Novel Amphiphilic Cyclobutene and Cyclobutane cis-C18 Fatty Acid Derivatives Inhibit Mycobacterium avium subsp. paratuberculosis Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Fatty Acid Derivatives
2.3. Drug Susceptibility Assays
3. Results
3.1. Inhibitory Activity of Fatty Acid Derivatives against Msm
3.2. Inhibitory Activity of Fatty Acid Derivatives against Map
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rathnaiah, G.; Zinniel, D.K.; Bannantine, J.P.; Stabel, J.R.; Grohn, Y.T.; Collins, M.T.; Barletta, R.G. Pathogenesis, molecular genetics, and genomics of Mycobacterium avium subsp. paratuberculosis, the etiologic agent of Johne’s disease. Front. Vet. Sci. 2017, 4, 187. [Google Scholar] [CrossRef] [PubMed]
- Kuenstner, J.T.; Naser, S.; Chamberlin, W.; Borody, T.; Graham, D.Y.; McNees, A.; Hermon-Taylor, J.; Hermon-Taylor, A.; Dow, C.T.; Thayer, W.; et al. The consensus from the Mycobacterium avium ssp. paratuberculosis (MAP) conference 2017. Front. Public Health 2017, 5, 208. [Google Scholar] [CrossRef]
- Sweeney, R.W.; Collins, M.T.; Koets, A.P.; McGuirk, S.M.; Roussel, A.J. Paratuberculosis (Johne’s disease) in cattle and other susceptible species. J. Vet. Intern. Med. 2012, 26, 1239–1250. [Google Scholar] [CrossRef]
- Harris, N.B.; Barletta, R.G. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 2001, 14, 489–512. [Google Scholar] [CrossRef] [PubMed]
- Fecteau, M.E.; Whitlock, R.H. Treatment and chemoprophylaxis for paratuberculosis. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.L.; Harris, N.B.; Barletta, R.G. Development of a firefly luciferase-based assay for determining antimicrobial susceptibility of Mycobacterium avium subsp. paratuberculosis. J. Clin. Microbiol. 1999, 37, 304–309. [Google Scholar] [PubMed]
- Brumbaugh, G.W.; Frelier, P.F.; Roussel, A.J., Jr.; Thomson, T.D. Prophylactic effect of monensin sodium against experimentally induced paratuberculosis in mice. Am. J. Vet. Res. 1992, 53, 544–546. [Google Scholar] [PubMed]
- Carballeira, N.M. New advances in fatty acids as antimalarial, antimycobacterial and antifungal agents. Prog. Lipid Res. 2008, 47, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Morbidoni, H.R.; Vilcheze, C.; Kremer, L.; Bittman, R.; Sacchettini, J.C.; Jacobs, W.R., Jr. Dual inhibition of mycobacterial fatty acid biosynthesis and degradation by 2-alkynoic acids. Chem. Biol. 2006, 13, 297–307. [Google Scholar] [CrossRef]
