Abstract
Superficial corneal ulcers that fail to heal within a normal time period and are refractory to conventional therapy in dogs are common in veterinary practice. Different etiologies can lead to this result, including spontaneous chronic corneal epithelial defects (SCCEDs) and ulcerative keratitis associated with bullous keratopathy. Thus, there is an urgent need to find new therapeutic approaches such as matrix therapy replacement. To determine the efficacy of a new ophthalmic treatment (Clerapliq®) for SCCEDs and ulcerative keratitis associated with bullous keratopathy, a total of 11 dogs referred to the clinic because of nonhealing erosive ulcers after a classic primary treatment were enrolled to get this new treatment. Dogs underwent ophthalmic exams and 7 dogs (10 eyes) were diagnosed with superficial ulceration and 4 dogs (5 eyes) with bullous keratopathy due to endothelial dystrophy/degeneration. They received eye drops of Clerapliq® every 3 days until recovery. The results showed that the corneas with recurrences of the ulcers were resolved predominantly by using Clerapliq® every 3 days in 83.3% of the cases during a period of treatment ranging between 6 to 35 days. Therefore, this new approach using matrix therapy regenerating technology in treating superficial ulcers and bullous keratopathy in dogs can be successfully considered as an adjunctive therapy.
1. Introduction
Over the past few years, a new type of matrix therapy agent named ReGeneraTing Agent (RGTA®) has provided encouraging results, accelerating the healing of chronic skin ulcers of diabetic or vascular origin [1,2,3,4]. RGTA® is a set of molecules, chemically engineered polymers, that are specifically designed to replace degraded heparan sulfate molecules in the injured matrix compartment. Therefore, they are considered as heparan sulfate mimetics based on their chemical structures and functions [3,4,5,6]. RGTA® protects naturally existing structural and signaling proteins, and in doing so, creates a cellular microenvironment favorable to healing, thereby enhancing the speed and quality of tissue repair [4,7].
Indeed, the importance of extracellular matrix (ECM) integrity in maintaining normal tissue function is highlighted by numerous pathologies and situations of acute and chronic injury associated with dysregulation or destruction of ECM components. Heparan sulfates (HS) are a key component of the ECM, where they fulfill important functions associated with tissue homeostasis. HS belong to the glycosaminoglycan (GAG) family. Their degradation following tissue injury disrupts this delicate equilibrium and may impair the healing process. RGTA® are specifically designed to replace degraded HS in injured tissues. The unique properties of RGTA®, such as resistance to degradation, binding, and protection of ECM structural and signaling proteins, permit the reconstruction of the ECM, restoring both structural and biochemical functions to this essential substrate, and facilitating the processes of tissue repair and regeneration [4].
Numerous situations implicating acute and chronic injuries are associated with dysregulation or destruction of ECM components. Heparan sulfates are the key components of the ECM, which tightly control tissue homeostasis. OTR4120 is a polysaccharide specifically designed (substituted with carboxymethyls and sulfates) to replace degraded heparan sulfates in injured tissues. The unique properties of OTR4120, as described above, permit the reconstruction of the ECM, restoring the homeostasis of the tissue, facilitating the processes of tissue repair and regeneration [1,4,8,9,10].
Superficial corneal ulcers that fail to heal within a normal time period and are refractory to conventional therapy are common in veterinary practice. Different etiologies can result in refractory corneal ulcers, such as morphologic and neurologic abnormalities of the eyelids, thick distichiasis or ectopic cilia, tear film abnormalities, deficiencies of corneal innervation, foreign bodies, microbial infection, spontaneous chronic corneal epithelial defects (SCCED), and ulcerative keratitis associated with bullous keratopathy [11].
SCCED are superficial epithelial defects that have not become infected, do not involve the corneal stroma, are bordered or partially covered with non-adherent epithelium, and fail to heal in a normal time period. The pathological mechanisms are not fully understood. Poorly adherent epithelium and epithelial dysmaturation at the periphery of lesions with varying degrees of leukocyte infiltration are currently associated with ECM disruption [12]. The basement membrane is typically absent or present in discontinuous segments within the lesion. A hyaline acellular zone in the anterior corneal stroma is commonly present in the area of the erosion [13]. Stromal fibroplasia, vascularization, and leukocyte infiltration have been observed in some specimens. Disorganized zones of sub-epithelial and epithelial hyperinnervation surround the epithelial defect. Matrix metalloproteinase (MMP) activity is elevated in affected corneas and epithelial–mesenchymal transition, the process in which anchored epithelial cells transform into migrating fibroblast-like cells to re-epithelialize corneal epithelial defects, is abnormal [14,15]. In summary, most dogs with SCCED do not have a normal basement membrane structure in the region of the epithelial defect and have other abnormalities in the subjacent extracellular matrix that may reflect a part of the underlying pathophysiology of chronic and recurrent erosions [12].
Bullous keratopathy is a sequela to severe or chronic corneal edema. Endothelial cell dysfunction (either degeneration or dystrophy) and the resultant corneal edema can lead to the formation of intra-epithelial or sub-epithelial bullae. These bullae are at risk of corneal ulceration due to structural weakening of the cornea or spontaneous rupture. These corneal ulcers tend to follow a prolonged healing course and reoccurrence is common [16,17,18].
Because the ECM is altered during the progression of both the bullous keratopathy and the SCCED, multiple treatment modalities have been considered. The management of SCCED treatments include medical therapies such as polysulfated glycosaminoglycans (PSGAGs), protease MMP inhibitors, topical epidermal growth factors, fibronectin and surgical therapy with epithelial debridement, especially diamond burr debridement (DBD), which is now becoming the standard but associated to medical therapies to enhance the healing process [15,19,20]. Indeed, DBD induces mechanical disruption that exposes the proteins of the ECM to reactivate the healing process, allowing exposure of the normal peripheral basal membrane (BM) to the growth of newly formed epithelium, enhancing its adhesion properties [13]. Indeed, removal of the epithelial BM during DBD enhances many wound healing processes in the cornea, including keratocyte apoptosis and nerve death. In addition, the BM controls cellular functions by binding and modulating the local concentrations of growth factors and cytokines, and is able to regulate cell polarity, cell adhesion, spreading, and migration via its effects on the cytoskeleton. [18]. Bullous keratopathy management approaches include mainly medical therapies, and different molecules have been proposed to restore the ECM, such as collagen cross-linking [21,22,23] or chondroitin sulfate [24]. The conclusions of these studies have not been conclusive or convincing. Thus, there is an urgent need to find new therapeutic approaches to replace the injured ECM in these pathologies.
In the domain of ophthalmology, an RGTA® family compound named OTR4120, a heparan sulfate mimetic, has been reported to show encouraging results for the treatment of corneal ulcers and dystrophies of various etiologies [10,25,26]. Furthermore, OTR4120 was described in a case report concerning one patient with a neurotrophic ulcer [26]. During the last few years, OTR4120 eye drops have been successfully used for the treatment of resistant corneal neurotrophic ulcers [8], as well as for the treatment of keratoconus in humans [9]. OTR4120 has been available on the European market for veterinary use as Clerapliq® for more than 5 years [8,9,25,26,27]. The mode of action of OTR4120 is to replace the destroyed HS and restore the ECM scaffold by organizing the collagens in the matrix and by protecting the growth factors and cytokines. This leads to the recreation of the physiological environment required for tissue repair and regeneration [3,4].
The aim of these case reports was to evaluate the beneficial impact of OTR4120 (Clerapliq®) to treat primary or secondary corneal epithelial erosion in dogs in the current ophthalmology veterinarian practice. The results show that improvement appeared in 6–35 days, with an average of 18.8 days of treatment once every 3 days (i.e., q3d) among the 11 dogs treated with Clerapliq® simultaneously with the usual treatment. Failure was observed only in 2 dogs due to other complications. In conclusion, these case reports show that Clerapliq® was successful for the treatment of primary or secondary epithelial erosion associated with the etiology treatment. Clerapliq® is indicated for analgesic purposes and for optimizing the time-space healing process of the cornea observed in 83.3% of the cases. Finally, Clerapliq® was easy and safe to use for the practitioner and the owner.
