Mycobacterium avium Subspecies paratuberculosis DNA and Antibodies in Dairy Goat Colostrum and Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Samples
- (I)
- Colostrum samples (n = 120) were collected from a convenience sample of dairy goats from each of the six dairy goat herds. It was intended to collect at least 150 samples, as this would allow to conclude with a level of confidence of 95% that the true proportion contaminated samples did not exceed a designMAP prevalence of 2% if none of the samples tested MAP positive. Preferably between 20 to 30 goats per herd were sampled by the farmer, of which 10 were one year old first lactation goats and the rest a representative representation of ages and/or lactations were collected as per written instruction. Samples were collected in provided vials, stored in the fridge, and picked up on farm by the first author for cooled transport to the GD-Animal Health laboratory.
- (II)
- Colostrum samples (n = 22) were collected from a convenience sample of ELISA-positive dairy goats with clinical signs consistent with paratuberculosis from two dairy goat herds (herds A and I). These samples were collected by the farmers as described above.
- (III)
- Milk samples (n = 202) from a convenience sampling of dairy goats from a single herd (herd B). These samples were collected by the farmers as described above.
- (IV)
- Post mortem collected milk samples (n = 27) from goats designated to be slaughtered from three herds (herd B, G, and H). These goats were selected by the dairy goat farmers based on clinical signs consistent with paratuberculosis and were euthanized on farm by the local veterinarian. After euthanisation, the cadavers were transported to the post mortem facility at GD-Animal Health. Milk samples were collected by milking both udder halves separately after disinfection and removal of the first milk. In addition, tissue samples were collected from the distal ileum, mid jejunum, ileocecal valve, draining mesenteric lymph nodes, as well as bilateral mammary tissue, and mammary lymph nodes. For each individual, four pooled tissue samples were created, consisting of samples from the intestines (distal ileum, mid jejunum, ileocecal valve), the draining mesenteric lymph nodes, bilateral mammary tissue, and the bilateral mammary lymph nodes, respectively.