- Sittiwong, W.; Zinniel, D.K.; Fenton, R.J.; Marshall, D.D.; Story, C.B.; Kim, B.; Lee, J.Y.; Powers, R.; Barletta, R.G.; Dussault, P.H. Development of cyclobutene- and cyclobutane-functionalized fatty acids with inhibitory activity against Mycobacterium tuberculosis. ChemMedChem 2014, 9, 1838–1849. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Wang, C.; Besra, G.S. Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis. Clin. Microbiol. Rev. 2005, 18, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Kremer, L.; Dover, L.G.; Morbidoni, H.R.; Vilcheze, C.; Maughan, W.N.; Baulard, A.; Tu, S.C.; Honore, N.; Deretic, V.; Sacchettini, J.C.; et al. Inhibition of InhA activity, but not KasA activity, induces formation of a KasA-containing complex in mycobacteria. J. Biol. Chem. 2003, 278, 20547–20554. [Google Scholar] [CrossRef] [PubMed]
- Druszczynska, M.; Kowalski, K.; Wawrocki, S.; Fol, M. Diversity and functionality of mycobacterial mycolic acids in relation to host-pathogen interactions. Curr. Med. Chem. 2017, 24, 4267–4278. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Naturally occurring bioactive Cyclobutane-containing (CBC) alkaloids in fungi, fungal endophytes, and plants. Phytomed. Int. J. Phytother. Phytopharmacol. 2014, 21, 1559–1581. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Y.; Gao, X.H.; Yue, J.M. Attractive natural products with strained cyclopropane and/or cyclobutane ring systems. Sci. China Chem. 2016, 59, 1126–1141. [Google Scholar] [CrossRef]
- Jamieson, E.R.; Lippard, S.J. Structure, recognition, and processing of cisplatin-DNA Adducts. Chem. Rev. 1999, 99, 2467–2498. [Google Scholar] [CrossRef]
- Yoon, T.P. Visible light photocatalysis: The development of photocatalytic radical ion cycloadditions. ACS Catal. 2013, 3, 895–902. [Google Scholar] [CrossRef]
- Namyslo, J.C.; Kaufmann, D.E. The application of cyclobutane derivatives in organic synthesis. Chem. Rev. 2003, 103, 1485–1537. [Google Scholar] [CrossRef]
- Sittiwong, W. Synthesis and Application of Four-Membered Carbocycle Amphiphiles. Ph.D. Thesis, University of Nebraska, Lincoln, NE, USA, 2014. [Google Scholar]
- Foley-Thomas, E.M.; Whipple, D.L.; Bermudez, L.E.; Barletta, R.G. Phage infection, transfection and transformation of Mycobacterium avium complex and Mycobacterium paratuberculosis. Microbiology 1995, 141, 1173–1181. [Google Scholar] [CrossRef]
- Snapper, S.B.; Melton, R.E.; Mustafa, S.; Kieser, T.; Jacobs, W.R., Jr. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 1990, 4, 1911–1919. [Google Scholar] [CrossRef]