2. Materials and Methods
2.1. Dogs
A total of 11 dogs were presented at the Eye Center for Animals Inc. (524 Moss Street, New Orleans, LA, USA) clinic for nonhealing corneal ulcers after failure of primary treatment by the referring veterinarian, usually by epithelial debridement, without evidence of defect resolution for several weeks to months. The dogs were then referred to the Eye Center for Animals. A total of 11 dogs (15 eyes) were evaluated in these case reports. All dogs underwent complete clinical evaluation and examination of both eyes. All dogs were privately owned pets. Owners reviewed and signed an informed-consent form before samples were collected, as well as consent for the use of Clerapliq®. All research was performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. Written informed consent to treat the dogs with Clerapliq® was obtained from all owners when they attended the Eye Center for Animals Inc. (524 Moss Street, New Orleans, LA, USA), in accordance with the relevant Louisiana state and USDA laws and regulations.
2.2. Clinical Evaluation
A total of 11 dogs were presented at the Eye Center for Animals Inc. clinic (New Orleans, LA, USA) for nonhealing corneal ulcers after failure of primary treatment by the referring veterinarian. Briefly, superficial corneal ulcers that fail to heal within a normal time period and are refractory to conventional therapy are common in veterinary practice. Different etiologies can result in refractory corneal ulcers: Morphological and neurological abnormalities of the eyelids, thick distichiasis or ectopic cilia, tear film abnormalities, deficiencies of corneal innervation, foreign bodies, microbial infection, spontaneous chronic corneal epithelial defects (SCCED), and ulcerative keratitis associated with bullae. All dogs underwent complete examination of both eyes. To assess the cause(s) of the treatment failure, complete eye exams were performed at the initial visit and then during follow-up. Clinical follow-up also included the evaluation of local side effects such as local edema, inflammation, pain, pruritus, and watery eyes, and systemic side effects such as body weight, food intake, behavior changes, body core temperature, skin rash, edema, and diarrhea.
2.3. Clinical Tests
Visual status, menace, and dazzle responses were assessed at the first visit. Palpebral and pupillary light reflexes were also recorded. Globe, adnexa, and conjunctiva status were examined, as well as the presence of discharge.
Then, each compartment of the eye, such as the cornea, anterior chamber, pupil, lens, vitreous, retina, and optic nerve, were examined at the first visit, as well as during follow-up, as follows.
To assess any retinopathy or optical nerve defects, each dog underwent slit-lamp examination (SL 15; Kowa Optimed Europe Ltd., Sandhurst, UK) after using mydriatic drops by assessing the vertical and horizontal cup-to-disc ratios, the visibility of the lamina cribosa, the color of the cup, the area of the vessels, their tortuosity and collateral vasculature, the contour of the neuroretinal rim and the four quadrants thickness, the retinal nerve localization and aspect with the red filter, the disc size along its axis, and the peripapillary development (e.g., normal or atrophic).
Indirect ophthalmoscopy (Omega 500, HEINE Optotechnik, Herrsching, Germany; 2.2 PanRetinal) and 20D lenses (Volk Optical Inc., Mentor, OH, USA) were used to assess the posterior segment.
Intraocular pressure (IOP) was measured using the TonoVet tonometer (TonoVet; ICare, Vantaa, Finland) at the first visit and during the follow-up of the animal.
2.4. Schirmer Tear Test
As a crude estimate of tear production, both eyes of each dog were evaluated using Schirmer test strips (Schirmer Tear Test; Intervet/Schering-Plough Animal Health, Roseland, NJ, USA). One test strip was placed in each conjunctival sac at the junction between the lateral one third and medial two thirds of the lower lid for 60 s, which is the standard time used for tear evaluation in dogs. Immediately after the 60-s period, the length of the moistened area of the test strip was measured and recorded in millimeters. Testing was performed before administration of any topical medication.
2.5. Fluorescein Stain
A strip containing fluorescein sodium (Acrivet-Veterinary Division, Hennigsdorf, Germany) was wetted with sterile irrigating solution, and 1 drop of fluorescein added to the affected eye. Then, the eye was washed-out with sterile irrigating solution before examination. The epithelial defect of each dog was then measured from epithelial edge to epithelial edge using a pair of calipers accurate to 0.1 mm. Two measurements of the ulcers were made. The first measurement was made at the greatest dimension of the erosion and the second at a 90° angle to the first measurement.
2.6. New Regenerative Matrix Treatment
A total of 11 dogs were presented at the Eye Center for Animals Inc. clinic for nonhealing corneal ulcer after failure of primary treatment by the referring veterinarian. All dogs underwent complete examination of both eyes; among the 11 dogs enrolled, 5 underwent a diamond burr debridement at the clinic, and then all dogs received the matrix therapy agent Clerapliq® eye drop every 3 days (1 drop, q3d) until ulcer healing (usually between 1–3 months). The structure, synthesis pathway, and potential applications of the active substance OTR4120 (CAS RN: 227322-59-0) are presented elsewhere [28], and it belongs to ReGeneraTing Agents (RGTA®) family. OTR4120 is already used in two commercially available products for the treatment of corneal lesions and chronic ulcers for humans and pets (Cacicol20® and Clerapliq®, respectively) in Europe, with more than 50,000 patients and pets treated in the last 5 years [28]. Briefly, Clerapliq® is a solution that contains OTR4120, an alpha 1-6 poly-(carboxymethyl-sufate)-glucose (Figure 1) with 4 mg/mL of dextran diluted in 9 mg/mL of sodium chloride solution. Clerapliq® is presented in a 0.33 mL sterile single-dose and provided by OTR3 (Paris, France).
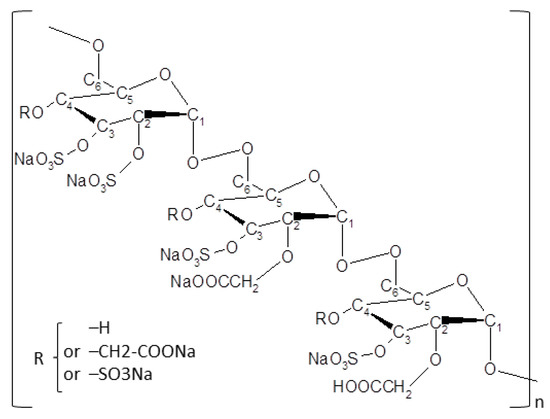
Figure 1.
Chemical structure of OTR4120. OTR4120 is a derivatized dextran with carboxymethyl and sulfate substitutions (dextran, hydrogen sulfate, carboxymethyl ether, sodium salt, CAS RN: 227322-59-0), known also as alpha 1-6 poly-(carboxymethyl-sulfate) glucose.
2.7. Statistical Analysis
The comparisons between the proportion of dogs that successfully responded to Cleraplic® was tested by using a chi-square test (χ2).
3. Results
3.1. New Regenerative Matrix Treatment
A new treatment based on matrix regeneration was used to help and accelerate the healing process. Each affected eye received 1 drop of Clerapliq® containing OTR4120 (Figure 1) every 3 days (i.e., q3d) during a mean time of 21.4 ± 15.9 days (i.e., ranging between 7 to 55 days; Table 1).

Table 1.
Case report populations and clinical data summarized from Appendix A.
3.2. Treatment and Follow-up
Dogs treated with Clerapliq® met the following criteria: Presence of a nonhealing corneal epithelial defect in one or both eyes for at least 3 weeks without notable progress towards resolution; absence of any identifiable underlying cause of the persistent defect (e.g., normal tear production; absence of lid conformational defects; normal lid function; no clinical evidence of sepsis; and absence of distichiasis, ectopic cilia, and foreign bodies); and absence of any clinical evidence of systemic disease. Only one dog (dog#8) developed local infection in the eye, leading to bacterial culture and an antibiogram test. The corneal ulcer healed very well after gentamicin treatment.
Among the 11 dogs enrolled, 7 dogs (10 eyes) were diagnosed with superficial ulceration and 4 dogs (5 eyes) were diagnosed with bullous keratopathy due to endothelial dystrophy. All animals received Clerapliq® (Table 1).
Among the 15 eyes treated with Clerapliq®, only 2 treated eyes failed to positively respond to the treatment (dog#5 and dog#6) and 3 dogs were lost to follow-up (Table 1). When diamond burr debridement was performed, Clerapliq® treatment started between 7 to 15 days after (median = 11 days).
The success of Clerapliq® treatment was observed in 13 among the 15 eyes treated (i.e., 86.6% success) with a mean treatment time of 15.6 ± 9.7 days (6–35 days). Because 3 dogs were lost to follow-up, the success of the treatment remains 10 among the 12 treated eyes, with follow-up leading to 83.3% success with a mean treatment time of 18.7 ± 10.5 days (6–35 days). The comparison of the dogs with success before (0%; 0/12) and after treatment with Clerapliq® (83.3 %; 10/12) showed a significant improvement (χ2 p-value = 3.5 × 10−5).
