Effects of a Change from an Indoor-Based Total Mixed Ration to a Rotational Pasture System Combined with a Moderate Concentrate Feed Supply on Immunological Cell and Blood Parameters of Dairy Cows
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments
2.2. Animal Measurements
2.2.1. Blood Sampling
2.2.2. Hematology
2.2.3. Serum Metabolites
2.2.4. Flow Cytometry
2.2.5. Enzyme Assays
2.3. Statistical Analyses
3. Results
3.1. Animal Performance
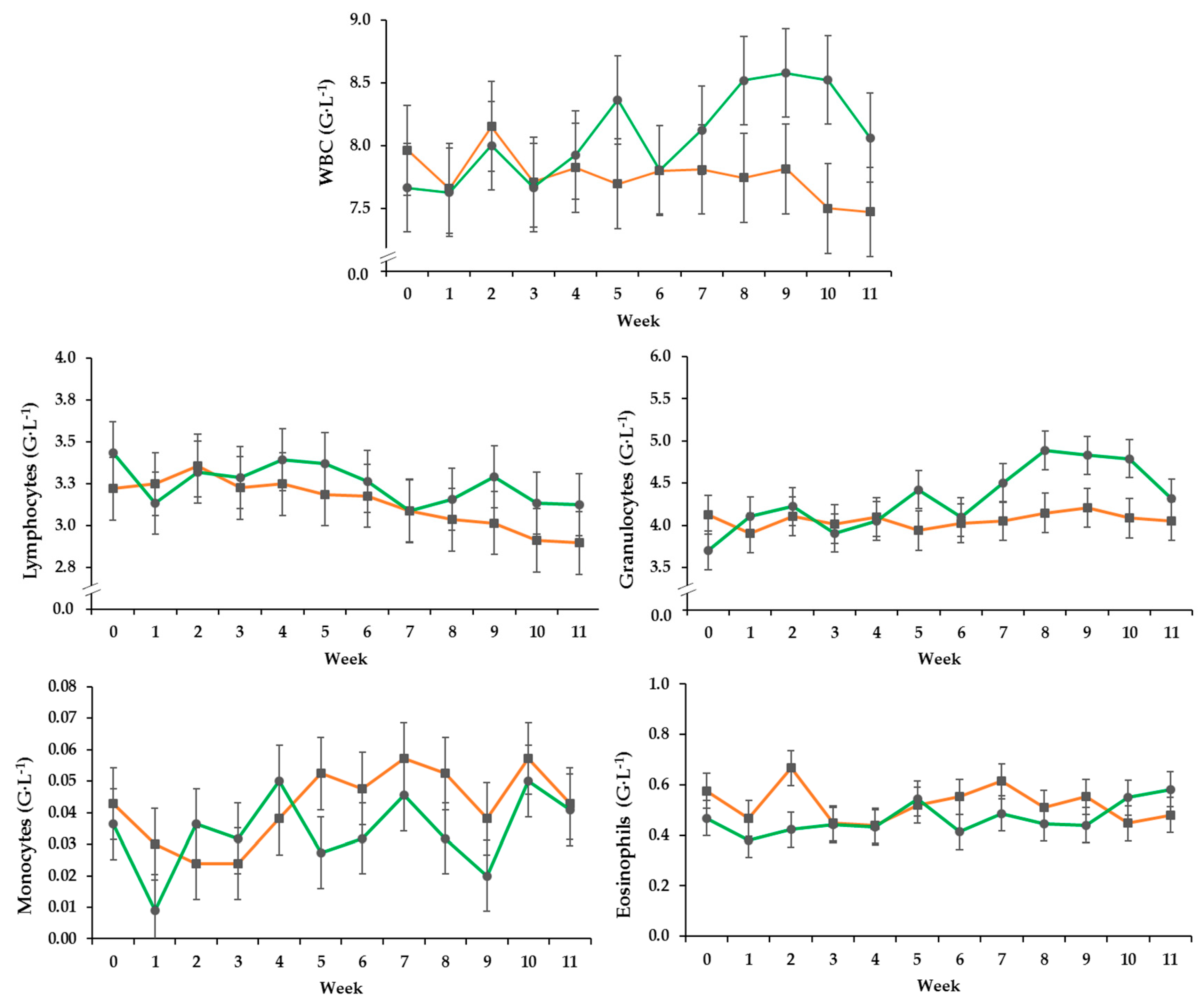
3.2. Hematology
3.3. Serum Metabolites
3.4. Flow Cytometry
3.4.1. T-Cell Phenotyping
3.4.2. DHR Assay
3.5. Enzyme Assay and Serum Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hartwiger, J.; Schären, M.; Gerhards, U.; Hüther, L.; Frahm, J.; von Soosten, D.; Kluess, J.; Bachmann, M.; Zeyner, A.; Meyer, U.; et al. Effects of a change from an indoor-based total mixed ration to a rotational pasture system combined with a moderate concentrate feed supply on the health and performance of dairy cows. Animals 2018, 8, 169. [Google Scholar] [CrossRef]
- Hartwiger, J.; Schären, M.; Potthoff, S.; Hüther, L.; Kersten, S.; von Soosten, D.; Beineke, A.; Meyer, U.; Breves, G.; Dänicke, S. Effects of a change from an indoor-based total mixed ration to a rotational pasture system combined with a moderate concentrate feed supply on rumen fermentation of dairy cows. Animals 2018, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Schären, M.; Jostmeier, S.; Ruesink, S.; Hüther, L.; Frahm, J.; Bulang, M.; Meyer, U.; Rehage, J.; Isselstein, J.; Breves, G.; et al. The effects of a ration change from a total mixed ration to pasture on health and production of dairy cows. J. Dairy Sci. 2016, 99, 1183–1200. [Google Scholar] [CrossRef]
- Schären, M.; Seyfang, G.M.; Steingass, H.; Dieho, K.; Dijkstra, J.; Hüther, L.; Frahm, J.; Beineke, A.; von Soosten, D.; Meyer, U.; et al. The effects of a ration change from a total mixed ration to pasture on rumen fermentation, volatile fatty acid absorption characteristics, and morphology of dairy cows. J. Dairy Sci. 2016, 99, 3549–3565. [Google Scholar] [CrossRef]
- Schären, M.; Kiri, K.; Riede, S.; Gardener, M.; Meyer, U.; Hummel, J.; Urich, T.; Breves, G.; Dänicke, S. Alterations in the rumen liquid-, particle- and epithelium-associated microbiota of dairy cows during the transition from a silage- and concentrate-based ration to pasture in spring. Front. Microbiol. 2017, 8, 744. [Google Scholar] [CrossRef] [PubMed]
- Drong, C.; Meyer, U.; Soosten, D.; Frahm, J.; Rehage, J.; Schirrmeier, H.; Beer, M.; Dänicke, S. Effects of monensin and essential oils on immunological, haematological and biochemical parameters of cows during the transition period. J. Anim. Physiol. Anim. Nutr. 2017, 101, 791–806. [Google Scholar] [CrossRef]