- Chacon, O.; Feng, Z.; Harris, N.B.; Caceres, N.E.; Adams, L.G.; Barletta, R.G. Mycobacterium smegmatis D-alanine racemase mutants are not dependent on D-alanine for growth. Antimicrob. Agents Chemother. 2002, 46, 47–54. [Google Scholar] [CrossRef]
- Davis, B.D.; Dubos, R.J. The binding of fatty acids by serum albumin, a protective growth factor in bacteriological media. J. Exp. Med. 1947, 86, 215–228. [Google Scholar] [CrossRef]
- Spector, A.A. Fatty acid binding to plasma albumin. J. Lipid Res. 1975, 16, 165–179. [Google Scholar]
- Fontana, A.; Spolaore, B.; Polverino de Laureto, P. The biological activities of protein/oleic acid complexes reside in the fatty acid. Biochim. Biophys. Acta 2013, 1834, 1125–1143. [Google Scholar] [CrossRef]
- Aldrich, C.; Bertozzi, C.; Georg, G.I.; Kiessling, L.; Lindsley, C.; Liotta, D.; Merz, K.M., Jr.; Schepartz, A.; Wang, S. The ecstasy and agony of assay interference compounds. J. Med. Chem. 2017, 60, 2165–2168. [Google Scholar] [CrossRef]
- McGovern, S.L.; Caselli, E.; Grigorieff, N.; Shoichet, B.K. A common mechanism underlying promiscuous inhibitors from virtual and high-throughput screening. J. Med. Chem. 2002, 45, 1712–1722. [Google Scholar] [CrossRef]
- McGovern, S.L.; Helfand, B.T.; Feng, B.; Shoichet, B.K. A specific mechanism of nonspecific inhibition. J. Med. Chem. 2003, 46, 4265–4272. [Google Scholar] [CrossRef]
- Ganesh, A.N.; Aman, A.; Logie, J.; Barthel, B.L.; Cogan, P.; Al-Awar, R.; Koch, T.H.; Shoichet, B.K.; Shoichet, M.S. Colloidal drug aggregate stability in high serum conditions and pharmacokinetic consequence. ACS Chem. Biol. 2019, 14, 751–757. [Google Scholar] [CrossRef]
- Nikolakopoulou, Z.; Shaikh, M.H.; Dehlawi, H.; Michael-Titus, A.T.; Parkinson, E.K. The induction of apoptosis in pre-malignant keratinocytes by omega-3 polyunsaturated fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) is inhibited by albumin. Toxicol. Lett. 2013, 218, 150–158. [Google Scholar] [CrossRef]
- Brennan, P.J.; Nikaido, H. The envelope of mycobacteria. Annu. Rev. Biochem. 1995, 64, 29–63. [Google Scholar] [CrossRef]
- Marrakchi, H.; Laneelle, M.A.; Daffe, M. Mycolic acids: Structures, biosynthesis, and beyond. Chem. Biol. 2014, 21, 67–85. [Google Scholar] [CrossRef]
- Liu, J.; Barry, C.E., III; Besra, G.S.; Nikaido, H. Mycolic acid structure determines the fluidity of the mycobacterial cell wall. J. Biol. Chem. 1996, 271, 29545–29551. [Google Scholar] [CrossRef]
- McGuire, A.M.; Weiner, B.; Park, S.T.; Wapinski, I.; Raman, S.; Dolganov, G.; Peterson, M.; Riley, R.; Zucker, J.; Abeel, T.; et al. Comparative analysis of Mycobacterium and related Actinomycetes yields insight into the evolution of Mycobacterium tuberculosis pathogenesis. BMC Genom. 2012, 13, 120. [Google Scholar] [CrossRef]