Finally, as expected, the dogs did not show any local (i.e., edema, inflammation, pain) or systemic (i.e., no loss of body weight; no decrease in food intake, behavior changes, no increase in body core temperature, skin rash, edema, no diarrhea) side effects after Clerapliq® treatment.
The clinical data are summarized in Table 1 and Supplementary Table S1.
All of the details for each clinical case can be found at the end of the manuscript in Appendix A.
4. Discussion
OTR4120 was tested for the first time in corneal ulcers and severe dystrophies resistant to standard therapies in humans [25]. The product was administered topically once a week for 1 month and resulted in significant reduction in pain, improvement of keratitis, and healing of the majority of corneal ulcers. Several case reports using RGTA® in cases of neurotrophic keratopathy and corneal ulcers since then have indicated a positive effect of the treatment [26,29,30,31]. The effectiveness of RGTA® in corneal neurotrophic ulcers of various primary etiologies was also examined in a larger study with 11 patients, where RGTA® treatment resulted in complete corneal healing in eight patients, with the remaining patients presenting the most severe cases [8]. More recently, the combination of OTR4120 (Cacicol20®) with a bandage contact lens in three patients with persistent epithelial defects promoted complete corneal epithelial healing in 4–21 days [28].
Also, dog#5 and dog#6 did not respond successfully to Clerapliq® (see Appendix A). They were treated for 55 and 40 days, respectively, and they still had persistent epithelial erosion. Failure of the treatment might be because of underlying causes such as aging, topical anti-inflammatory or immunomodulation medications, or canine herpes virus-1 (CHV) infection [32,33,34]. However, based on the clinical data, the main failure of the treatment in these two dogs is most likely due to severe edema preventing healing. Interestingly, previous reports have shown that OTR4120 (Cacicol®) has antiviral effects and promotes corneal regeneration in herpes neurotrophic ulcers [26,35], suggesting that Clerapliq® treatment might be efficient in CHV-infected dogs.
Also, because 3 dogs were lost to follow-up, we did not know if they relapsed. We assumed they did not relapse, and because of the success of the treatment, they did not show up. Whatever the case, this loss to follow-up did not change the percentage of success (86.6% vs. 83.3% with 3 dogs missing).
In a recent publication, OTR4120 was found to be no more effective than hyaluronic acid, considered as a placebo, for healing SCCED [36]. We believe that hand removal of the hyaline acellular zone in the anterior corneal stroma may be difficult to fully achieve, and for this reason, diamond burr is preferred. Removal of the hyaline acellular zone is a prerequisite for successful SCCED management and is an absolute prerequisite for Clerapliq® efficacy, in order for OTR4120 to reach the heparan binding sites available in the ECM of the wounded cornea and to restore the healing process. Thus, improper hyaline acellular zone removal may explain the lack of efficacy of OTR4120. Also, it should be noted that local immunosuppression medications should be avoided during treatment with Clerapliq®.
Finally, the Clerapliq® treatment used in these case reports did not result in any local or systemic side effects in treated dogs. This is in accordance with the safety data obtained from more than thousands of dogs and cats treated by Clerapliq® in Europe (TVM) and with the publications on Clerapliq® [36]. Also, as mentioned in the introduction, the equivalent of Cleraplic® in humans is called Cacicol®, which is a medical device that has been successfully used for more than 50,000 patients with no reported local or systemic side effects [37,38,39].
5. Conclusions
Taken together, the corneas with recurrences of the ulcer were resolved predominantly by using the matrix therapy agent eye drop Clerapliq® every 3 days during a period ranging between 6 to 35 days with 83.3% success. Local immunosuppression medications should be avoided during the use of Clerapliq® in order to successfully treat recurrence of corneal ulcer. Therefore, this new approach using matrix therapy regenerating technology in treating superficial ulcers and bullous keratopathy in dogs can be successfully considered as an adjunctive therapy.
Supplementary Materials
The following are available online at https://www.mdpi.com/2306-7381/6/4/103/s1, Table S1: Characteristics of the case population.
Author Contributions
Investigation, J.A.M.; resources, J.A.M. and D.B.; writing—original draft preparation, J.A.M.; writing—review and editing, J.A.M., D.B., and F.C.; data collection, J.A.M.; data analysis, F.C.; visualization—tables and figures, F.C.; supervision, D.B.
Funding
This research received no external funding.
Conflicts of Interest
J.A.M. declares no conflicts of interest. D.B. is OTR3 founder and inventor, with patents and a significant OTR3 shareholder. F.C. is an employee of OTR3. Clerapliq® is a veterinary comfort product available in the EU distributed by TVM company for OTR3.
Abbreviations
| KCS | Keratoconjunctivitis sicca or dry eye syndrome |
| OD (oculus dexter) | Right eye |
| OS (oculus sinister) | Left eye |
| OU (oculus uterque) | Both eyes |
| PO | per os |
| q3d | Quaque 3 die = every 3 days |
Appendix A
This appendix combines all of the clinical data, clinical tests, treatments, and follow-up of from each clinical case included in these case reports.
Patient 1
- 13 years old, Shih-Tzu, female/spayed.
- History: KCS (i.e., dry eye syndrome), Glaucoma.
- Prior Treatments: OU: Dorzolamide, Neopolydex, cyclosporine 2%, and artificial tears.
- Diagnostics: 8/31/15 OS superficial ulcer.
- Treatments: OU: Dorzolamide, cyclosporine 2%, and artificial tears.
- OS: Clerapliq® 1 drop q3days, Ofloxacin.
- Recheck: 9/17/15 OS ulcer healed.
- No more rechecks.
Patient 2
- 10 years old, Boston Terrier, male/intact.
- History: OD squinting and erosion treated by referring veterinarian two months prior to presentation.
- 6/29/2015 Grid Keratotomy.
- Prior Treatments: Atropine/terramycin/serum/remand/flurbiprofen.
- Findings: OD supero-temporal paracentral 3 mm erosion with poorly adherent margins, temporal paracentral 2–3+ edema.
- Diagnostics: 7/24/2015 OD endothelial dystrophy and persistent erosion.
- Treatments: 7/24/2015 OD: Diamond burr debridement; Ofloxacin 1; NaCl 5% solution.
- Systemic: Tramadol 50 mg PO as needed for pain.
- Recheck: 8/6/2015 persistent ulcer.
- Treatments: Diamond burr debridement repeated and continued above topical meds.
- Recheck: 8/17/2015 OD complete epithelialization but nonadherent central to paracentral; 3+ edema; peripheral superficial vascularization.
- Treatments: OD: Clerapliq® 1 drop q3d; ofloxacin; NaCl 5% ointment; strip.
- Systemic: Tramadol 50 mg as needed for pain.
- Recheck: 8/31/2015 OD complete epithelialization with fluorescein stipple over vascular ridge with bullae in temporal paracentral region, adherent epithelium challenged with cotton tip applicator, 3+ edema.
- Treatments: OD: Clerapliq® 1 drop q3d; Neopolydex; ofloxacin; NaCl 5% ointment; strip.
- Systemic: Tramadol 50 mg PO as needed for pain.
- Recheck: 9/21/2015 complete epithelialization with no fluorescein stipple and no bullae, adherent epithelium in all quadrants, 2+ edema, attenuated vascularization.
- Treatments: None.
Patient 3
- 7 years old, French Bulldog, male/neutered.
- History: 8/20/2015 diagnosed with OD erosion by referring veterinarian.
- Treatments: Tobramycin q4h and serum q4h.
- Findings: OD temporal paracentral corneal erosion with nonadherent margins and subepithelial fluorescein uptake, 1+ edema, 9:00 peripheral superficial pigmentation.
- Diagnostics: 8/24/2015 OD persistent epithelial erosion.
- Treatments: OD: Geographic diamond burr debridement; tobramycin; serum; Remand, corneal repair gel.
- Systemic: Clavamox 125 mg until gone; Rimadyl 25 mg until gone; tramadol 50 mg as needed for pain.
- Recheck: 8/25/2015 owner noted increased discharge.
- Treatments: OD: Ofloxacin; Remend corneal repair gel.
- Systemic: Clavamox 125 mg until gone; Rimadyl 25 mg; tramadol 50 mg for pain.
- Recheck: 9/2/2015 OD smaller erosion of temporal quadrants with partially adherent margins on its superonasal margin, 1+ edema, 9:00 previously observed pigmentation.
- Treatments: OD: Clerapliq® 1 drop q3d; Ofloxacin; Remand corneal repair gel.
- Systemic: Tramadol 50 mg as needed for pain.
