Role of Tibial Tuberosity Fracture/Fissure through the Maquet Hole in Stifle Osteoarthritis after Porous Tibial Tuberosity Advancement in Dogs at Mid-Term Follow-Up
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
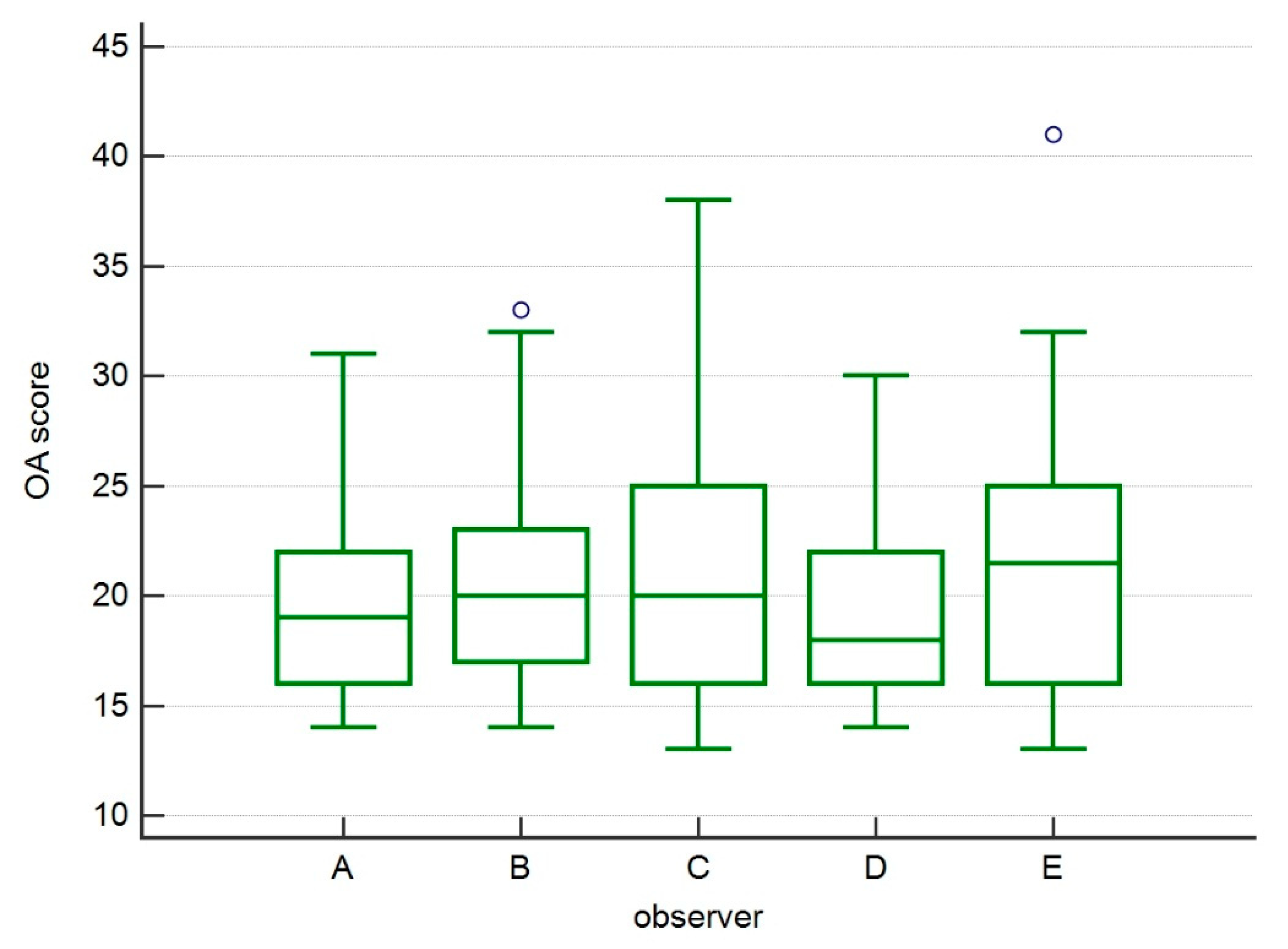
2.2. Evaluation of Osteoarthritis
2.3. Statistical Analysis
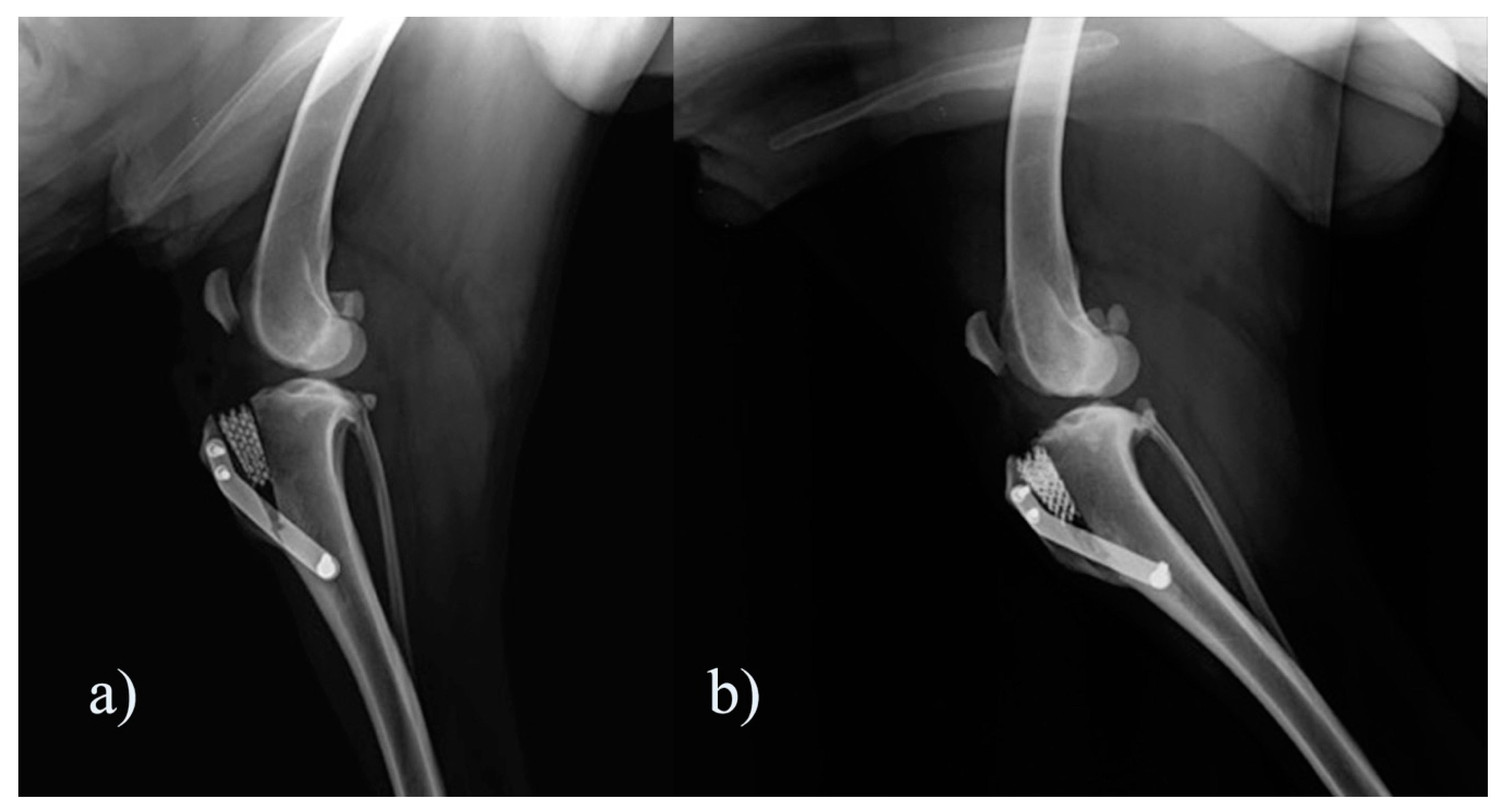
3. Results
3.1. Population
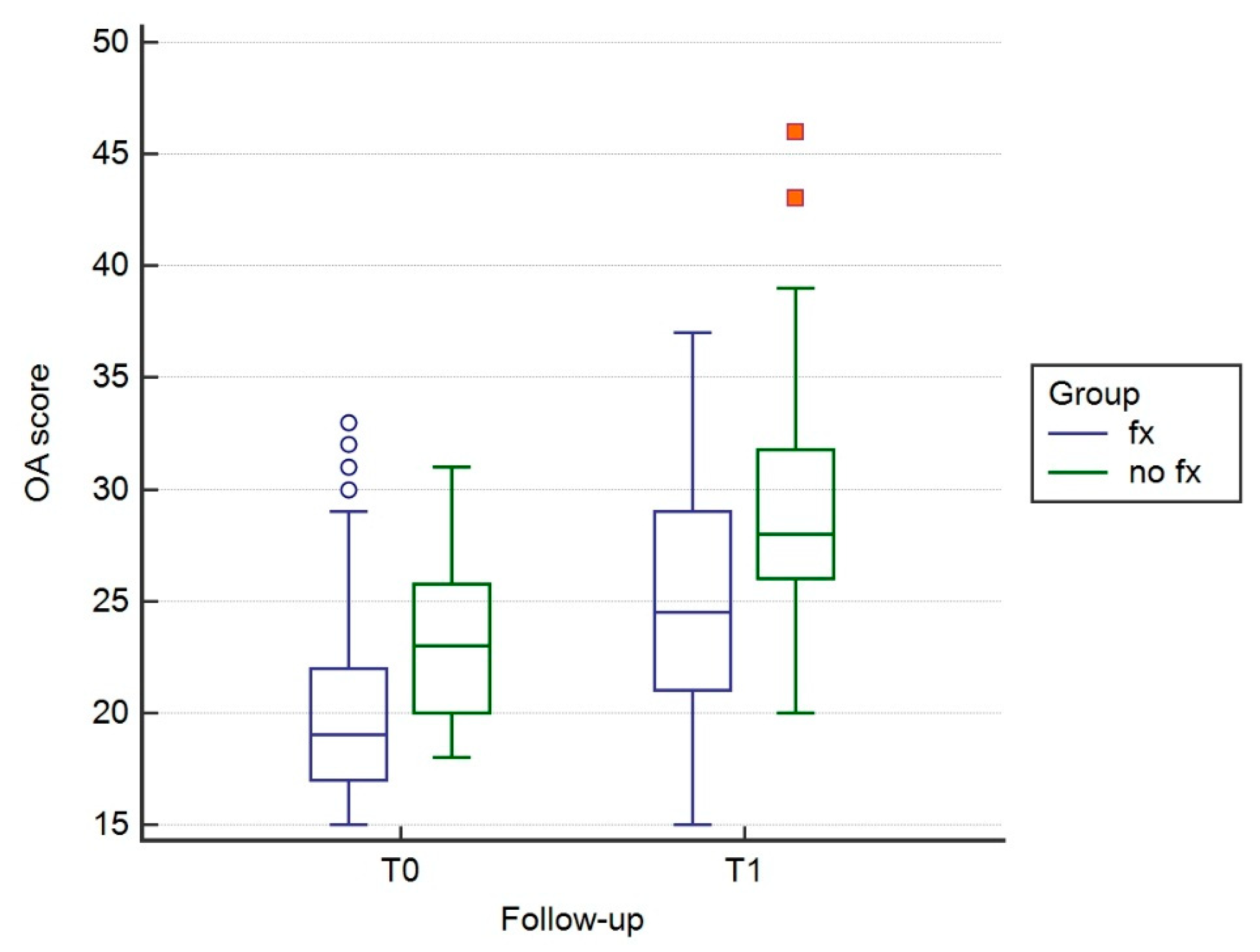
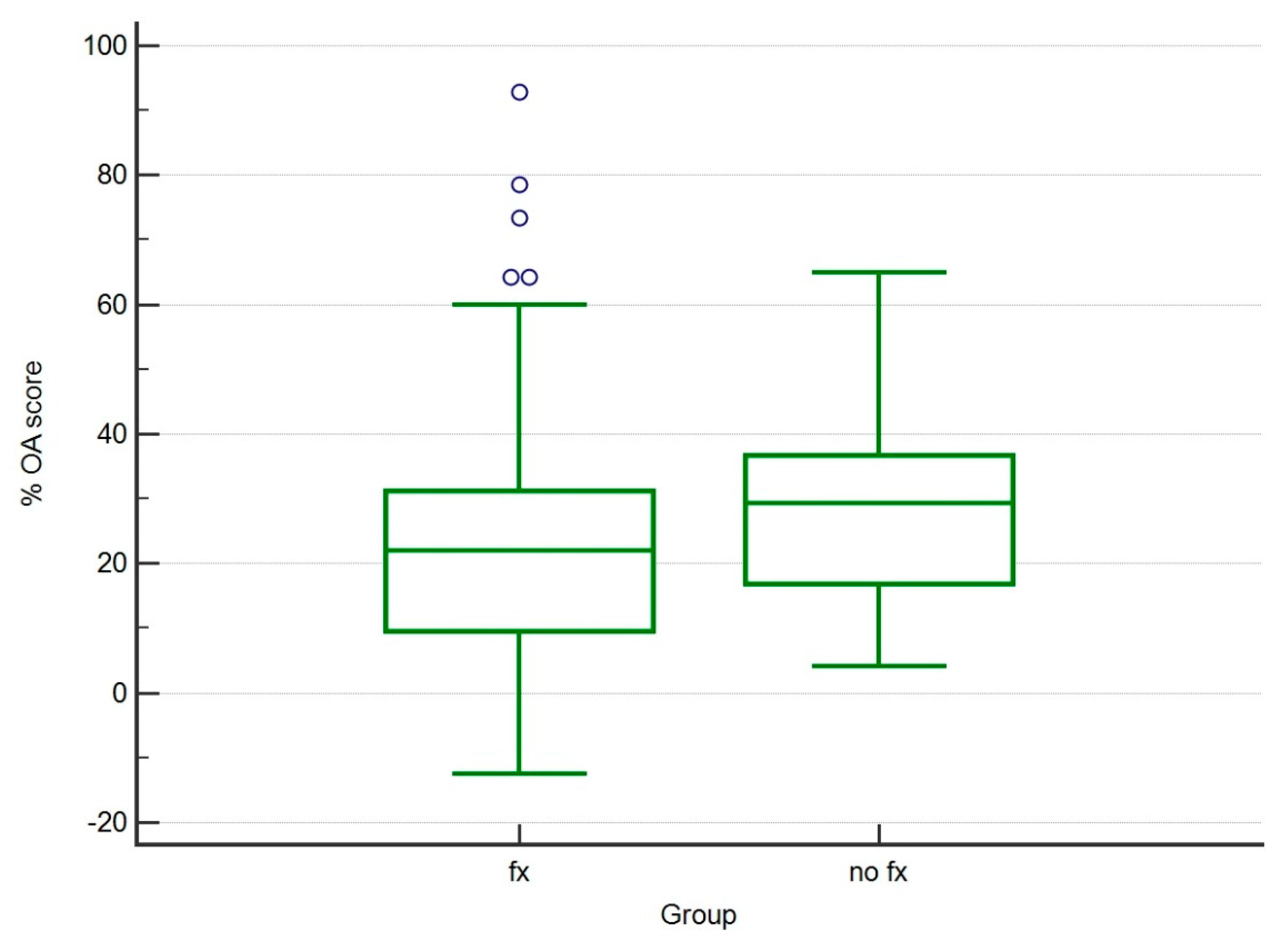
3.2. Evaluation of Osteoarthritis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Apelt, D.; Kowaleski, M.P.; Boudrieau, R.J. Effect of tibial tuberosity advancement on cranial tibial subluxation in canine cranial cruciate-deficient stifle joints: An in vitro experimental study. Vet. Surg. 2007, 36, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Leach, E.S.; Krotscheck, U.; Goode, K.J.; Hayes, G.M.; Bottcher, P. Long-term effects of tibial plateau leveling osteotomy and tibial tuberosity advancement on tibial plateau subchondral bone density in dogs. Vet. Surg. 2018, 47, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Pinna, S.; Lanzi, F.; Cordella, A.; Diana, A. Relationship between the stage of osteoarthritis before and six months after tibial tuberosity advancement procedure in dogs. PLoS ONE 2019, 14, e0219849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montavon, P.M.D.; Damur, D.M.; Tepic, S. Advancement of the tibialtuberosity for the treatment of cranial cruciate deficient canine stifle. In Proceedings of the 1st World Orth Vet Congress, Munich, Germany, 5–8 September 2002. [Google Scholar]