- Marri, P.R.; Bannantine, J.P.; Golding, G.B. Comparative genomics of metabolic pathways in Mycobacterium species: gene duplication, gene decay and lateral gene transfer. FEMS Microbiol. Rev. 2006, 30, 906–925. [Google Scholar] [CrossRef]
- Belardinelli, J.M.; Yazidi, A.; Yang, L.; Fabre, L.; Li, W.; Jacques, B.; Angala, S.K.; Rouiller, I.; Zgurskaya, H.I.; Sygusch, J.; et al. Structure-function profile of MmpL3, the essential mycolic acid transporter from Mycobacterium tuberculosis. ACS Infect. Dis. 2016, 2, 702–713. [Google Scholar] [CrossRef]
- Mycobrowser. Available online: https://mycobrowser.epfl.ch/ (accessed on 26 April 2019).
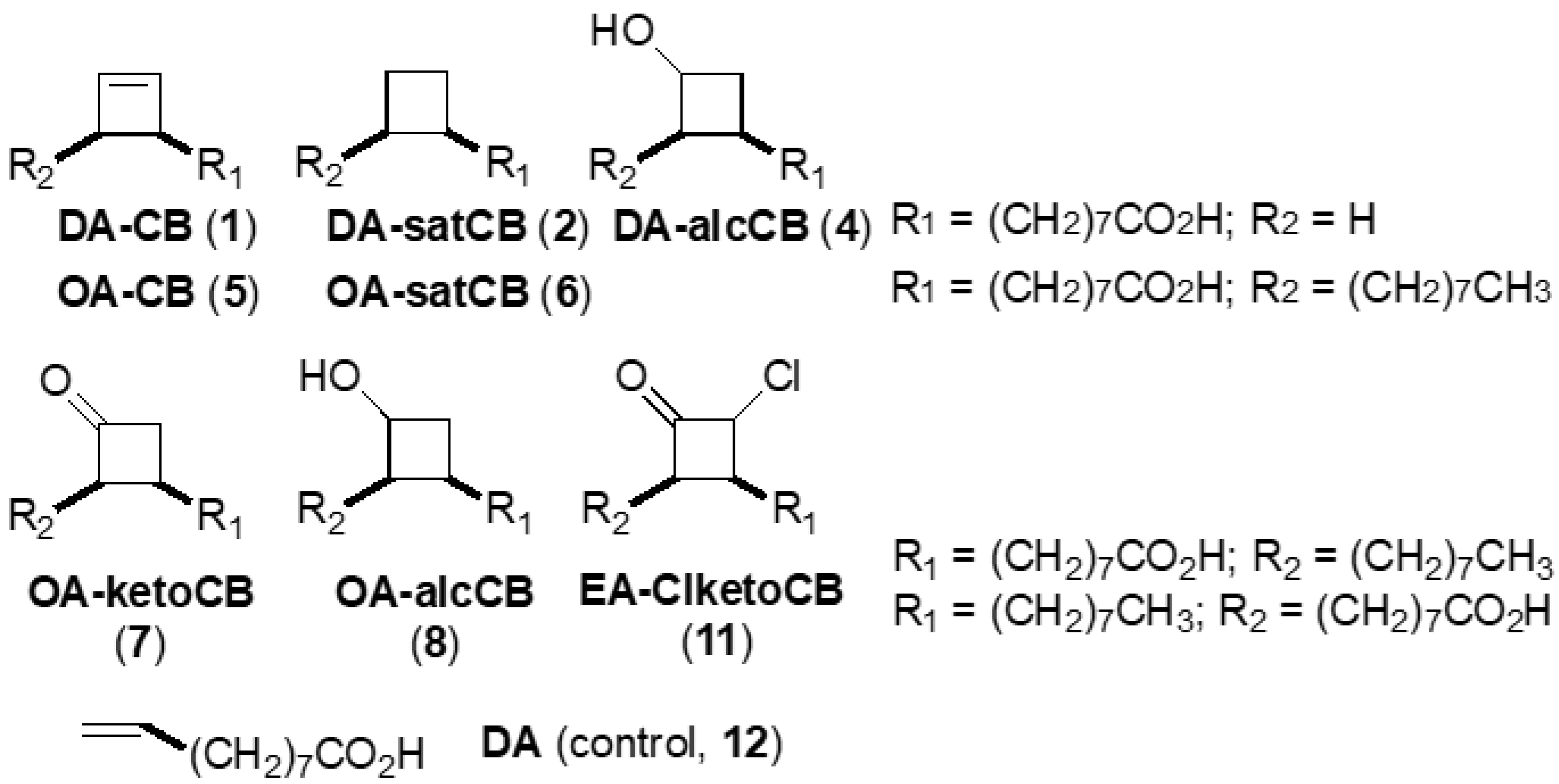
| No. | Abbreviated Name 2 | Compound Full Chemical Name | Formula Weight | Scaffold, Functionality |
|---|---|---|---|---|
| 1 | DA-CB | 8-(2-Cyclobuten-1-yl)octanoic acid | 196.3 | C10, alkene |
| 2 | DA-satCB | 8-Cyclobutyloctanoic acid | 198.3 | C10, alkane |
| 4 1 | DA-alcCB | 3-(Octanoic acid)cyclobutanol | 214.3 | C10, alcohol |
| 5 | OA-CB | 1-(Octanoic acid-8-yl)-2-octylcyclobutene | 308.5 | cis-C18, alkene |
| 6 | OA-satCB | cis-9,10-Ethanooctadecenoic acid | 310.5 | cis-C18, alkane |
| 7 | OA-ketoCB | 3-(Octanoic acid-8-yl)-2-octylcyclobutanone | 324.5 | cis-C18, ketone |
| 8 | OA-alcCB | 3-(Octanoic acid-8-yl)-2-octylcyclobutanol | 326.5 | cis-C18, alcohol |
| 11 | EA-ClketoCB | 2-Chloro-3-(octanoic acid-8-yl)-4-octylcyclobutanone | 358.9 | trans-C18, chloroketone |
| 12 | DA | Decenoic acid | 170.2 | C10, alkene (acyclic) |
| DCS | D-cycloserine | 102.1 |
| No. | Minimal Media 1 | No BSA 2 | 0.125% BSA | 0.25% BSA | 0.5% BSA | No HSA 2 | 0.125% HSA | 0.25% HSA | 0.5% HSA |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 512 (2608) | 512 (2608) | 512 (2608) | 512 (2608) | 512 (2608) | 512 (2608) | 1024 (5217) | 1024 (5217) | 1024 (5217) |
| 2 | 512 (2582) | 256 (1291) | 512 (2582) | 1024 (5164) | 1024 (5164) | 512 (2582) | 512 (2582) | 1024 (5164) | 1024 (5164) |
| 5 | 128 (415) | 1024 (3319) | 2048 (6639) | 2048 (6639) | 2048 (6639) | 512 (1660) | 1024 (3319) | 2048 (6639) | >2048 (>6639) |
| 6 | 256 (824) | 512 (1649) | 1024 (3298) | 1024 (3298) | 2048 (6596) | 512 (1649) | 2048 (6596) | 2048 (6596) | 2048 (6596) |
| 7 | 128 (394) | 256 (789) | 512 (1578) | 1024 (3156) | 1024 (3156) | 256 (789) | 512 (1578) | 1024 (3156) | 1024 (3156) |