2.2. Laboratory Analyses
2.3. Data Analyses
2.4. Animal Welfare and Ethical Considerations
3. Results
3.1. Colostrum
3.2. Milk
3.3. Milk and Tissue Samples Collected Post Mortem
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Windsor, P.A. Paratuberculosis in sheep and goats. Vet. Microbiol. 2015, 181, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Sigurethardottir, O.G.; Valheim, M.; Press, C.M. Establishment of Mycobacterium avium subsp. paratuberculosis infection in the intestine of ruminants. Adv. Drug Deliv. Rev. 2004, 56, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Schuiling, E.; Groenveld, J. Geitenziektes en Hun Schade; WUR: Wageningen, The Netherlands, 2005; Available online: https://edepot.wur.nl/15424 (accessed on 5 February 2019).
- Gonda, M.G.; Chang, Y.M.; Shook, G.E.; Collins, M.T.; Kirkpatrick, B.W. Effect of Mycobacterium paratuberculosis infection on production, reproduction, and health traits in US Holsteins. Prev. Vet. Med. 2007, 80, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J.; Vaughan, J.A.; Stiles, P.L.; Noske, P.J.; Tizard, M.L.; Prowse, S.J.; Michalski, W.P.; Butler, K.L.; Jones, S.L. A long-term bacteriological and immunological study in Holstein-Friesian cattle experimentally infected with Mycobacterium avium subsp. paratuberculosis and necropsy culture results for Holstein-Friesian cattle, Merino sheep and Angora goats. Vet. Microbiol. 2007, 122, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, R.W. Transmission of Paratuberculosis. Vet. Clin. N. Am. Food Anim. Pract. 1996, 12, 305–312. [Google Scholar] [CrossRef]
- Waddell, L.A.; Rajic, A.; Stark, K.D.; McEwen, S.A. The potential Public Health Impact of Mycobacterium avium ssp. paratuberculosis: Global Opinion Survey of Topic Specialists. Zoonoses Public Health 2016, 63, 212–222. [Google Scholar] [CrossRef]
- Barkema, H.W.; Orsel, K.; Nielsen, S.S.; Koets, A.P.; Rutten, V.; Bannantine, J.P.; Keefe, G.P.; Kelton, D.F.; Wells, S.J.; Whittington, R.J.; et al. Knowledge gaps that hamper prevention and control of Mycobacterium avium subspecies paratuberculosis infection. Transbound. Emerg. Dis. 2018, 65, 125–148. [Google Scholar] [CrossRef]