- Recheck: 9/8/2015 OD adherent epithelium challenged with cotton tip applicator, 1+ edema, 9:00 pigmentation, 3+ paracentral superficial vascularization.
- Diagnostics: Healed persistent erosion.
- Treatments: OD: Neopolydex suspension.
- Systemic: Collar.
- Recheck: 10/1/2015 OD 1+ edema most prominent in temporal quadrants, temporal superficial pigmentation, attenuated paracentral superficial vascularization.
Patient 4
- 9 years old, Griffon, male/intact.
- History: Presented with persistent ulcer OD diagnosed by referring veterinarian on 10/21,
- OU endothelial dystrophy with cloudiness that owner had noticed for the past 3 months.
- Prior treatments: OD: BNP; ketorolac.
- OS: Rimadyl; cefpodoxime;
- OU: Schirmer tear test: 25 mm/min
- Findings: OU: 2+ diffuse edema;
- OD: Multiple erosion across palpebral fissure with poorly adherent epithelium in those regions, superonasal paracentral superficial vascularization, temporal paracentral focal superficial to deep scar, Seidel-negative.
- Diagnostics: 7/27/2015 OD: persistent erosion.
- OU: Corneal endothelial dystrophy.
- Treatments: OD: Diamond burr debridement; ofloxacin; 5% NaCl solution.
- Systemic: Tramadol 3 tabs for pain; Rimadyl; cefpodoxime as instructed until gone.
- Recheck: 8/10/2015 OU: 2+ diffuse edema.
- OD: Temporal paracentral erosion with poorly adherent epithelium margins, multifocal erosions at 1:00 periphery, 3+ paracentral superficial vascularization, temporal paracentral focal superficial to deep scar.
- Treatments: OD: Repeated diamond burr debridement; ofloxacin; 5% NaCl solution.
- Systemic: Tramadol 3 tabs for pain.
- Recheck: 8/26/2015 OU: 2+ diffuse edema.
- OD: Diffuse superficial vascularization and haze, temporal paracentral focal superficial to deep scar.
- OS: Nasal paracentral 3 mm erosion with nonadherent epithelium.
- Treatments: OS diamond burr debridement; E-collar; neopolydex suspension; ofloxacin; 5% NaCl solution.
- Systemic: Tramadol 3 tabs for pain.
- Recheck: 9/14/2015 OU: 2+ diffuse edema.
- OD: Attenuating diffuse superficial vascularization and haze, temporal paracentral focal superficial to deep scar.
- OS: Epithelial erosion of the superior quadrants with nonadherent epithelial margins on the superior periphery only (debrided to adherent margins with sterile cotton applicator), 3+ peripheral vascularization.
- Treatments: OD: Neopolydex suspension; ofloxacin; tobramycin.
- OS: 5% NaCl solution; Clerapliq® 1 drop q3d.
- Systemic: Tramadol 50 mg 3 for pain.
- Recheck: 9/14/2015 OU: 2+ diffuse edema.
- OD: Attenuated diffuse superficial vascularization and haze, temporal paracentral focal superficial to deep scar.
- OS: Epithelial erosion of the superotemporal quadrants with nonadherent epithelial margins, debrided to adherent margins with sterile cotton applicators, 3+ peripheral vascularization with raised vascularization in superotemporal periphery.
- Treatments: OU: neopolydex suspension.
- OS: Tobramycin; 5% NaCl solution; Clerapliq® 1 drop q3d.
- Systemic: Tramadol 50 mg 3 tabs for pain.
- Recheck: 9/24/2015 OU: 2+ diffuse edema.
- OD: Attenuating diffuse superficial vascularization and haze.
- OS: Focal epithelial erosion of the superotemporal quadrant with more adherent epithelial margins, attenuating raised vascularization, erosions in palpebral fissure with nonadherent margins.
- Treatments: OU: Neopolydex susp 1 drop OU q24h.
- OS: Diamond burr debridement; tobramycin; 5% NaCl solution; Clerapliq® 1 drop q3d.
- Systemic: Tramadol 50 mg 3 tabs for pain.
- Recheck: 10/5/2015 OU: 2+ diffuse edema.
- OD: Attenuating diffuse superficial vascularization and haze.
- OS: Attenuating vascularization, no bullae, diffuse superficial haze.
- Treatments: OD: Neopolydex suspension.
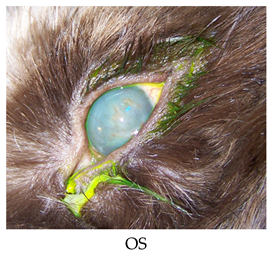
- Recheck: 11/3/2015 OU: 2+ diffuse edema, no bullae, attenuated diffuse superficial haze, and vascularization (mostly ghost vessels).
- Treatments: None.
- Diagnostics: OU: Healed persistent epithelial erosion, post-op diamond burr debridement, corneal endothelial dystrophy with predisposition to recurrent erosions.
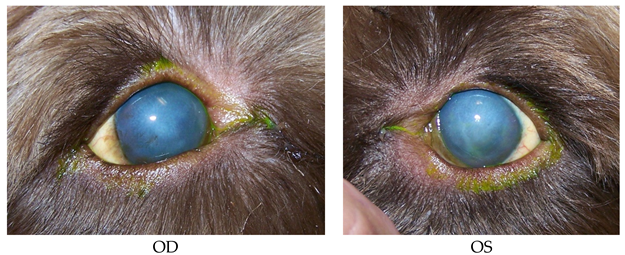
Patient 5: (post-cataract surgery)
- 15 years old, Chihuahua, female/spayed.
- History: Cataract Sx OD on 8/26/2015. OD TPA intracamaral injection on 9/8/2015.
- Prior diagnostics: OD: Post-operation of phacoemulsification and lens implant.
- OS: Mature cataract.
- Prior treatments: OD: Restart dorzolamide/timolol; tropicamide 1%; Genteal Gel drops; ketorolac 0.5%.
- OS: Neopolydex suspension.
- Systemic: Metacam; tramadol 50 mg 1/4 tab for pain; E-collar.
- Findings on 9/9/2015: OD: Seidel-negative incision, central erosion.
- OS: Central erosion.
- Diagnostics: OU: Nocturnal exposure keratitis and erosions.
- OD: Post-op phacoemulsification and lens implant, transient ocular hypertension.
- OS: Mature cataract.
- Treatments: OU: Start Clerapliq® 1 drop q3d; GenTeal Gel drops; artificial tear ointment.
- OD: Dorzolamide/timolol; tropicamide 1%; ketorolac 0.5%, neopolydex suspension.
- OS: Ofloxacin; ketorolac 0.5%.
- Systemic: Metacam; tramadol 50 mg 1/4 tab for pain.
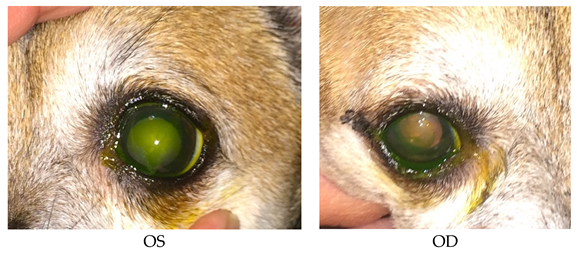
- Recheck: 9/15/2015 OD: Seidel-negative incision, central erosion with adherent margins.
- OS: Healed central erosion with 1+ haze.
- Treatments: OU: Clerapliq® 1 drop q3days; GenTeal Gel drops; artificial tear ointment.
- OD: Dorzolamide/timolol; tropicamide 1%; ketorolac 0.5%; neopolydex suspension.
- OS: Ofloxacin; ketorolac 0.5%.
- Systemic: Metacam; tramadol 50 mg for pain; E-collar.
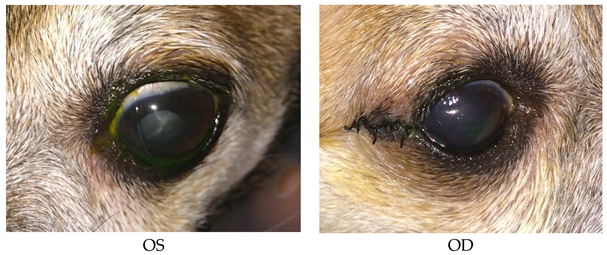
- Recheck: 9/24/15 OD: Seidel-negative vascularized incision, central erosion with adherent margins and edema.
- OS: Healed central erosion with 1+ haze.
- Treatments: OU: Artificial tear ointment.