- Tizard, I.R. Veterinary Immunology: An Introduction; Saunders: Philadelphia, PA, USA, 2004. [Google Scholar]
- Tuo, W.; Li, L.; Lv, Y.; Carrillo, J.; Brown, D.; Davis, W.C.; Song, J.; Zarlenga, D.; Xiao, Z. Abomasal mucosal immune responses of cattle with limited or continuous exposure to pasture-borne gastrointestinal nematode parasite infection. Vet. Parasitol. 2016, 229, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Meyer, U.; Kersten, S.; Frahm, J. Animal models to study the impact of nutrition on the immune system of the transition cow. Res. Vet. Sci. 2018, 116, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Ingvartsen, K.L.; Moyes, K. Nutrition, immune function and health of dairy cattle. Animal 2012, 7, 112–122. [Google Scholar] [CrossRef]
- Scalia, D.; Lacetera, N.; Bernabucci, U.; Demeyere, K.; Duchateau, L.; Burvenich, C. In Vitro Effects of Nonesterified Fatty Acids on Bovine Neutrophils Oxidative Burst and Viability1. J. Dairy Sci. 2006, 89, 147–154. [Google Scholar] [CrossRef]
- Trevisi, E.; Amadori, M.; Riva, F.; Bertoni, G.; Bani, P. Evaluation of innate immune responses in bovine forestomachs. Res. Vet. Sci. 2014, 96, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, E.; Riva, F.; Filipe, J.F.S.; Massara, M.; Minuti, A.; Bani, P.; Amadori, M. Innate immune responses to metabolic stress can be detected in rumen fluids. Res. Vet. Sci. 2018, 117, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Contreras, G.A.; Sordillo, L.M. Lipid mobilization and inflammatory responses during the transition period of dairy cows. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Ametaj, B.N.; Bradford, B.J.; Bobe, G.; Nafikov, R.A.; Lu, Y.; Young, J.W.; Beitz, D.C. Strong relationships between mediators of the acute phase response and fatty liver in dairy cows. Can. J. Anim. Sci. 2005, 85, 165–175. [Google Scholar] [CrossRef]
- Badawy, A.A.B. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int. J. Tryptophan Res. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Raphael, W.; Sordillo, L.M. Dietary polyunsaturated fatty acids and inflammation: The role of phospholipid biosynthesis. Int. J. Mol. Sci. 2013, 14, 21167–21188. [Google Scholar] [CrossRef]
- Celi, P. Biomarkers of oxidative stress in ruminant medicine. Immunopharmacol. Immunotoxicol. 2011, 33, 233–240. [Google Scholar] [CrossRef]
- Lynch, A.; Kerry, J.P.; Buckley, D.J.; Morrissey, P.A.; Lopez-Bote, C. Use of high pressure liquid chromatography (HPLC) for the determination of cda-tocopherol levels in forage (silage/grass) samples collected from different regions in Ireland. Food Chem. 2001, 72, 521–524. [Google Scholar] [CrossRef]