- Groenendaal, H.; Zagmutt, F.J. Scenario analysis of changes in consumption of dairy products caused by a hypothetical causal link between Mycobacterium avium subspecies paratuberculosis and Crohn’s disease. J. Dairy Sci. 2008, 91, 3245–3258. [Google Scholar] [CrossRef]
- Whittington, R.; Donat, K.; Weber, M.F.; Kelton, D.; Nielsen, S.S.; Eisenberg, S.; Arrigoni, N.; Juste, R.; Saez, J.L.; Dhand, N.; et al. Control of paratuberculosis: Who, why and how. A review of 48 countries. BMC Vet. Res. 2019, 15, 198. [Google Scholar] [CrossRef]
- Weber, M.F.; Schaik, G. van Results of the Dutch bulk milk quality assurance programme for paratuberculosis. In Proceedings of the 9th International Colloquium on Paratuberculosis, Tsukuba, Japan, 28 October–2 November 2007; Nielsen, S.S., Ed.; International Association for Paratuberculosis, Inc.: Derio, Spain, 2007; pp. 324–327. [Google Scholar]
- Weber, M.F. Milk quality assurance for paratuberculosis in the national Dutch dairy herd. In The Paratuberculosis Newsletter; International Association for Paratuberculosis, Inc.: Derio, Spain, 2008. [Google Scholar]
- Geraghty, T.; Graham, D.A.; Mullowney, P.; More, S.J. A review of bovine Johne’s disease control activities in 6 endemically infected countries. Prev. Vet. Med. 2014, 116, 1–11. [Google Scholar] [CrossRef][Green Version]
- Nagel-Alne, G.E.; Asheim, L.J.; Hardaker, J.B.; Solverod, L.; Lindheim, D.; Valle, P.S. The Norwegian Healthier Goats programme--a financial cost-benefit analysis. Prev. Vet. Med. 2014, 114, 96–105. [Google Scholar] [CrossRef]
- Luttikholt, S.; Lievaart-Peterson, K.; Gonggrijp, M.; Aalberts, M.; van Schaik, G.; Vellema, P. Mycobacterium avium subsp. paratuberculosis ELISA Responses in Milk Samples from Vaccinated and Nonvaccinated Dairy Goat Herds in The Netherlands. Vet. Sci. 2019, 6, 58. [Google Scholar] [CrossRef]
- Stabel, J.R.; Goff, J.P. Efficacy of immunologic assays for the detection of Johne’s disease in dairy cows fed additional energy during the periparturient period. J. Vet. Diagn. Investig. 2004, 16, 412–420. [Google Scholar] [CrossRef]
- Streeter, R.N.; Hoffsis, G.F.; Bech-Nielsen, S.; Shulaw, W.P.; Rings, D.M. Isolation of Mycobacterium paratuberculosis from colostrum and milk of subclinically infected cows. Am. J. Vet. Res. 1995, 56, 1322–1324. [Google Scholar]
- Sweeney, R.W.; Whitlock, R.H.; Rosenberger, A.E. Mycobacterium paratuberculosis cultured from milk and supramammary lymph nodes of infected asymptomatic cows. J. Clin. Microbiol. 1992, 30, 166–171. [Google Scholar]