- OD: Clerapliq® 1 drop OD q3days; ofloxacin; dorzolamide/timolol; tropicamide 1%; Genteal Gel drops; ketorolac 0.5%; neopolydex suspension.
- OS: Ketorolac 0.5%.
- Systemic: Metacam; tramadol 50 mg 1/4 tab for pain; E-collar.
- Diagnostics: OU: Nocturnal exposure keratitis and erosions.
- OD: Post-op phacoemulsification and lens implant, transient ocular hypertension.
- OS: Mature cataract.
- Recheck: 10/2/15 OD: Seidel-negative vascularized incision with suture remnants, smaller 2 mm central erosion with surrounding haze and reduced edema.
- OS: Healed central erosion with 1+ haze.
- Treatments: OU: Artificial tear ointment 1/2 inch strip at bed time;
- OD: Clerapliq® 1 dropq3days; ofloxacin; dorzolamide/timolol; tropicamide 1%; GenTeal Gel drops; ketorolac 0.5%, neopolydex suspension.
- OS: Ketorolac 0.5%.
- Systemic: Metacam; tramadol 50 mg 1/4 tab; E-collar.
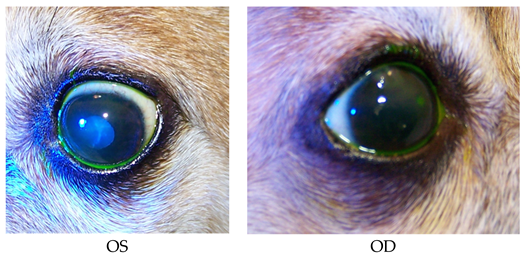
- Recheck: 10/20/15 OD: Seidel-negative vascularized incision scar with superficial pigment, 2 mm central erosion with pinpoint leukoma and surrounding adherent epithelial margins.
- OS: Central 1+ superficial haze with thin epithelium.
- Treatments: OU: Artificial tear ointment.
- OD: Clerapliq® 1 drop q3days until gone; ofloxacin tobramycin; dorzolamide/timolol; tropicamide 1%; Genteal Gel drops; ketorolac 0.5%; neopolydex suspension.
- OS: Ketorolac 0.5%.
- Systemic: Tramadol 50 mg 1/4 tab for pain; E-collar.
- Recheck: 11/3/2015 OD: Seidel-negative vascularized incision scar with superficial pigment, 2 mm central superficial ulcer with pinpoint leukoma and surrounding marginally adherent epithelial margins.
- OS: Central 1+ superficial haze with multifocal subepithelial fluorescein uptake.
- Treatments: OU: tobramycin; Genteal Gel drops; artificial tear ointment.
- OD: Central to paracentral diamond burr debridement; ketorolac 0.5%.
- OS: Geographic diamond burr debridement.; ketorolac 0.5%; neopolydex suspension.
- Systemic: Tramadol 50 mg 1/4 tab for pain; E-collar.
- Ran out of Clerapliq® samples.
- Patient 5 developed melting ulcers in both eyes after diamond burr debridement.
Patient 6
- 13 years old, Poodle mix, male/neutered.
- History: Presented OD squinting/epiphora x2d; coughing for 2 months.
- Prior treatments: OD: ofloxacin, doxycycline, hydroxyzine, and cough tabs (dextromethorphane/guafesesin).
- Findings: OD: Diffuse edema, temporal paracentral superficial vascularization, and erosion with poorly adherent margins debrided to adherent margins with sterile cotton applicators.
- OS: 2+ diffuse edema most prominent in temporal quadrants with multifocal bullae and paracentral superficial vascularization and traces of pigment.
- Diagnostics: OU: Corneal endothelial dystrophy with risk of recurrent erosions.
- OD: Corneal epithelial erosion.
- Treatments: OD: Ofloxacin; 5% NaCl solution.
- Systemic: E-collar; other medicines as per referring veterinarian.
- Recheck: 8/25/2015 OD: diffuse edema, temporal paracentral superficial vascularization, and erosion with nonadherent margins.
- OS: 2+ diffuse edema most prominent in temporal quadrants with multifocal bullae and paracentral superficial vascularization and traces of pigment.
- Treatments: OD: Geographic diamond burr debridement; ofloxacin; 5% NaCl solution ointment;
- Systemic: Tramadol 50 mg 1 tab for pain; E-collar; other medicines as per referring veterinarian.
- Recheck: 8/27/2015 OD: diffuse edema, epithelialization of entire cornea, except for temporal quadrant with superficial vascularization.
- OS: 2+ diffuse edema most prominent in temporal quadrants with multifocal bullae and paracentral superficial vascularization and traces of pigment.
- Treatments: OD: Ofloxacin; 5% NaCl solution ointment.
- Systemic: Tramadol 50 mg 1 tab for pain; E-collar; other medicines as per referring veterinarian.
- Recheck: 9/9/2015 OD: diffuse edema, central to paracentral erosion with nonadherent margins, temporal superficial vascularization.
- OS: 2+ diffuse edema most prominent in temporal quadrants, no bullae and paracentral superficial vascularization and traces of pigment.
- Treatments: OD: Clerapliq® 1 drop q3d; ofloxacin; 5% NaCl solution ointment.
- Systemic: Tramadol 50 mg 1 tab for pain; E-collar; other medicines as per referring veterinarian.
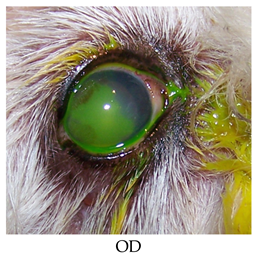
- Recheck: 9/21/2015 OD: Diffuse edema, temporal paracentral large bullae with fluorescein stipple and 2 focal erosions in inferior quadrant, diffuse superficial vascularization.
- OS: 2+ diffuse edema most prominent in temporal quadrants, nasal paracentral bullae with fluorescein stipple and paracentral superficial vascularization and traces of pigment.
- Treatments: OU: Clerapliq® 1 drop q3d.
- OD: Ofloxacin; 5% NaCl solution ointment.
- Systemic: E-collar.
- Recheck: 10/19/2015 OD: Diffuse edema, temporal paracentral erosion with nonadherent margins, diffuse superficial vascularization.
- OS: 2+ diffuse edema most prominent in temporal quadrants, nasal paracentral linear break in epithelium with subepithelial fluorescein uptake, paracentral superficial vascularization and traces of pigment.
- Treatments: OU: Ofloxacin; 5% NaCl solution ointment; no more Clerapliq® samples.
- Systemic: E-collar PRN for rubbing.
- Follow-up: Some erosions recurred, and some were persisting OU for which I discussed thermo-keratoplasty or conjunctival pedicle grafting (keratoleptnysis), which may help minimize progressive corneal edema.
Patient 7
- 12 years old Boston Terrier, male/neutered.
- History: OD cloudy more than a year; OD red and squinting early last week
- Prior treatments: OD: With BNPH and changed on 9/5/2015 to ofloxacin and atropine, Remand; corneal repair gel; rimadyl and tramadol.
- Findings: OD: Central to paracentral 3+ edema, central bullae with fairly adherent epithelial cells challenged with sterile cotton applicator.
- OS: Within normal limits.
- Diagnostics on 9/15/2015: OU: Iris cysts.
- OD: Corneal endothelial dystrophy with predisposition to recurrent erosions, emerging Meibomian mass.
- OS: Zonulysis.
- Treatments: OD: Clerapliq® 1 drop q3d; ofloxacin; 5% NaCl solution ointment; Remand; corneal repair gel;
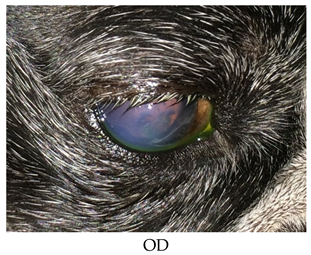
- Recheck on 9/28/2015: OD: Central to paracentral 3+ edema, no bullae, 2 curvilinear epithelial breaks where lid mass apposes with fairly adherent epithelial cells challenged with sterile cotton applicator;
- OS: Within normal limits.
- Treatments: OD: Clerapliq® 1 drop q3d; ofloxacin; 5% NaCl solution ointment; Remand; corneal repair gel
- Lost to follow up.
Patient 8
- 14 years old, miniature Poodle, female/spayed, diabetic.
- History: Bilateral cataract section on 8/18/2015.
- Since 8/31/2015: OU: Seidel-negative incision, 1+ diffuse edema, central erosion.
- Prior treatments: OU: ketorolac 0.5%; tropicamide 1%; dorzolamide/timolol; GenTeal Gel drops; neopolydex suspension.