- Yue, Y.; Hymoller, L.; Jensen, S.K.; Lauridsen, C. Effect of vitamin D treatments on plasma metabolism and immune parameters of healthy dairy cows. Arch. Anim. Nutr. 2018, 72, 205–220. [Google Scholar] [CrossRef] [PubMed]
- VDLUFA. Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschunganstalten. Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch), Bd. III Die Chemische Untersuchung von Futtermitteln; VDLUFA-Verlag: Darmstadt, Germany, 2012. [Google Scholar]
- Heublein, C.; Dohme-Meier, F.; Sudekum, K.H.; Bruckmaier, R.M.; Thanner, S.; Schori, F. Impact of cow strain and concentrate supplementation on grazing behaviour, milk yield and metabolic state of dairy cows in an organic pasture-based feeding system. Animal 2017, 11, 1163–1173. [Google Scholar] [CrossRef]
- Gorniak, T.; Meyer, U.; Sudekum, K.H.; Danicke, S. Impact of mild heat stress on dry matter intake, milk yield and milk composition in mid-lactation Holstein dairy cows in a temperate climate. Arch. Anim. Nutr. 2014, 68, 358–369. [Google Scholar] [CrossRef]
- Kellaway, R.; Colditz, P. The effect of heat stress on growth and nitrogen metabolism in Friesian and F 1 Brahman x Friesian heifers. Aust. J. Agric. Res. 1975, 26, 615–622. [Google Scholar] [CrossRef]
- Collier, R.; Zimbelman, R.; Rhoads, R.; Rhoads, M.; Baumgard, L. A re-evaluation of the impact of temperature humidity index (THI) and black globe humidity index (BGHI) on milk production in high producing dairy cows. In Proceedings of the Western Dairy Management Conference, Reno, NV, USA, 9–11 March 2009; pp. 113–125. [Google Scholar]
- Khan, A.; Khan, M.I.; Iqbal, Z.; Shah, Y.; Ahmad, L.; Watson, D.G. An optimized and validated RP-HPLC/UV detection method for simultaneous determination of all-trans-retinol (vitamin A) and alpha-tocopherol (vitamin E) in human serum: Comparison of different particulate reversed-phase HPLC columns. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 2339–2347. [Google Scholar] [CrossRef]
- Gimeno, E.; Castellote, A.I.; Lamuela-Raventos, R.M.; de la Torre-Boronat, M.C.; Lopez-Sabater, M.C. Rapid high-performance liquid chromatographic method for the simultaneous determination of retinol, alpha-tocopherol and beta-carotene in human plasma and low-density lipoproteins. J. Chromatogr. B Biomed. Sci. 2001, 758, 315–322. [Google Scholar] [CrossRef]
- Hiss, S.; Mielenz, M.; Bruckmaier, R.M.; Sauerwein, H. Haptoglobin concentrations in blood and milk after endotoxin challenge and quantification of mammary Hp mRNA expression. J. Dairy Sci. 2004, 87, 3778–3784. [Google Scholar] [CrossRef]
- Hiss, S.; Weinkauf, C.; Hachenberg, S.; Sauerwein, H. Short communication: Relationship between metabolic status and the milk concentrations of haptoglobin and lactoferrin in dairy cows during early lactation. J. Dairy Sci. 2009, 92, 4439–4443. [Google Scholar] [CrossRef]