- O’Brien, J.P.; Sherman, D.M. Serum immunoglobulin concentrations of newborn goat kids and subsequent kid survival through weaning. Small Rumin. Res. 1993, 11, 71–77. [Google Scholar] [CrossRef]
- Zadoks, R.N.; Orsel, K.; Verwer, C.; de Winter, A.; van Amerongen, J.J.; Wensing, T. Serum gammaglobulin titer in goat kids after colostrum administration: Effect of commercial colostrum replacers. Tijdschr. Voor Diergeneeskd. 2001, 126, 646–650. [Google Scholar]
- Constant, S.B.; LeBlanc, M.M.; Klapstein, E.F.; Beebe, D.E.; Leneau, H.M.; Nunier, C.J. Serum immunoglobulin G concentration in goat kids fed colostrum or a colostrum substitute. J. Am. Vet. Med. Assoc. 1994, 205, 1759–1762. [Google Scholar]
- Herthnek, D.; Englund, S.; Willemsen, P.T.; Bolske, G. Sensitive detection of Mycobacterium avium subsp. paratuberculosis in bovine semen by real-time PCR. J. Appl. Microbiol. 2006, 100, 1095–1102. [Google Scholar] [CrossRef]
- Eisenberg, S.W.; Koets, A.P.; Nielen, M.; Heederik, D.; Mortier, R.; De Buck, J.; Orsel, K. Intestinal infection following aerosol challenge of calves with Mycobacterium avium subspecies paratuberculosis. Vet. Res. 2011, 42, 117. [Google Scholar] [CrossRef]
- Kluge, J.P.; Merkal, R.S.; Monlux, W.S.; Larsen, A.B.; Kopecky, K.E.; Ramsey, F.K.; Lehmann, R.P. Experimental paratuberculosis in sheep after oral, intratracheal, or intravenous inoculation lesions and demonstration of etiologic agent. Am. J. Vet. Res. 1968, 29, 953–962. [Google Scholar]
- Koets, A.P.R.L.; Ruul, R.; Dinkla, A.; Eisenberg, S.; Lievaart-Peterson, K. Effects of age and environment on adaptive immune responses to Mycobacterium avium ssp. paratuberculosis (MAP) vaccination in dairy goats in relation to paratuberculosis control strategies. Vet. Sci. 2019, 6, 62. [Google Scholar] [CrossRef]
- Godden, S.M.; Smith, S.; Feirtag, J.M.; Green, L.R.; Wells, S.J.; Fetrow, J.P. Effect of on-farm commercial batch pasteurization of colostrum on colostrum and serum immunoglobulin concentrations in dairy calves. J. Dairy Sci. 2003, 86, 1503–1512. [Google Scholar] [CrossRef]
- Stabel, J.R.; Hurd, S.; Calvente, L.; Rosenbusch, R.F. Destruction of Mycobacterium paratuberculosis, Salmonella spp. and Mycoplasma spp. in raw milk by a commercial on-farm high-temperature, short-time pasteurizer. J. Dairy Sci. 2004, 87, 2177–2183. [Google Scholar] [CrossRef]