- Etchepareborde, S.; Mills, J.; Busoni, V.; Brunel, L.; Balligand, M. Theoretical discrepancy between cage size and efficient tibial tuberosity advancement in dogs treated for cranial cruciate ligament rupture. Vet. Comp. Orthop. Traumatol. 2011, 24, 27–31. [Google Scholar] [PubMed] [Green Version]
- Retallack, L.M.; Daye, R.M. A modified Maquet-tibial tuberosity advancement technique for treatment of canine cranial cruciate ligament disease: Short term outcome and complications. Vet. Surg. 2018, 47, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, D.E.; Miller, J.M.; Ober, C.P.; Lanz, O.I.; Martin, R.A.; Shires, P.K. Tibial tuberosity advancement in 65 canine stifles. Vet. Comp. Orthop. Traumatol. 2006, 19, 219–227. [Google Scholar]
- Lafaver, S.; Miller, N.A.; Stubbs, W.P.; Taylor, R.A.; Boudrieau, R.J. Tibial tuberosity advancement for stabilization of the canine cranial cruciate ligament-deficient stifle joint: Surgical technique, early results, and complications in 101 dogs. Vet. Surg. 2007, 36, 573–586. [Google Scholar] [CrossRef]
- Stein, S.; Schmoekel, H. Short-term and eight to 12 months results of a tibial tuberosity advancement as treatment of canine cranial cruciate ligament damage. J. Small Anim. Pract. 2008, 49, 398–404. [Google Scholar] [CrossRef]