- Skinner, J.G. International standardization of acute phase proteins. Vet. Clin. Pathol. 2001, 30, 2–7. [Google Scholar] [CrossRef]
- Hüther, L.; Hartwiger, J.; Drong, C.; Meyer, U.; Dänicke, S. Simultaneous determination of tryptophan, kynurenine and niacin in serum of periparturient dairy cows by high-performance liquid chromatography with diode array detection. J. Vet. Sci. Med. Diagn. 2016, 5, 6. [Google Scholar] [CrossRef]
- Dänicke, S.; Winkler, J.; Meyer, U.; Frahm, J.; Kersten, S. Haematological, clinical–chemical and immunological consequences of feeding Fusarium toxin contaminated diets to early lactating dairy cows. Mycotoxin Res. 2017, 33, 1–13. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [CrossRef]
- Dirksen, G.; Gründer, H.D.; Stöber, M. Die klinische Untersuchung des Rindes: Begründet von Gustav Rosenberger; Enke: Erlangen, Germany, 2012. [Google Scholar]
- Murata, H.; Shimada, N.; Yoshioka, M. Current research on acute phase proteins in veterinary diagnosis: An overview. Vet. J. 2004, 168, 28–40. [Google Scholar] [CrossRef]
- Lomborg, S.R.; Nielsen, L.R.; Heegaard, P.M.; Jacobsen, S. Acute phase proteins in cattle after exposure to complex stress. Vet. Res. Commun. 2008, 32, 575–582. [Google Scholar] [CrossRef]
- Danscher, A.M.; Thoefner, M.B.; Heegaard, P.M.H.; Ekstrøm, C.T.; Jacobsen, S. Acute phase protein response during acute ruminal acidosis in cattle. Livest. Sci. 2011, 135, 62–69. [Google Scholar] [CrossRef]
- Zebeli, Q.; Dijkstra, J.; Tafaj, M.; Steingass, H.; Ametaj, B.N.; Drochner, W. Modeling the adequacy of dietary fiber in dairy cows based on the responses of ruminal pH and milk fat production to composition of the diet. J. Dairy Sci. 2008, 91, 2046–2066. [Google Scholar] [CrossRef]
- Saremi, B.; Al-Dawood, A.; Winand, S.; Muller, U.; Pappritz, J.; von Soosten, D.; Rehage, J.; Danicke, S.; Haussler, S.; Mielenz, M.; et al. Bovine haptoglobin as an adipokine: Serum concentrations and tissue expression in dairy cows receiving a conjugated linoleic acids supplement throughout lactation. Vet. Immunol. Immunopathol. 2012, 146, 201–211. [Google Scholar] [CrossRef]
- Hayaishi, O. Properties and function of indoleamine 2,3-dioxygenase. J. Biochem. 1976, 79, 13–21. [Google Scholar] [CrossRef]
- Thomas, S.R.; Stocker, R. Redox reactions related to indoleamine 2,3-dioxygenase and tryptophan metabolism along the kynurenine pathway. Redox Rep. 1999, 4, 199–220. [Google Scholar] [CrossRef]
- Schröcksnadel, K.; Wirleitner, B.; Winkler, C.; Fuchs, D. Monitoring tryptophan metabolism in chronic immune activation. Clin. Chim. Acta 2006, 364, 82–90. [Google Scholar] [CrossRef]