- Garin-Bastuji, B.; Perrin, B.; Thorel, M.-F.; Martel, J.L. Evaluation of γ-rays irradiation of cows’ colostrum for Brucella abortus, Escherichia coli K99, Salmonella dublin and Mycobacterium paratuberculosis decontamination. Lett. Appl. Microbiol. 1990, 11, 163–166. [Google Scholar] [CrossRef]
- Verhegghe, M.; Rasschaert, G.; Herman, L.; Goossens, K.; Vandaele, L.; De Bleecker, K.; Vlaemynck, G.; Heyndrickx, M.; De Block, J. Reduction of Mycobacterium avium ssp. paratuberculosis in colostrum: Development and validation of 2 methods, one based on curdling and one based on centrifugation. J. Dairy Sci. 2017, 100, 3497–3512. [Google Scholar] [CrossRef]
- Mercier, P.; Freret, S.; Laroucau, K.; Gautier, M.P.; Bremaud, I.; Bertin, C.; Rossignol, C.; Souriau, A.; Guilloteau, L.A. A longitudinal study of the Mycobacterium avium subspecies paratuberculosis infection status in young goats and their mothers. Veterinary microbiology 2016, 195, 9–16. [Google Scholar] [CrossRef]
- Verin, R.; Perroni, M.; Rossi, G.; De Grossi, L.; Botta, R.; De Sanctis, B.; Rocca, S.; Cubeddu, T.; Crosby-Durrani, H.; Taccini, E. Paratuberculosis in sheep: Histochemical, immunohistochemical and in situ hybridization evidence of in utero and milk transmission. Res. Vet. Sci. 2016, 106, 173–179. [Google Scholar] [CrossRef]
- Muehlherr, J.E.; Zweifel, C.; Corti, S.; Blanco, J.E.; Stephan, R. Microbiological quality of raw goat’s and ewe’s bulk-tank milk in Switzerland. J. Dairy Sci. 2003, 86, 3849–3856. [Google Scholar] [CrossRef]
- Djonne, B.; Jensen, M.R.; Grant, I.R.; Holstad, G. Detection by immunomagnetic PCR of Mycobacterium avium subsp. paratuberculosis in milk from dairy goats in Norway. Vet. Microbiol. 2003, 92, 135–143. [Google Scholar] [CrossRef]
- Bauman, C.A.; Jones-Bitton, A.; Jansen, J.; Kelton, D.; Menzies, P. Evaluation of bulk tank milk PCR and bulk tank milk modified ELISA tests for the detection of paratuberculosis at the herd level in goat and sheep dairies in Ontario, Canada. J. Dairy Sci. 2019, 102, 511–520. [Google Scholar] [CrossRef]