- Voss, K.; Damur, D.M.; Guerrero, T.; Haessig, M.; Montavon, P.M. Force plate gait analysis to assess limb function after tibial tuberosity advancement in dogs with cranial cruciate ligament disease. Vet. Comp. Orthop. Traumatol. 2008, 21, 243–249. [Google Scholar]
- Steinberg, E.J.; Prata, R.G.; Palazzini, K.; Brown, D.C. Tibial tuberosity advancement for treatment of CrCL injury: Complications and owner satisfaction. J. Am. Anim. Hosp. Assoc. 2011, 47, 250–257. [Google Scholar] [CrossRef]
- De Lima Dantas, B.; Sul, R.; Parkin, T.; Calvo, I. Incidence of complications associated with tibial tuberosity advancement in Boxer dogs. Vet. Comp. Orthop. Traumatol. 2016, 29, 39–45. [Google Scholar] [PubMed]
- Dyall, B.; Schmokel, H. Tibial tuberosity advancement in small-breed dogs using TTA Rapid implants: Complications and outcome. J. Small Anim. Pract. 2017, 58, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Hans, E.C.; Barnhart, M.D.; Kennedy, S.C.; Naber, S.J. Comparison of complications following tibial tuberosity advancement and tibial plateau levelling osteotomy in very large and giant dogs 50 kg or more in body weight. Vet. Comp. Orthop. Traumatol. 2017, 30, 299–305. [Google Scholar] [PubMed]
- Adamiak, Z.; Sobolewski, A.; Walus, G.; Zhalniarovich, Y.; Glodek, J. Single-stage Bilateral Tibial Tuberosity Advancement with Cranial Fixation in an English Bulldog—A Case Report. Top. Companion Anim. Med. 2018, 33, 63–64. [Google Scholar] [CrossRef]
