoth, E.; Masembe, C.; Knobel, D.; Ssematimba, A. Estimating the Basic Reproductive Number (R0) for African Swine Fever Virus (ASFV) Transmission between Pig Herds in Uganda. PLoS ONE 2015, 10, e0125842. [Google Scholar] [CrossRef] [PubMed]
- Korennoy, F.I.; Gulenkin, V.M.; Gogin, A.E.; Vergne, T.; Karaulov, A.K. Estimating the Basic Reproductive Number for African Swine Fever Using the Ukrainian Historical Epidemic of 1977. Transbound. Emerg. Dis. 2017, 64, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Vynnycky, E.; White, R. An Introduction to Infectious Disease Modelling; OUP Oxford: New York, NY, USA, 2010; ISBN 978-0-19-151136-3. [Google Scholar]
- Sánchez-Cordón, P.J.; Nunez, A.; Neimanis, A.; Wikström-Lassa, E.; Montoya, M.; Crooke, H.; Gavier-Widén, D. African Swine Fever: Disease Dynamics in Wild Boar Experimentally Infected with ASFV Isolates Belonging to Genotype I and II. Viruses 2019, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.; Conraths, F.J.; Blome, S.; Staubach, C.; Sauter-Louis, C. African Swine Fever: Fast and Furious or Slow and Steady? Viruses 2019, 11, 866. [Google Scholar] [CrossRef] [PubMed]
- Bassi, E.; Battocchio, D.; Marcon, A.; Stahlberg, S.; Apollonio, M. Scavenging on Ungulate Carcasses in a Mountain Forest Area in Northern Italy. Mamm. Study 2018, 43, 33–43. [Google Scholar] [CrossRef]
- Iglesias, I.; Perez, A.M.; Sánchez-Vizcaíno, J.M.; Muñoz, M.J.; Martínez, M.; Torre, A.D.L. Reproductive ratio for the local spread of highly pathogenic avian influenza in wild bird populations of Europe, 2005–2008. Epidemiol. Infect. 2011, 139, 99–104. [Google Scholar] [CrossRef] [PubMed]
| Equation | Doubling Time | R0 | |
|---|---|---|---|
| Czech Rep. | y = ex*0.158 | 4.39 | 1.95 |
| Belgium | y = e3.206 + x*0.108 | 6.43 | 1.65 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcon, A.; Linden, A.; Satran, P.; Gervasi, V.; Licoppe, A.; Guberti, V. R0 Estimation for the African Swine Fever Epidemics in Wild Boar of Czech Republic and Belgium. Vet. Sci. 2020, 7, 2. https://doi.org/10.3390/vetsci7010002
Marcon A, Linden A, Satran P, Gervasi V, Licoppe A, Guberti V. R0 Estimation for the African Swine Fever Epidemics in Wild Boar of Czech Republic and Belgium. Veterinary Sciences. 2020; 7(1):2. https://doi.org/10.3390/vetsci7010002
Chicago/Turabian StyleMarcon, Andrea, Annick Linden, Petr Satran, Vincenzo Gervasi, Alain Licoppe, and Vittorio Guberti. 2020. "R0 Estimation for the African Swine Fever Epidemics in Wild Boar of Czech Republic and Belgium" Veterinary Sciences 7, no. 1: 2. https://doi.org/10.3390/vetsci7010002
APA StyleMarcon, A., Linden, A., Satran, P., Gervasi, V., Licoppe, A., & Guberti, V. (2020). R0 Estimation for the African Swine Fever Epidemics in Wild Boar of Czech Republic and Belgium. Veterinary Sciences, 7(1), 2. https://doi.org/10.3390/vetsci7010002