- Systemic: Metacam after surgery; Ocu-Glo; Tramadol 50 mg 1/2 tab for pain.
- Recheck: 9/9/2015 OU: Seidel-negative incision, 1+ diffuse edema.
- OD: Central erosion with nonadherent margins debrided to adherent margins with sterile cotton applicator; Schirmer tear test OD: 11 mm/min; anterior chamber: Trace flare, no cells, no lipid-laden aqueous.
- OS: Central linear erosion with adherent epithelium challenged with sterile cotton applicator. Schirmer tear test OS: 12 mm/min; anterior chamber: Trace flare, no cells/lipid.
- Treatments: OU: Ketorolac 0.5%; tropicamide 1%; dorzolamide/timolol; GenTeal Gel drops 1; neopolydex; ofloxacin; prednisolone acetate suspension 1%; start cyclosporine 2%.
- Systemic: Tramadol 50 mg 1/2 tab for pain; Ocu-Glo.
- Recheck: 9/17/2015 OU: Seidel-negative incision, 2+ diffuse edema.
- OD: Central erosion with nonadherent margins debrided to adherent margins with sterile cotton applicator.
- OS: 1+ central edema, central focal erosion with fairly adherent epithelium challenged with sterile cotton applicator.
- Treatments: OU: Clerapliq® 1 drop q3d; ketorolac 0.5%; tropicamide 1%; GenTeal Gel drops; ofloxacin; prednisolone acetate suspension 1%; cyclosporine 2%.
- Systemic: Tramadol 50 mg 1/2 tab for pain; Ocu-Glo.
- Recheck: 10/1/2015 OU: Seidel-negative vascularized incision with suture remnants, 1-2+ diffuse edema.
- OD: Deep stromal ulcer with deepest portion epithelialized and inferior portion with keratomalacia; Schirmer tear test: 18 mm/min.
- OS: 1+ central edema, central superficial haze; Schirmer tear test: 15 mm/min.
- 10/1/2015: Corneal culture and cytology: Septic neutrophilic inflammation with epithelial dysplasia. Enterococcus species 4+.
- Treatments: OU: Cyclosporine 2%; ketorolac 0.5%; tropicamide 1%.
- OD: Clerapliq® 1 drop q3d; gentamicin 20 mg/0.2 mL sub-conjunctival; cefazolin 50 mg/mL; 1.2% gentamicin; GenTeal Gel drops 1 drop OD tid.
- OS: Ofloxacin; prednisolone acetate suspension 1% only.
- Systemic: E-collar; tramadol 50 mg 1/2 tab for pain; Ocu-Glo.
- Comments: The erosion OS healed, but the erosion OD became infected with a deep melting ulcer that is partially epithelialized.
- Recheck: 10/5/2015 OU: Seidel-negative vascularized incision scar, 1+ diffuse edema.
- OD: Deep stromal ulcer with deepest portion epithelialized and inferior portion with reduced keratomalacia and increased epithelialization.
- OS: 1+ central edema, central superficial haze.
- Treatments: OU: Ketorolac 0.5%; tropicamide 1%; cyclosporine 2%; tobramycin.
- OD: Clerapliq® 1 drop q3d; cefazolin; 1.2% gentamicin; ofloxacin.
- OS: GenTeal Gel drops; prednisolone acetate suspension 1%.
- Systemic: E-collar; Ocu-Glo; tramadol 50 mg 1/2 tab for pain.
- Comments: The melting ulcer is healing well and will probably not need a conjunctival graft. The cytology was negative for fungus, and the culture revealed enterococcus resistant to fluoroquinolones and cephalosporins.
- Recheck: 10/12/15 OU: Seidel-negative vascularized incision scar, reduced diffuse edema.
- OD: Deep stromal scar complete and adherent epithelium challenged with sterile cotton applicator; Schirmer tear test: 18 mm/min.
- OS: 1+ central edema, central superficial haze with adherent epithelium challenged with sterile cotton applicator. Schirmer tear test: 14 mm/min.
- Diagnostics: OU: Post-op phacoemulsification and lens implant for diabetic cataract, healed persistent corneal erosion, KCS (i.e., dry eye syndrome).
- OD: Healed melting ulcer.
- Treatments: OU: Serum; tobramycin; ketorolac 0.5%; tropicamide 1%; GenTeal Gel drops; cyclosporine 2%;
- OS: Prednisolone acetate suspension 1%.
- Systemic: E-collar; Ocu-Glo.
- Comments: Both corneas have epithelialized with adherent but thin central epithelium. The infection appears cleared.
- Recheck: 10/19/2015. OU: Seidel-negative vascularized incision scar, reduced diffuse edema.
- OD: Deep stromal scar complete and adherent epithelium challenged with sterile cotton applicator.
- OS: 1+ central edema, central superficial haze with adherent epithelium challenged with sterile cotton applicator.
- Treatments: OU: Tobramycin; gentamicin ointment; ketorolac 0.5%; tropicamide 1%; GenTeal Gel drops; d/c prednisolone acetate; cyclosporine 2%;.
- Systemic: E-collar; Ocu-Glo.
- Recheck: 10/26/2015 OU: Seidel-negative vascularized incision scar, reduced diffuse edema.
- OD: Deep stromal scar complete and adherent epithelium.
- OS: 1+ central edema, 11:00 paracentral anterior stromal ulcer with adherent margins, central superficial haze with adherent epithelium challenged with sterile cotton applicator.
- Treatments: OU: Ketorolac 0.5%; tropicamide 1%; GenTeal Gel drops; cyclosporine 2%.
- OD: Restart prednisolone acetate suspension 1%.
- OS: Tobramycin; gentamicin ointment 1.
- Systemic: E-collar; Ocu-Glo.
- Recheck: 11/2/2015 OU: Seidel-negative vascularized incision scar, reduced diffuse edema.
- OD: Deep stromal scar complete but thin epithelium.
- OS: 1+ central edema, 11:00 paracentral anterior stromal ulcer site with thin epithelium, central superficial haze.
- Diagnostics: OU: Post-op phacoemulsification and lens implant for diabetic cataract, persistent corneal erosion, KCS (i.e., dry eye syndrome).
- OD: Healed melting ulcer.
- OS: Healing superficial ulcer.
- Treatments: OU: Chloramphenicol 1% ointment; d/c prednisolone acetate suspension; ketorolac 0.5%; tropicamide 1%; GenTeal Gel drops; cyclosporine 2%.
- OS: Ulcer healed and rechecks continue for cataracterisation.
- Systemic: E-collar; Ocu-Glo.
Patient 9
- 15 years old, miniature Poodle, male/neutered.
- History: Cataract Surgery OD on 9/9/2015. On 9/22/2015, both corneas have persistent epithelial erosions which may eventually need diamond burr debridement.
- Prior treatments: OU: Cyclosporine 2%; Genteal Gel drops; neopolydex suspension.
- OD: Ketorolac 0.5%.
- OS: Ofloxacin.
- Systemic: Rimadyl 25 mg 1 tab; E-collar.
- Findings: OU: Attenuated superior paracentral superficial vascularization.
- OD: Seidel-negative incision, reduced edema, faint central superficial haze/lipid/mineral, central 3 mm erosion with nonadherent margins.
- OS: Central focal erosion with poorly adherent epithelium.
- Diagnostics: OD: Post-op phacoemulsification and lens implantation.
- OS: Hypermature cataract and lens induced uveitis.
- OU: Severe iris atrophy, suspected nocturnal lagophthalmia.
- Treatments: OU: Clerapliq® 1 drop q3d; cyclosporine 2%; GenTeal Gel drops; neopolydex suspension.
- OD: Ketorolac 0.5%.
- OS: Ofloxacin 1 drop.
- Sytsemic: Rimadyl 25 mg 1 tab; E-collar.
- Recheck: 10/5/2015 OU: Central fluorescein stipple in palpebral fissure with little subepithelial uptake and more adherent margins, attenuated superior paracentral superficial vascularization.
- OD: Seidel-negative partially vascularized incision, reduced edema, faint central superficial haze/lipid/mineral.
- Treatments: OU: Cyclosporine 2%; GenTeal Gel drops; neopolydex suspension.
- OD: Ketorolac 0.5%.
- OS: Ofloxacin 1 drop.
- Systemic: Rimadyl 25 mg 1 tab; E-collar.
- Recheck: 10/20/15 OD: Seidel-negative partially vascularized incision scar, reduced edema, faint central superficial haze/lipid/mineral, central thin epithelium, attenuated superior paracentral superficial vascularization.