| 8 | 256 (784) | 256 (784) | 512 (1568) | 1024 (3136) | 1024 (3136) | 256 (784) | 1024 (3136) | 1024 (3136) | 2048 (6272) |
| 11 | 128 (357) | 1024 (2853) | 2048 (5706) | >2048 (>5706) | >2048 (>5706) | 512 (1426) | 1024 (2853) | 2048 (5706) | 2048 (5706) |
| 12 | 512 (3007) | 1024 (6015) | 1024 (6015) | 1024 (6015) | 1024 (6015) | 1024 (6015) | 2048 (12029) | 2048 (12029) | 2048 (12029) |
| DCS | 64 (627) | 128 (1254) | 128 (1254) | 128 (1254) | 128 (1254) | 128 (1254) | 128 (1254) | 128 (1254) | 128 (1254) |
| Compound # | Map K-10 Week 5 | Map K-10 Week 7 | Mtb CDC1551 1 Week 7 | Mtb H37Rv 1 Week 7 |
|---|---|---|---|---|
| 1 | 512 (2608) | 256 (1304) | 16 (82) | 16 (82) |
| 2 | 256 (1291) | 256 (1291) | 4 (20) | 4 (20) |
| 4 | >1024 (>4778) | >1024 (>4778) | 64 (299) | 128 (597) |
| 5 | 128 (415) | 128 (415) | 128 (415) | 16 (52) |
| 6 | 512 (1649) | 256 (824) | 8 (26) | 16 (52) |
| 7 | 128 (394) | 128 (394) | 64 (197) | 32 (99) |
| 8 | 128 (392) | 128 (392) | 8 (24) | 2 (6) |
| 11 | 256 (713) | 256 (713) | 256 (713) | 256 (713) |
| 12 | 1024 (6015) | 512 (3007) | 32 (188) | 32 (188) |
| DCS | 256 (2508) | 128 (1254) | 4 (39) | 8 (78) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zinniel, D.K.; Sittiwong, W.; Marshall, D.D.; Rathnaiah, G.; Sakallioglu, I.T.; Powers, R.; Dussault, P.H.; Barletta, R.G. Novel Amphiphilic Cyclobutene and Cyclobutane cis-C18 Fatty Acid Derivatives Inhibit Mycobacterium avium subsp. paratuberculosis Growth. Vet. Sci. 2019, 6, 46. https://doi.org/10.3390/vetsci6020046
Zinniel DK, Sittiwong W, Marshall DD, Rathnaiah G, Sakallioglu IT, Powers R, Dussault PH, Barletta RG. Novel Amphiphilic Cyclobutene and Cyclobutane cis-C18 Fatty Acid Derivatives Inhibit Mycobacterium avium subsp. paratuberculosis Growth. Veterinary Sciences. 2019; 6(2):46. https://doi.org/10.3390/vetsci6020046
Chicago/Turabian StyleZinniel, Denise K., Wantanee Sittiwong, Darrell D. Marshall, Govardhan Rathnaiah, Isin T. Sakallioglu, Robert Powers, Patrick H. Dussault, and Raúl G. Barletta. 2019. "Novel Amphiphilic Cyclobutene and Cyclobutane cis-C18 Fatty Acid Derivatives Inhibit Mycobacterium avium subsp. paratuberculosis Growth" Veterinary Sciences 6, no. 2: 46. https://doi.org/10.3390/vetsci6020046
APA StyleZinniel, D. K., Sittiwong, W., Marshall, D. D., Rathnaiah, G., Sakallioglu, I. T., Powers, R., Dussault, P. H., & Barletta, R. G. (2019). Novel Amphiphilic Cyclobutene and Cyclobutane cis-C18 Fatty Acid Derivatives Inhibit Mycobacterium avium subsp. paratuberculosis Growth. Veterinary Sciences, 6(2), 46. https://doi.org/10.3390/vetsci6020046