- Moyes, K.M.; Drackley, J.K.; Salak-Johnson, J.L.; Morin, D.E.; Hope, J.C.; Loor, J.J. Dietary-induced negative energy balance has minimal effects on innate immunity during a Streptococcus uberis mastitis challenge in dairy cows during midlactation. J. Dairy Sci. 2009, 92, 4301–4316. [Google Scholar] [CrossRef]
- Inaba, H.; Cao, X.; Pounds, S.; Pui, C.H.; Rubnitz, J.E.; Ribeiro, R.C.; Razzouk, B.I. Randomized trial of 2 dosages of prophylactic granulocyte-colony-stimulating factor after induction chemotherapy in pediatric acute myeloid leukemia. Cancer 2011, 117, 1313–1320. [Google Scholar] [CrossRef]
- El-Benna, J.; Hurtado-Nedelec, M.; Marzaioli, V.; Marie, J.-C.; Gougerot-Pocidalo, M.-A.; Dang, P.M.-C. Priming of the neutrophil respiratory burst: Role in host defense and inflammation. Immunol. Rev. 2016, 273, 180–193. [Google Scholar] [CrossRef]
- Celi, P.; Cowieson, A.J.; Fru-Nji, F.; Kluenter, A.M.; Verlhac, V. The growing importance of defining gut “health” in animal nutrition and health. J. Anim. Sci. 2016, 94, 214. [Google Scholar] [CrossRef]
- Krehbiel, C.R.; Zhang, G. Modulating gut health in beef, dairy cattle vital productivity. Feedstuffs Repr. 2017, 89, 3. [Google Scholar]
- Steele, M.A.; Malmuthuge, N.; Guan, L.L. Opportunities to Improve Gut Health in Ruminant Production Systems. In Proceedings of the Cornell Nutrition Conference for Feed Manufacturers, Department of Animal Science in the College of Agriculture and Life Sciences at Cornell University, New York, NY, USA, 19 October 2015. [Google Scholar]
- Sies, H.; Stahl, W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am. J. Clin. Nutr. 1995, 62, 1315S–1321S. [Google Scholar] [CrossRef]
- Bernabucci, U.; Ronchi, B.; Lacetera, N.; Nardone, A. Influence of body condition score on relationships between metabolic status and oxidative stress in periparturient dairy cows. J. Dairy Sci. 2005, 88, 2017–2026. [Google Scholar] [CrossRef]
- Kay, J.K.; Roche, J.R.; Kolver, E.S.; Thomson, N.A.; Baumgard, L.H. A comparison between feeding systems (pasture and TMR) and the effect of vitamin E supplementation on plasma and milk fatty acid profiles in dairy cows. J. Dairy Res. 2005, 72, 322–332. [Google Scholar] [CrossRef]
- Lewis, G. Applied Geochemistry in the 1980s; Thornton, I., Howarth, R.J., Eds.; Springer Science & Business Media: London, UK, 1986; pp. 267–349. [Google Scholar]
- Politis, I.; Bizelis, I.; Tsiaras, A.; Baldi, A. Effect of vitamin E supplementation on neutrophil function, milk composition and plasmin activity in dairy cows in a commercial herd. J. Dairy Res. 2004, 71, 273–278. [Google Scholar] [CrossRef]
- Allison, R.D.; Laven, R.A. Effect of vitamin E supplementation on the health and fertility of dairy cows: A review. Vet. Rec. 2000, 147, 703–708. [Google Scholar]