- Stewart, D.J.; Vaughan, J.A.; Stiles, P.L.; Noske, P.J.; Tizard, M.L.; Prowse, S.J.; Michalski, W.P.; Butler, K.L.; Jones, S.L. A long-term study in Angora goats experimentally infected with Mycobacterium avium subsp. paratuberculosis: Clinical disease, faecal culture and immunological studies. Vet. Microbiol. 2006, 113, 13–24. [Google Scholar] [CrossRef]
- Aly, S.S.; Thurmond, M.C. Evaluation of Mycobacterium avium subsp paratuberculosis infection of dairy cows attributable to infection status of the dam. J. Am. Vet. Med Assoc. 2005, 227, 450–454. [Google Scholar] [CrossRef]
- Benedictus, A.; Mitchell, R.M.; Linde-Widmann, M.; Sweeney, R.; Fyock, T.; Schukken, Y.H.; Whitlock, R.H. Transmission parameters of Mycobacterium avium subspecies paratuberculosis infections in a dairy herd going through a control program. Prev. Vet. Med. 2008, 83, 215–227. [Google Scholar] [CrossRef]
- Park, H.E.; Park, H.T.; Jung, Y.H.; Yoo, H.S. Establishment a real-time reverse transcription PCR based on host biomarkers for the detection of the subclinical cases of Mycobacterium avium subsp. paratuberculosis. PLoS ONE 2017, 12, e0178336. [Google Scholar] [CrossRef]
- Mitchell, R.M.; Beaver, A.; Knupfer, E.; Pradhan, A.K.; Fyock, T.; Whitlock, R.H.; Schukken, Y.H. Elucidating Transmission Patterns of Endemic Mycobacterium avium subsp. paratuberculosis Using Molecular Epidemiology. Vet. Sci. 2019, 6, 32. [Google Scholar] [CrossRef]
- Mitchell, R.M.; Medley, G.F.; Collins, M.T.; Schukken, Y.H. A meta-analysis of the effect of dose and age at exposure on shedding of Mycobacterium avium subspecies paratuberculosis (MAP) in experimentally infected calves and cows. Epidemiol. Infect. 2012, 140, 231–246. [Google Scholar] [CrossRef]
- Eisenberg, S.W.; Rutten, V.P.; Koets, A.P. Dam Mycobacterium avium subspecies paratuberculosis (MAP) infection status does not predetermine calves for future shedding when raised in a contaminated environment: A cohort study. Vet. Res. 2015, 46, 70. [Google Scholar] [CrossRef]
- Pithua, P.; Godden, S.M.; Wells, S.J.; Stabel, J.R. Evaluation of the risk of paratuberculosis in adult cows fed Mycobacterium avium subsp paratuberculosis DNA-positive or -negative colostrum as calves. Am. J. Vet. Res. 2011, 72, 1456–1464. [Google Scholar] [CrossRef]
- Pithua, P.; Wells, S.J.; Sreevatsan, S.; Godden, S.M. Lack of evidence for fecal shedding of Mycobacterium avium subsp. paratuberculosis in calves born to fecal culture positive dams. Prev. Vet. Med. 2010, 93, 242–245. [Google Scholar] [CrossRef]