- OS: Central thin epithelium, attenuated superior paracentral superficial vascularization.
- Diagnostics: OU: Severe iris atrophy, suspected nocturnal lagophthalmia.
- OD: Post-op phacoemulsification and lens implantation.
- OS: Hypermature cataract and lens induced uveitis.
- Treatment: OU: Cyclosporine 2%; GenTeal Gel drops; ketorolac 0.5%.
- OD: Ofloxacin 1 drop.
- OS: Neopolydex suspension.
- Systemic: E-collar.
- Recheck on 11/3/2015: OD: Seidel-negative partially vascularized incision scar, reduced edema, central anterior stromal ulcer with superficial haze/lipid/mineral, attenuated superior paracentral superficial vascularization.
- OS: Central thin epithelium, attenuated superior paracentral superficial vascularization.
- Diagnostics: OU: Severe iris atrophy, suspected nocturnal lagophthalmia.
- OD: Post-op phacoemulsification and lens implantation.
- OS: Hypermature cataract and lens induced uveitis.
- Treatment: OU: Cyclosporine 2%; GenTeal Gel drops; ketorolac 0.5%.
- OD: Gentamicin 20 mg/0.2 mL subconjunctival; ofloxacin.
- OS: Neopolydex suspension.
- Systemic: E-collar.
- Comments: Patient 9 is doing well, almost being 2 months after cataract surgery, but the right cornea re-ulcerated for which a culture is pending. Patient 9 melting ulcer was healed on antibiotics selected based on culture results.
Patient 10
- 12 years old, Pomeranian mix, female/intact.
- History: OD KCS (i.e., dry eye syndrome), post-op conjunctival pedicle graft for penetrating ulcer, mature cataract.
- OS: Immature cataract.
- Prior treatments: OU: ketorolac 0.5%.
- OD: Cyclosporine 2%.
- Systemic: Ocu-Glo.
- Findings on 9/23/2015: OD: Seidel-negative intact and viable conjunctival pedicle graft with surrounding vascularization, reduced edema in inferior quadrants.
- OS: Superotemporal paracentral pinpoint erosion, attenuated superficial scar/pigment/vascularization and edema most prominent temporally, reduced superficial lipid.
- Diagnostics: OD: KCS (i.e., dry eye syndrome), post-op conjunctival pedicle graft for penetrating ulcer, mature cataract.
- OS: Epithelial erosion, immature cataract.
- Treatments: OU: Ketorolac 0.5%.
- OD: Cyclosporine 2%.
- OS: Clerapliq® 1 drop q3d; ofloxacin.
- Systemic: Ocu-Glo.
- Recheck on 10/1/2015: OD: Seidel-negative intact and viable conjunctival pedicle graft with surrounding vascularization, reduced edema in inferior quadrants.
- OS: Healed superotemporal paracentral pinpoint erosion, superior paracentral faint fluorescein stipple with adherent epithelium challenged with sterile cotton applicators, attenuated superficial scar/pigment/vascularization and edema most prominent temporally, reduced superficial lipid.
- Diagnostics: OD: KCS (i.e., dry eye syndrome), post-op conjunctival pedicle graft for penetrating ulcer, mature cataract.
- OS: Epithelial erosion, immature cataract.
- Treatments: OU: Cyclosporine 2%; ketorolac 0.5%.
- OS: Clerapliq® 1 drop q3d; ofloxacin.
- Systemic: Ocu-Glo.
- Comments: The left corneal erosion is essentially healed.
- Lost to follow up
Patient 11
- 14 years old, Dachshund, female/spayed.
- History: Lost to follow-up since 2011, when there was good vision but diminished at night with small retinal vessels suspecting early progressive retinal atrophy; owner stopped topical NSAID and Ocu-Glo years ago.
- OU: Cloudy for a few months and red; no squinting; good navigation at home without bumping into things but hesitates in the dark.
- Prior treatments: OU: neopolydex suspension.
- OS: Latanoprost.
- Systemic: Ocu-Glo.
- Findings on 9/24/2015: OU: Seidel-negative incision scar, diffuse edema, traces of superficial pigment peripherally, reduced central cholesterol.
- OD: Central focal erosion with nonadherent margins.
- Diagnostics: OU: Post-op phacoemulsification and lens implant, corneal endothelial dystrophy; progressive retinal atrophy.
- OD: Persistent epithelial erosion.
- OS: Suspected glaucoma.
- Treatments: OU: Ketorolac 0.5%.
- OD: Diamond burr debridement centrally and nasally; Clerapliq® 1 drop q3d; neopolydex; ofloxacin.
- OS: Latanoprost.
- Systemic: Ocu-Glo; E-collar.
- Recheck: 10/1/2015 OU: Seidel-negative incision scar, diffuse edema, traces of superficial pigment peripherally, reduced central cholesterol.
- OD: Central multifocal stipple with bullae.
- Diagnostics: OU: Post-op phacoemulsification and lens implant, corneal endothelial dystrophy; progressive retinal atrophy.
- OD: Healing persistent epithelial erosion.
- OS: Suspected glaucoma.
- Treatment: OU: Ketorolac 0.5%.
- OD: Clerapliq® 1 drop q3d; ofloxacin.
- OS: Latanoprost.
- Systemic: Ocu-Glo; E-collar.
- Comments: The central erosion OD is almost healed.
- Lost to follow up!
References
- Groah, S.L.; Libin, A.; Spungen, M.; Nguyen, K.L.; Woods, E.; Nabili, M.; Ramella-Roman, J.; Barritault, D. Regenerating matrix-based therapy for chronic wound healing: A prospective within-subject pilot study. Int. Wound J. 2011, 8, 85–95. [Google Scholar] [CrossRef]
- Garcia-Filipe, S.; Barbier-Chassefiere, V.; Alexakis, C.; Huet, E.; Ledoux, D.; Kerros, M.E.; Petit, E.; Barritault, D.; Caruelle, J.P.; Kern, P. RGTA OTR4120, a heparan sulfate mimetic, is a possible long-term active agent to heal burned skin. J. Biomed. Mater. Res. A 2007, 80, 75–84. [Google Scholar] [CrossRef]
- Barritault, D.; Desgranges, P.; Meddahi-Pelle, A.; Denoix, J.M.; Saffar, J.L. RGTA((R))-based matrix therapy —A new branch of regenerative medicine in locomotion. Jt. Bone Spine 2017, 84, 283–292. [Google Scholar] [CrossRef]
- Barritault, D.; Gilbert-Sirieix, M.; Rice, K.L.; Sineriz, F.; Papy-Garcia, D.; Baudouin, C.; Desgranges, P.; Zakine, G.; Saffar, J.L.; van Neck, J. RGTA((R)) or ReGeneraTing Agents mimic heparan sulfate in regenerative medicine: From concept to curing patients. Glycoconj. J. 2017, 34, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Jacquet-Guibon, S.; Dupays, A.G.; Coudry, V.; Crevier-Denoix, N.; Leroy, S.; Sineriz, F.; Chiappini, F.; Barritault, D.; Denoix, J.M. Randomized controlled trial demonstrates the benefit of RGTA(R) based matrix therapy to treat tendinopathies in racing horses. PLoS ONE 2018, 13, e0191796. [Google Scholar] [CrossRef] [PubMed]
- Ouidja, M.O.; Petit, E.; Kerros, M.E.; Ikeda, Y.; Morin, C.; Carpentier, G.; Barritault, D.; Brugere-Picoux, J.; Deslys, J.P.; Adjou, K.; et al. Structure-activity studies of heparan mimetic polyanions for anti-prion therapies. Biochem. Biophys. Res. Commun. 2007, 363, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Hayek, S.; Dibo, S.; Baroud, J.; Ibrahim, A.; Barritault, D. Refractory sickle cell leg ulcer: Is heparan sulphate a new hope? Int. Wound J. 2016, 13, 35–38. [Google Scholar] [CrossRef]
- Aifa, A.; Gueudry, J.; Portmann, A.; Delcampe, A.; Muraine, M. Topical treatment with a new matrix therapy agent (RGTA) for the treatment of corneal neurotrophic ulcers. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8181–8185. [Google Scholar] [CrossRef]
- Bata, A.M.; Witkowska, K.J.; Wozniak, P.A.; Fondi, K.; Schmidinger, G.; Pircher, N.; Szegedi, S.; Aranha Dos Santos, V.; Pantalon, A.; Werkmeister, R.M.; et al. Effect of a Matrix Therapy Agent on Corneal Epithelial Healing After Standard Collagen Cross-linking in Patients With Keratoconus: A Randomized Clinical Trial. JAMA Ophthalmol. 2016, 134, 1169–1176. [Google Scholar] [CrossRef]