- McDowell, L.R.; Wilkinson, N.; Madison, R.; Felix, T. Vitamins and minerals functioning as antioxidants with supplementation considerations. In Proceedings of the Florida Ruminant Nutrition Symposium. Best Western Gateway Grand, Gainesville, FL, USA, 30–31 January 2007; pp. 30–31. [Google Scholar]
- Politis, I.; Hidiroglou, M.; Batra, T.R.; Gilmore, J.A.; Gorewit, R.C.; Scherf, H. Effects of vitamin E on immune function of dairy cows. Am. J. Vet. Res. 1995, 56, 179–184. [Google Scholar]
- Descalzo, A.M.; Rossetti, L.; Páez, R.; Grigioni, G.; García, P.T.; Costabel, L.; Negri, L.; Antonacci, L.; Salado, E.; Bretschneider, G.; et al. Differential Characteristics of Milk Produced in Grazing Systems and Their Impact on Dairy Products. In Milk Production—Advanced Genetic Traits, Cellular Mechanism, Animal Management and Health; Chaiyabutr, N., Ed.; InTech: Rijeka, Croacia, 2012; pp. 339–368. [Google Scholar]
- Brown, A.J.; Dusso, A.; Slatopolsky, E. Vitamin D. Am. J. Physiol. 1999, 277, F157–F175. [Google Scholar] [CrossRef]
- Hymøller, L.; Jensen, S.K. Plasma transport of ergocalciferol and cholecalciferol and their 25-hydroxylated metabolites in dairy cows. Domest. Anim. Endocrinol. 2017, 59, 44–52. [Google Scholar] [CrossRef]
- Horst, R.L.; Goff, J.P.; Reinhardt, T.A. Role of vitamin D in calcium homeostasis and its use in prevention of bovine periparturient paresis. Acta Vet. Scand. Suppl. 2003, 97, 35–50. [Google Scholar]
- Waters, W.R.; Nonnecke, B.J.; Rahner, T.E.; Palmer, M.V.; Whipple, D.L.; Horst, R.L. Modulation of Mycobacterium bovis-specific responses of bovine peripheral blood mononuclear cells by 1, 25-dihydroxyvitamin D3. J. Clin. Diagn. Lab. Immunol. 2001, 8, 1204–1212. [Google Scholar]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef]
- Nelson, C.D.; Reinhardt, T.A.; Lippolis, J.D.; Sacco, R.E.; Nonnecke, B.J. Vitamin D signaling in the bovine immune system: A model for understanding human vitamin D requirements. Nutrients 2012, 4, 181–196. [Google Scholar] [CrossRef]

| Type of Feeding | Item 2 (g·kg−1 of DM, Unless Otherwise Noted) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM (g·kg−1) | Ash | CP | uCP | NEL * | Sugar | Starch | RNB | CF | NDF | ADF | EE | |
| TMR 1 (CG/PG) | 471 ± 6 | 66 ± 0 | 158 ± 1 | 152 ± 0.5 | 6.8 ± 0 | 18 ± 0 | 253 ± 2.5 | 1.1 ± 0.3 | 184 ± 0 | 363 ± 0 | 205 ± 1.5 | 37 ± 0.5 |
| Pasture (PG) | 174 ± 23 | 94 ± 9 | 188 ± 19 | 142 ± 7 | 6.2 ± 0.3 | 114 ± 45 | -- | 6.2 ± 1.9 | 231 ± 34 | 471 ± 40 | 254 ± 37 | 41 ± 5.8 |
| Concentrate (PG) | 899 | 108 | 93 | 148 | 7.6 | 27 | 579 | 8.9 | 31.5 | 124 | 42 | 30 |
| Variable 1 | Group 2 | Week | PSEM 3 | p-Value | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | G | T | GxT | |||