- Whittington, R.J.; Marshall, D.J.; Nicholls, P.J.; Marsh, I.B.; Reddacliff, L.A. Survival and dormancy of Mycobacterium avium subsp. paratuberculosis in the environment. Appl. Environ. Microbiol. 2004, 70, 2989–3004. [Google Scholar] [CrossRef]
- Eisenberg, S.W.; Koets, A.P.; Hoeboer, J.; Bouman, M.; Heederik, D.; Nielen, M. Presence of Mycobacterium avium subsp. paratuberculosis in environmental samples collected on commercial Dutch dairy farms. Appl. Environ. Microbiol. 2010, 76, 6310–6312. [Google Scholar] [CrossRef][Green Version]
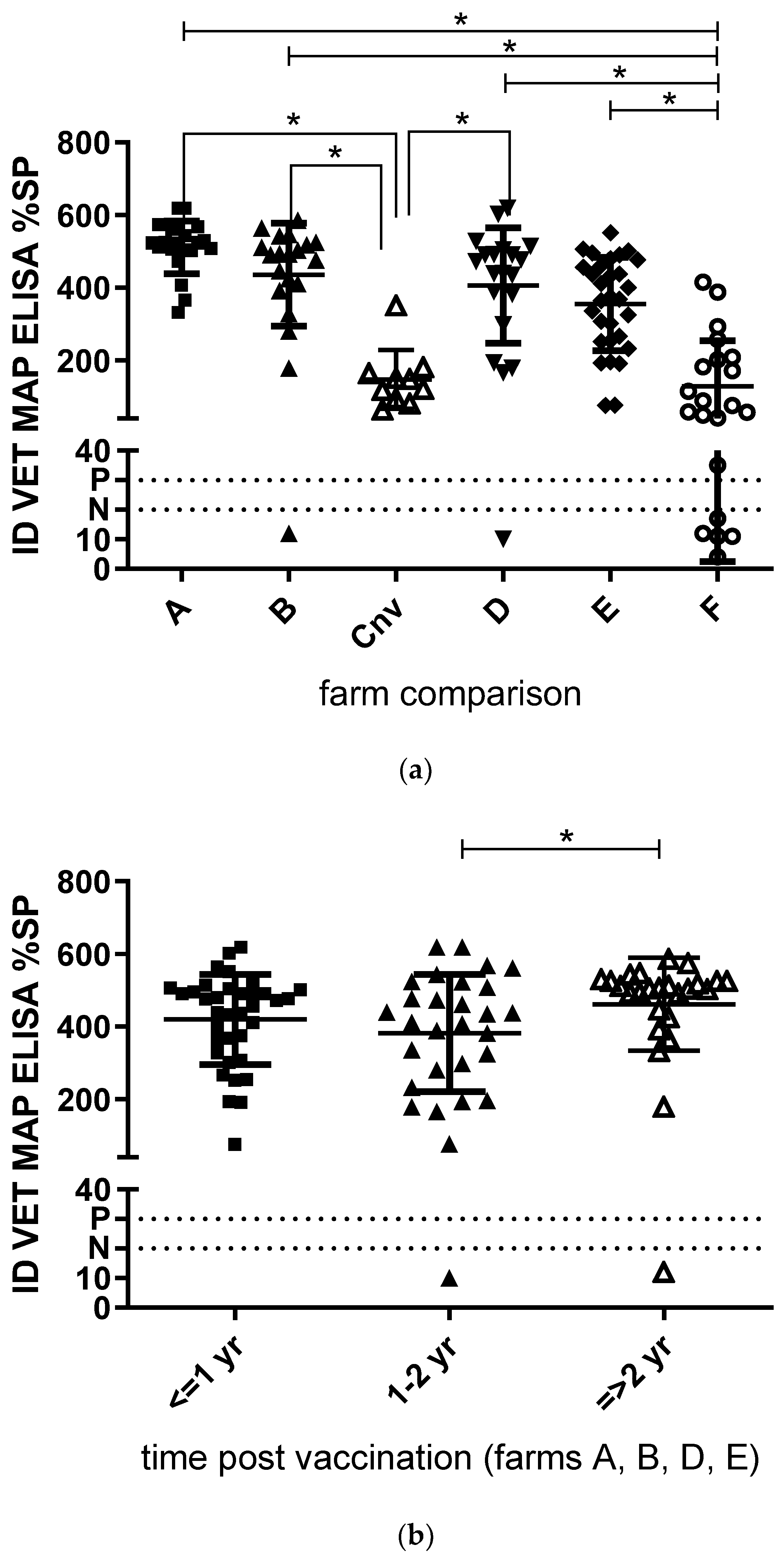
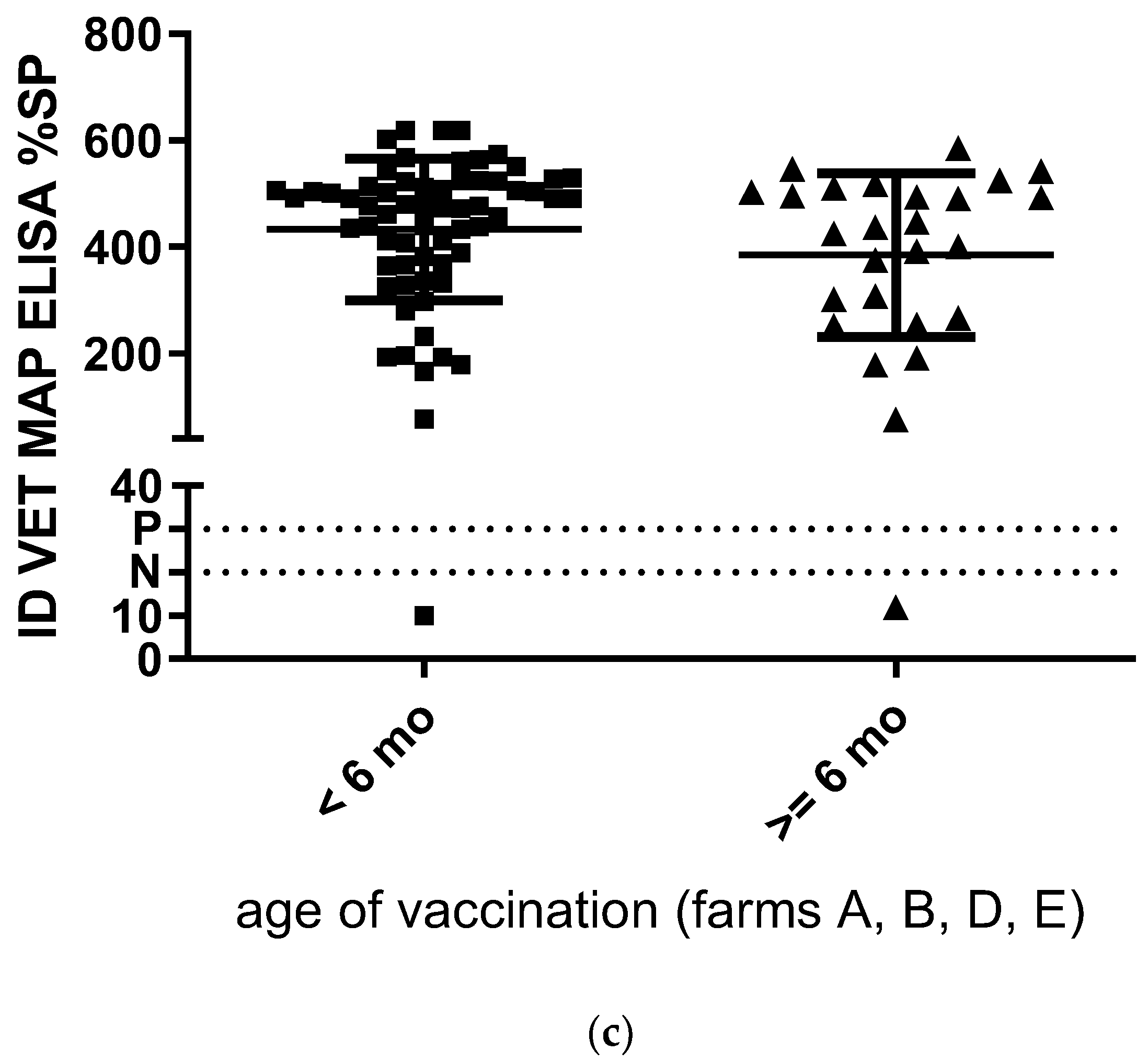
| Herd | Herd Size | MAP History | Age at MAP Vaccination | Sample Type | Age at Sampling (no. of Goats × Age in Years) | Lactation at Sampling (no. of Goats × Lactation) |
|---|---|---|---|---|---|---|
| A | 840 Total 411 > 1 yo 429 < 1 yo | Known (post mortem) | At approx. 2 months | Colostrum | Colostrum: 10 × 1, 4 × 2, 1 × 3, 2 × 4, 1 × 5, 2 × 6. | 10 × 1st, 5 × 2nd, 1 × 3rd, 3 × 4th, 1 × 5th. |
| B | 962 Total 662 > 1 yo 300 < 1 yo | Known (post mortem and serology) | Between 1 to 8 months | Colostrum Milk Goats for post mortem | Colostrum: 6 × 1, 2 × 3, 2 × 4, 4 × 5, 2 × 6, 3 × 8. Mortem: 1 × 2, 1 × 5, 1 × 6, 1 × 8, 1 × 9. | 6 × 1st, 6 × 2nd, 3 × 3rd, 5 × 4th. |
| Cnv | 1727 Total 1292 > 1 yo 435 < 1 yo | Unsuspected | Not applicable | Colostrum | Colostrum: 10 × 1. | 10 × 1st |
| D | 1673 Total 1287 > 1 yo 386 < 1 yo | Known (post mortem) | At approx. 3 months of age | Colostrum | Colostrum: 11 × 1, 8 × 2, 1 × 3. | 19 × 1st, 1 × 2nd. |
| E | 1533 Total 1153 > 1 yo 380 < 1 yo | Suspected | 18 as goat kids 12 as adults | Colostrum | Colostrum: 8 × 1, 22 × 2. | 30 × 1st |
| Fpv | 1226 Total 878 > 1 yo 348 < 1 yo | Known (serology) | Unknown | Colostrum | 17 × no age data provided | 17 × no lactation data provided |
| G | 577 Total 453 > 1 yo 124 < 1 yo | Known (post mortem and serology) | Partly | Goats for post mortem | Post mortem: 1 × 4, 1 × 6, 1 × 9, 2 × 10, 1 × 11, 1 × 13. | Unknown |
| H | 554 Total 435 > 1 yo 119 < 1 yo | Known (serology and post mortem) | Not applicable | Goats for post mortem | Post mortem: 1 × 2, 3 × 3, 1 × 4, 3 × 7, 1 × 9, and 6 × unknown. | Unknown |