- Brignole-Baudouin, F.; Warnet, J.M.; Barritault, D.; Baudouin, C. RGTA-based matrix therapy in severe experimental corneal lesions: Safety and efficacy studies. J. Fr. Ophtalmol. 2013, 36, 740–747. [Google Scholar] [CrossRef]
- Kirschner, S.E. Persistent corneal ulcers. What to do when ulcers won’t heal. Vet. Clin. N. Am. Small Anim. Pract. 1990, 20, 627–642. [Google Scholar] [CrossRef]
- Bentley, E.; Abrams, G.A.; Covitz, D.; Cook, C.S.; Fischer, C.A.; Hacker, D.; Stuhr, C.M.; Reid, T.W.; Murphy, C.J. Morphology and immunohistochemistry of spontaneous chronic corneal epithelial defects (SCCED) in dogs. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2262–2269. [Google Scholar]
- Da Silva, E.G.; Powell, C.C.; Gionfriddo, J.R.; Ehrhart, E.J.; Hill, A.E. Histologic evaluation of the immediate effects of diamond burr debridement in experimental superficial corneal wounds in dogs. Vet. Ophthalmol. 2011, 14, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.T.; Kambampati, R.; Murphy, C.J.; Bentley, E. Expression of matrix metalloproteinase 2 and 9 in experimentally wounded canine corneas and spontaneous chronic corneal epithelial defects. Cornea 2007, 26, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Bentley, E. Spontaneous chronic corneal epithelial defects in dogs: A review. J. Am. Anim. Hosp. Assoc. 2005, 41, 158–165. [Google Scholar] [CrossRef]
- Cooley, P.L.; Dice, P.F., II. Corneal dystrophy in the dog and cat. Vet. Clin. N. Am. Small. Anim. Pract. 1990, 20, 681–692. [Google Scholar] [CrossRef]
- Rosenthal, J.J. Bullous keratopathy: A latent complication of chronic corneal disease. Vet. Med. Small Anim. Clin. 1974, 69, 181–182. [Google Scholar]
- Torricelli, A.A.; Singh, V.; Santhiago, M.R.; Wilson, S.E. The corneal epithelial basement membrane: Structure, function, and disease. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6390–6400. [Google Scholar] [CrossRef]
- Wu, D.; Smith, S.M.; M. Stine, J.; M. Michau, T.; Miller, T.R.; Pederson, S.L.; Freeman, K.S. Treatment of spontaneous chronic corneal epithelial defects (SCCEDs) with diamond burr debridement vs. combination diamond burr debridement and superficial grid keratotomy. Vet. Ophthalmol. 2018, 21, 622–631. [Google Scholar] [CrossRef]
- Miller, W.W. Use of polysulfated glycosaminoglycans in treatment of persistent corneal erosions in dogs—A pilot clinical study. Vet. Med. 1996, 916–922. [Google Scholar]
- Famose, F. Evaluation of accelerated corneal collagen cross-linking for the treatment of bullous keratopathy in eight dogs (10 eyes). Vet. Ophthalmol. 2016, 19, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Pot, S.A.; Gallhofer, N.S.; Walser-Reinhardt, L.; Hafezi, F.; Spiess, B.M. Treatment of bullous keratopathy with corneal collagen cross-linking in two dogs. Vet. Ophthalmol. 2015, 18, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Spiess, B.M.; Pot, S.A.; Florin, M.; Hafezi, F. Corneal collagen cross-linking (CXL) for the treatment of melting keratitis in cats and dogs: A pilot study. Vet. Ophthalmol. 2014, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C.; Munger, R.J.; Ring, R.D.; Scarlett, J.M. Efficacy of two chondroitin sulfate ophthalmic solutions in the therapy of spontaneous chronic corneal epithelial defects and ulcerative keratitis associated with bullous keratopathy in dogs. Vet. Ophthalmol. 2006, 9, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Chebbi, C.K.; Kichenin, K.; Amar, N.; Nourry, H.; Warnet, J.M.; Barritault, D.; Baudouin, C. Pilot study of a new matrix therapy agent (RGTA OTR4120) in treatment-resistant corneal ulcers and corneal dystrophy. J. Fr. Ophtalmol. 2008, 31, 465–471. [Google Scholar]
- De Monchy, I.; Labbe, A.; Pogorzalek, N.; Gendron, G.; M’Garrech, M.; Kaswin, G.; Labetoulle, M. Management of herpes zoster neurotrophic ulcer using a new matrix therapy agent (RGTA): A case report. J. Fr. Ophtalmol. 2012, 35, 187. [Google Scholar] [CrossRef]
- Michaud, B. Medical management of corneal ulcers: Use of RGTA Prise en charge médicale des ulcères cornéens: Utilisation des RGTA(r). L’essentiel Vétérinaire 2013, 285, 16–24. [Google Scholar]
- Kymionis, G.D.; Liakopoulos, D.A.; Grentzelos, M.A.; Tsoulnaras, K.I.; Detorakis, E.T.; Cochener, B.; Tsilimbaris, M.K. Effect of the Regenerative Agent Poly(Carboxymethylglucose Sulfate) on Corneal Wound Healing After Corneal Cross-Linking for Keratoconus. Cornea 2015, 34, 928–931. [Google Scholar] [CrossRef]
- Xeroudaki, M.; Peebo, B.; Germundsson, J.; Fagerholm, P.; Lagali, N. RGTA in corneal wound healing after transepithelial laser ablation in a rabbit model: A randomized, blinded, placebo-controlled study. Acta. Ophthalmol. 2016, 94, 685–691. [Google Scholar] [CrossRef]
- Pison, A.; Feumi, C.; Bourges, J.L. Healing of a resistant neurotrophic corneal ulcer using a new matrix therapy agent. J. Fr. Ophtalmol. 2014, 37, e101–e104. [Google Scholar] [CrossRef]
- Mateo, A.; Abadia, B.; Calvo, P.; Minguez, E.; Pablo, L.; Del Castillo, J.M. Treatment of Acanthamoeba neurotrophic corneal ulcer with topical matrix therapy. J. Ophthalmic Inflamm. Infect. 2015, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C. Canine herpesvirus-1 ocular diseases of mature dogs. N. Z. Vet. J. 2013, 61, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C.; Kim, S.G.; Dubovi, E.J. Outbreak of ocular disease associated with naturally-acquired canine herpesvirus-1 infection in a closed domestic dog colony. Vet. Ophthalmol. 2009, 12, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C.; Riis, R.C.; Kern, T.J.; Haley, N.J.; Schatzberg, S.J. Corneal ulceration associated with naturally occurring canine herpesvirus-1 infection in two adult dogs. J. Am. Vet. Med. Assoc. 2006, 229, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Deback, C.; Rousseau, A.; Breckler, M.; Molet, L.; Boutolleau, D.; Burrel, S.; Roque-Afonso, A.M.; Labetoulle, M. Antiviral effects of Cacicol((R)), a heparan sulfate biomimetic for corneal regeneration therapy, for herpes simplex virus type-1 and varicella zoster virus infection. Antivir. Ther. 2018, 23, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Sebbag, L.; Allbaugh, R.; Strong, T.; Strauss, R.; Wehrman, R.; Foote, B.; Peterson, C.; Ben-Shlomo, G. Lack of effect of a topical regenerative agent on re-epithelialization rate of canine spontaneous chronic corneal epithelial defects: A randomized, double-masked, placebo-controlled study. Vet. J. 2018, 233, 63–65. [Google Scholar] [CrossRef]
- Gumus, K.; Guerra, M.G.; de Melo Marques, S.H.; Karakucuk, S.; Barritault, D. A New Matrix Therapy Agent for Faster Corneal Healing and Less Ocular Discomfort Following Epi-off Accelerated Corneal Cross-linking in Progressive Keratoconus. J. Refract. Surg. 2017, 33, 163–170. [Google Scholar] [CrossRef]
- Robciuc, A.; Arvola, R.P.J.; Jauhiainen, M.; Holopainen, J.M. Matrix regeneration agents improve wound healing in non-stressed human corneal epithelial cells. Eye 2018, 32, 813–819. [Google Scholar] [CrossRef]
- Utine, C.A.; Engin Durmaz, C.; Kocak, N. Corneal matrix repair therapy with the regenerating agent in neurotrophic persistent epithelial defects. Int. J. Ophthalmol. 2017, 10, 1935–1939. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).