| DMI (kg/d) 4 | CG | 20.5 | 21.1 | 22.2 | 21.5 | 22.4 | 22.4 | 23.0 | 21.8 | 21.7 | 23.4 | 22.3 | 20.8 | 0.9 | <0.001 | <0.001 | <0.001 |
| DMI (kg/d) 5 | PG | 20.3 | 20.7 | 20.1 | 19.6 | 18.5 | 17.8 | 18.4 | 16.3 | 18.4 | 16.1 | 17.5 | 17.2 | ||||
| Milk yield | CG | 29.3 | 28.3 | 27.5 | 28.8 | 28.9 | 28.9 | 28.6 | 28.1 | 28.4 | 28.0 | 27.3 | 27.2 | 2.7 | 0.013 | <0.001 | <0.001 |
| (kg/d) | PG | 29.1 | 29.4 | 29.8 | 29.4 | 26.0 | 27.1 | 25.5 | 25.1 | 24.7 | 22.6 | 23.8 | 22.8 | ||||
| Body weight | CG | 620 | 623 | 624 | 621 | 621 | 627 | 627 | 633 | 636 | 645 6 | 653 | 654 | 13 | 0.079 | <0.001 | <0.001 |
| (kg) | PG | 620 | 619 | 607 | 600 | 593 | 588 | 578 | 581 | 584 | 597 6 | 610 | 612 | ||||
| NEB (MJ NEL·day−1) | CG | −5.5 | −2.0 | 6.0 | 0.4 | 5.5 | 6.8 | 12.8 | 4.9 | 2.1 | 13.4 | 6.8 | −3.5 | 2.3 | 0.003 | <0.001 | <0.001 |
| PG | −2.2 | −2.1 | −1.0 | −4.2 | −3.9 | −3.6 | −3.7 | −4.3 | −4.1 | −3.9 | −4.3 | −4.1 | |||||
| Variable | PSEM 1 | p-Value | ||
|---|---|---|---|---|
| Group (G) | Time (T) | G × T | ||
| WBC (G·L−1) * | 0.34 | 0.554 | 0.038 | 0.002 |
| Lymphocytes (G·L−1) | 0.19 | 0.674 | <0.001 | 0.249 |
| Granulocytes (G·L−1) | 0.20 | 0.345 | 0.002 | 0.012 |
| Monocytes (G·L−1) | 0.01 | 0.163 | 0.021 | 0.710 |
| Eosinophils (G·L−1) | 0.07 | 0.082 | 0.595 | 0.683 |
| Variable 1 | Group 2 | 0 | 6 | 11 | PSEM 3 | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| Group (G) | Time (T) | G × T | ||||||
| Haptoglobin 4 (μg·mL−1) | CG | 204 | 108 | 239 | 136 | 0.602 | 0.820 | 0.058 |
| PG | 192 | 482 | 92 | |||||
| Tryptophan (Trp) (mg·L−1) | CG | 5.7 | 5.8 | 6.4 | 0.19 | 0.571 | <0.001 | 0.186 |
| PG | 5.6 | 5.2 | 6.1 | |||||
| Kynurenine (Kyn) (mg·L−1) | CG | 1.05 | 1.10 | 1.24 | 0.05 | 0.208 | <0.001 | 0.422 |
| PG | 0.95 | 0.93 | 1.29 | |||||
| Trp/Kyn | CG | 0.19 | 0.18 | 0.20 | 0.01 | 0.391 | 0.012 | 0.434 |
| PG | 0.24 | 0.19 | 0.20 | |||||
| Variable 1 | Group 2 | Week | PSEM 3 | Group (G) | Time (T) | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| 0 | 6 | 11 | G × T | |||||
| CD4+ (G·L−1) * | CG | 0.77 | 0.86 | 0.79 | 0.02 | 0.052 | 0.005 | 0.129 |
| PG | 0.78 | 0.79 | 0.76 | |||||
| CD4+ (%) | CG | 23.4 | 26.7 | 27.1 | 0.43 | 0.009 | <0.001 | 0.008 |
| PG | 23.7 | 25.2 | 24.9 | |||||
| CD8+ (G·L−1) * | CG | 0.35 | 0.41 | 0.36 | 0.07 | 0.032 | 0.000 | 0.245 |
| PG | 0.35 | 0.37 | 0.34 | |||||
| CD8+ (%) | CG | 10.8 | 12.7 | 12.2 | 0.21 | 0.002 | <0.001 | 0.008 |
| PG | 10.7 | 11.9 | 10.9 | |||||
| CD4+/CD8+ | CG | 2.31 | 2.26 | 2.35 | 0.05 | 0.407 | 0.001 | 0.455 |
| PG | 2.32 | 2.24 | 2.47 | |||||
| Variable 1 | PSEM 2 | p-Value | ||
|---|---|---|---|---|
| Group (G) | Time (T) | G × T | ||
| Basal unstimulated | ||||
| ROS+PMN (G·L−1) 3 | 0.05 | <0.001 | <0.001 | <0.001 |
| ROS+PMN (%) | 1.10 | <0.001 | <0.001 | 0.001 |