| I | 902 Total 676 > 1 yo 226 < 1 yo | Known (serology and post mortem) | As goat kids | Colostrum | 22 × no age data provided | 22 × no lactation data provided |
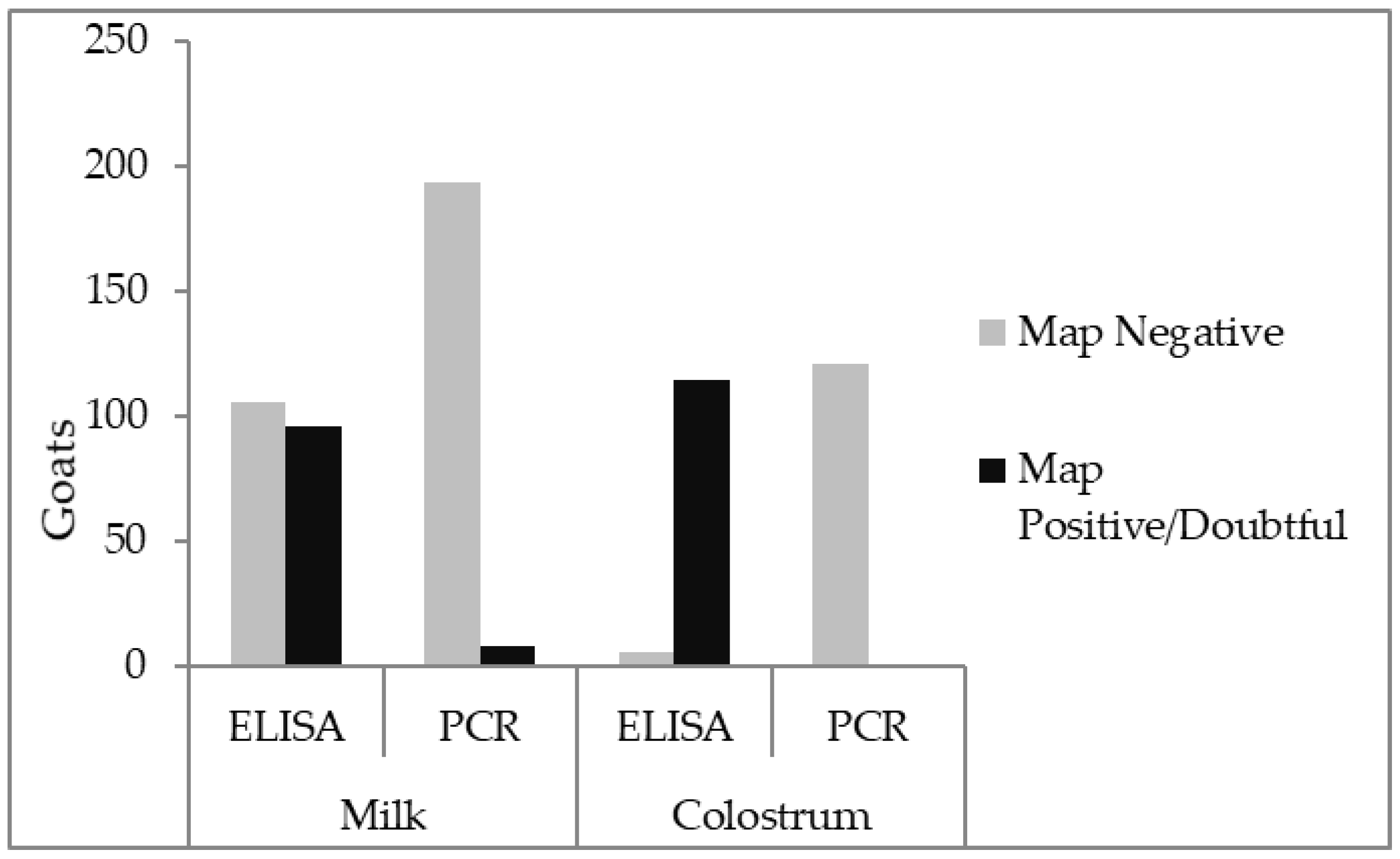
| PCR Negative | PCR Dubieus | PCR Positive | Total | |
|---|---|---|---|---|
| ELISA negative | 102 | 2 | 2 | 106 |
| ELISA dubious | 22 | 0 | 1 | 23 |
| ELISA positive | 70 | 3 | 0 | 73 |
| Total | 194 | 5 | 3 | 202 |
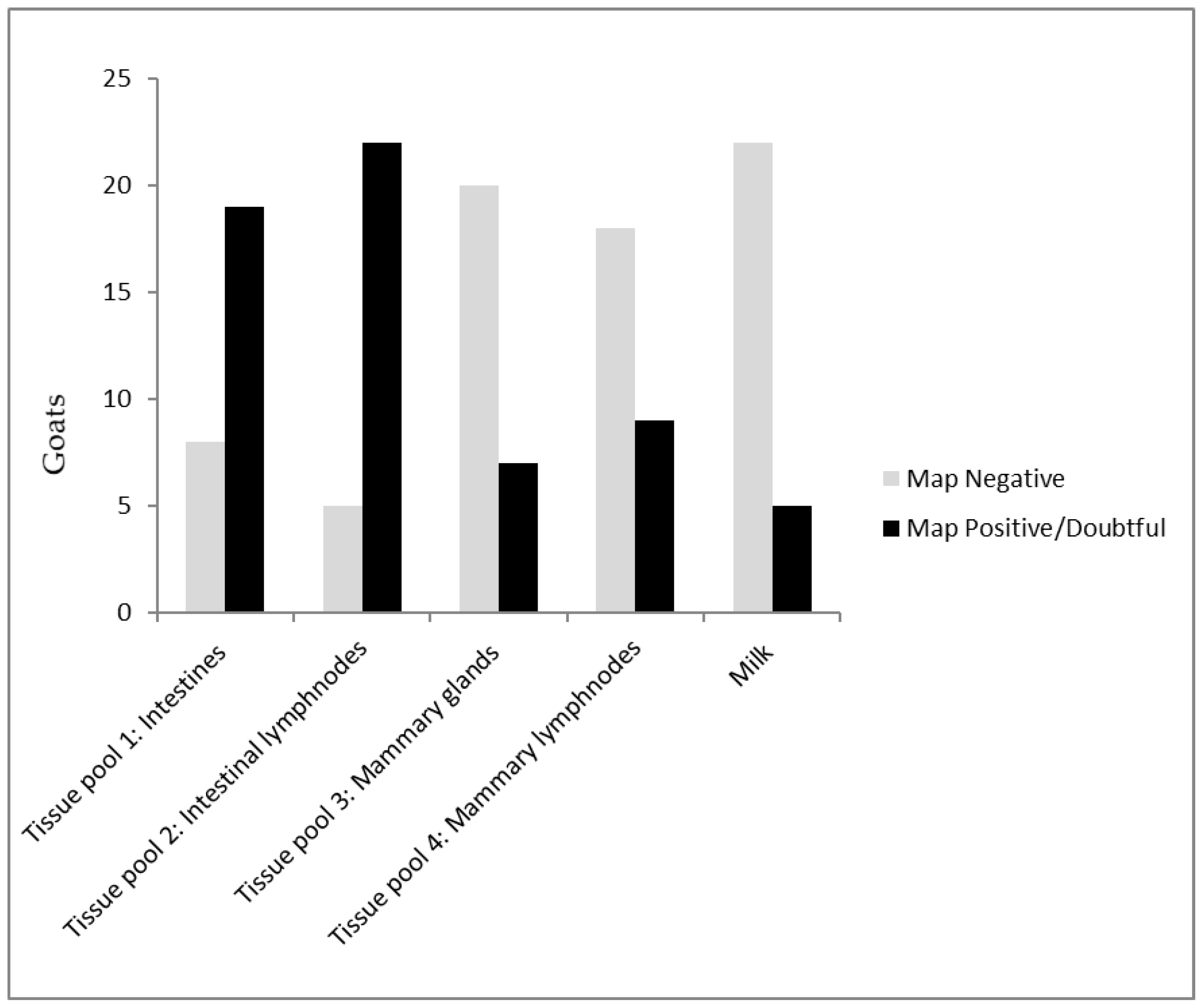
| Pooled Tissue Samples | Milk L | Milk R | Number of Goats | ||||
|---|---|---|---|---|---|---|---|
| Results Combination | Intestines | Mesenteric Lnn | Mammary Lnn | Mammary Tissue | |||
| 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| 2 | 1 | 1 | 1 | 1 | 0 | 0 | 3 |
| 3 | 1 | 1 | 0 | 0 | 1 | 1 | 2 |
| 4 | 1 | 1 | 1 | 0 | 0 | 0 | 3 |
| 5 | 1 | 1 | 0 | 1 | 0 | 0 | 2 |
| 6 | 1 | 1 | 0 | 0 | 1 | 0 | 1 |
| 7 | 1 | 0 | 1 | 1 | 0 | 0 | 1 |
| 8 | 1 | 1 | 0 | 0 | 0 | 0 | 5 |
| 9 | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| 10 | 0 | 1 | 0 | 0 | 0 | 0 | 5 |
| 11 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Total: 19 | Total: 22 | Total: 9 | Total: 7 | Total: 4 | Total: 3 | Total no. of goats: 27 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lievaart-Peterson, K.; Luttikholt, S.; Gonggrijp, M.; Ruuls, R.; Ravesloot, L.; Koets, A.P. Mycobacterium avium Subspecies paratuberculosis DNA and Antibodies in Dairy Goat Colostrum and Milk. Vet. Sci. 2019, 6, 96. https://doi.org/10.3390/vetsci6040096
Lievaart-Peterson K, Luttikholt S, Gonggrijp M, Ruuls R, Ravesloot L, Koets AP. Mycobacterium avium Subspecies paratuberculosis DNA and Antibodies in Dairy Goat Colostrum and Milk. Veterinary Sciences. 2019; 6(4):96. https://doi.org/10.3390/vetsci6040096
Chicago/Turabian StyleLievaart-Peterson, Karianne, Saskia Luttikholt, Maaike Gonggrijp, Robin Ruuls, Lars Ravesloot, and Ad P. Koets. 2019. "Mycobacterium avium Subspecies paratuberculosis DNA and Antibodies in Dairy Goat Colostrum and Milk" Veterinary Sciences 6, no. 4: 96. https://doi.org/10.3390/vetsci6040096
APA StyleLievaart-Peterson, K., Luttikholt, S., Gonggrijp, M., Ruuls, R., Ravesloot, L., & Koets, A. P. (2019). Mycobacterium avium Subspecies paratuberculosis DNA and Antibodies in Dairy Goat Colostrum and Milk. Veterinary Sciences, 6(4), 96. https://doi.org/10.3390/vetsci6040096