- Serratore, V.R.; Barnhart, M.D. Results and complications after removal of tibial tuberosity advancement cage for treatment of surgical site infections: A retrospective study. Vet. Surg. 2018, 47, 768–773. [Google Scholar] [CrossRef]
- Calvo, I.; Aisa, J.; Chase, D.; Garcia-Fernandez, P.; San Roman, F.; Bennett, D. Tibial tuberosity fracture as a complication of tibial tuberosity advancement. Vet. Comp. Orthop. Traumatol. 2014, 27, 148–154. [Google Scholar]
- Wolf, R.E.; Scavelli, T.D.; Hoelzler, M.G.; Fulcher, R.P.; Bastian, R.P. Surgical and postoperative complications associated with tibial tuberosity advancement for cranial cruciate ligament rupture in dogs: 458 cases (2007–2009). J. Am. Vet. Med. Assoc. 2012, 240, 1481–1487. [Google Scholar] [CrossRef]
- Trisciuzzi, R.; Fracassi, L.; Martin, H.A.; Monopoli Forleo, D.; Amat, D.; Santos-Ruiz, L.; De Palma, E.; Crovace, A.M. 41 Cases of Treatment of Cranial Cruciate Ligament Rupture with Porous TTA: Three Years of Follow Up. Vet. Sci. 2019, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Wessely, M.; Bruhschwein, A.; Schnabl-Feichter, E. Evaluation of Intra- and Inter-observer Measurement Variability of a Radiographic Stifle Osteoarthritis Scoring System in Dogs. Vet. Comp. Orthop. Traumatol. 2017, 30, 377–384. [Google Scholar] [CrossRef]
- Lineberger, J.A.; Allen, D.A.; Wilson, E.R.; Tobias, T.A.; Shaiken, L.G.; Shiroma, J.T.; Biller, D.S.; Lehenbauer, T.W. Comparison of radiographic arthritic changes associated with two variations of tibial plateau leveling osteotomy. Vet. Comp. Orthop. Traumatol. 2005, 18, 13–17. [Google Scholar]