| ROS+PMN (MFI) 4 | 546 | 0.873 | 0.001 | 0.363 |
| TPA stimulated | ||||
| ROS+PMN (%) | 0.67 | 0.399 | <0.001 | 0.003 |
| ROS+PMN (MFI) | 2437 | 0.002 | <0.001 | <0.001 |
| Variable 1 | Group 2 | 0 | 6 | 11 | PSEM 3 | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| Group (G) | Time (T) | G × T | ||||||
| HGB in EL (g·dL−1) | CG | 2.63 | 2.48 | 2.33 | 0.09 | 0.006 | 0.372 | 0.016 |
| PG | 2.51 | 2.82 | 2.70 | |||||
| SOD (mU·g Hb−1) # | CG | 3.3 | 3.5 | 4.0 | 0.19 | 0.759 | <0.001 | 0.099 |
| PG | 3.6 | 3.1 | 4.4 | |||||
| GPx (mU·mg Hb−1) # | CG | 225 | 280 | 218 | 8.8 | 0.964 | 0.001 | 0.002 |
| PG | 237 | 242 | 242 | |||||
| Vitamin E (mg·L−1) | CG | 6.47 | 5.34 | 6.34 | 0.27 | <0.001 | <0.001 | <0.001 |
| PG | 6.48 | 8.18 | 8.79 | |||||
| Vitamin E/cholesterol | CG | 0.53 | 0.48 | 0.57 | 0.03 | <0.05 | <0.001 | <0.001 |
| PG | 0.50 | 0.59 | 0.68 | |||||
| 25CHO (μg·L−1) | CG | 38.7 | 35.2 | 29.2 | 1.4 | <0.001 | <0.001 | <0.001 |
| PG | 36.8 | 52.6 | 61.0 | |||||
| 25ERG (μg·L−1) | CG | 16.3 | 19.3 | 18.8 | 0.6 | <0.001 | <0.001 | <0.001 |
| PG | 15.3 | 16.1 | 10.8 | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartwiger, J.; Schären, M.; Frahm, J.; Kersten, S.; Hüther, L.; Sauerwein, H.; Meyer, U.; Breves, G.; Dänicke, S. Effects of a Change from an Indoor-Based Total Mixed Ration to a Rotational Pasture System Combined with a Moderate Concentrate Feed Supply on Immunological Cell and Blood Parameters of Dairy Cows. Vet. Sci. 2019, 6, 47. https://doi.org/10.3390/vetsci6020047
Hartwiger J, Schären M, Frahm J, Kersten S, Hüther L, Sauerwein H, Meyer U, Breves G, Dänicke S. Effects of a Change from an Indoor-Based Total Mixed Ration to a Rotational Pasture System Combined with a Moderate Concentrate Feed Supply on Immunological Cell and Blood Parameters of Dairy Cows. Veterinary Sciences. 2019; 6(2):47. https://doi.org/10.3390/vetsci6020047
Chicago/Turabian StyleHartwiger, Julia, Melanie Schären, Jana Frahm, Susanne Kersten, Liane Hüther, Helga Sauerwein, Ulrich Meyer, Gerhard Breves, and Sven Dänicke. 2019. "Effects of a Change from an Indoor-Based Total Mixed Ration to a Rotational Pasture System Combined with a Moderate Concentrate Feed Supply on Immunological Cell and Blood Parameters of Dairy Cows" Veterinary Sciences 6, no. 2: 47. https://doi.org/10.3390/vetsci6020047
APA StyleHartwiger, J., Schären, M., Frahm, J., Kersten, S., Hüther, L., Sauerwein, H., Meyer, U., Breves, G., & Dänicke, S. (2019). Effects of a Change from an Indoor-Based Total Mixed Ration to a Rotational Pasture System Combined with a Moderate Concentrate Feed Supply on Immunological Cell and Blood Parameters of Dairy Cows. Veterinary Sciences, 6(2), 47. https://doi.org/10.3390/vetsci6020047