- Morgan, J.P.; Voss, K.; Damur, D.M.; Guerrero, T.; Haessig, M.; Montavon, P.M. Correlation of radiographic changes after tibial tuberosity advancement in dogs with cranial cruciate-deficient stifles with functional outcome. Vet. Surg. 2010, 39, 425–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, T.L.; Allen, D.A.; Monteith, G.J. Clinical assessment following tibial tuberosity advancement in 28 stifles at 6 months and 1 year after surgery. Can. Vet. J. 2013, 54, 249–254. [Google Scholar] [PubMed]
- Lefebvre, M.D.; Broux, O.R.; Barthelemy, N.P.; Hamon, M.; Moyse, E.V.; Bouvy, B.M.; Balligand, M.H. Risk factors for tibial damage associated with the modified Maquet technique in 174 stifles. Vet. Surg. 2018, 47, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudrieau, R.J. Tibial plateau leveling osteotomy or tibial tuberosity advancement? Vet. Surg. 2009, 38, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Proot, J.L.; Corr, S.A. Clinical audit for the tibial tuberosity advancement procedure: Establishing the learning curve and monitoring ongoing performance for the tibial tuberosity advancement procedure using the cumulative summation technique. Vet. Comp. Orthop. Traumatol. 2013, 26, 280–284. [Google Scholar] [PubMed]
| Group | Follow-Up | Mean | SD |
|---|---|---|---|
| No Fx | T0 | 19.3 | 3.2751 |
| T1 | 24.9 | 5.6253 | |
| Fx | T0 | 17 | 3.6978 |
| T1 | 21.1 | 4.8414 |
| Group | % OA Score Increment | SD |
|---|---|---|
| No Fx | 29.20 | 15.02 |
| Fx | 25.31 | 22.89 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crovace, A.M.; Staffieri, F.; Monopoli, D.; Artiles, A.; Fracassi, L.; Crovace, A.; Lacitignola, L. Role of Tibial Tuberosity Fracture/Fissure through the Maquet Hole in Stifle Osteoarthritis after Porous Tibial Tuberosity Advancement in Dogs at Mid-Term Follow-Up. Vet. Sci. 2020, 7, 1. https://doi.org/10.3390/vetsci7010001
Crovace AM, Staffieri F, Monopoli D, Artiles A, Fracassi L, Crovace A, Lacitignola L. Role of Tibial Tuberosity Fracture/Fissure through the Maquet Hole in Stifle Osteoarthritis after Porous Tibial Tuberosity Advancement in Dogs at Mid-Term Follow-Up. Veterinary Sciences. 2020; 7(1):1. https://doi.org/10.3390/vetsci7010001
Chicago/Turabian StyleCrovace, Alberto Maria, Francesco Staffieri, Donato Monopoli, Alejandro Artiles, Laura Fracassi, Antonio Crovace, and Luca Lacitignola. 2020. "Role of Tibial Tuberosity Fracture/Fissure through the Maquet Hole in Stifle Osteoarthritis after Porous Tibial Tuberosity Advancement in Dogs at Mid-Term Follow-Up" Veterinary Sciences 7, no. 1: 1. https://doi.org/10.3390/vetsci7010001
APA StyleCrovace, A. M., Staffieri, F., Monopoli, D., Artiles, A., Fracassi, L., Crovace, A., & Lacitignola, L. (2020). Role of Tibial Tuberosity Fracture/Fissure through the Maquet Hole in Stifle Osteoarthritis after Porous Tibial Tuberosity Advancement in Dogs at Mid-Term Follow-Up. Veterinary Sciences, 7(1), 1. https://doi.org/10.3390/vetsci7010001






