Risk Factors for Development of Canine and Human Osteosarcoma: A Comparative Review
Abstract
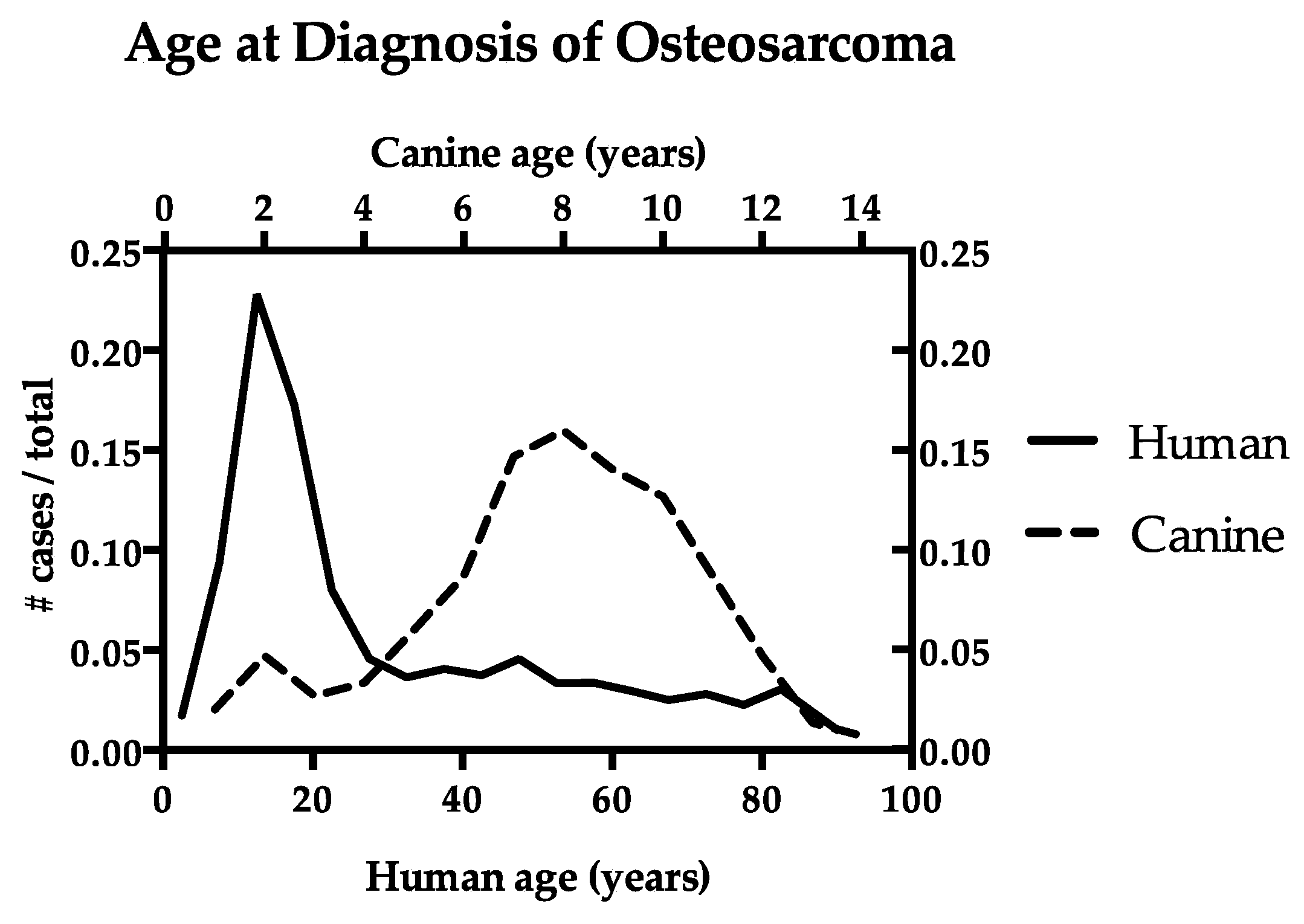
1. Introduction
2. Hormonal Influence
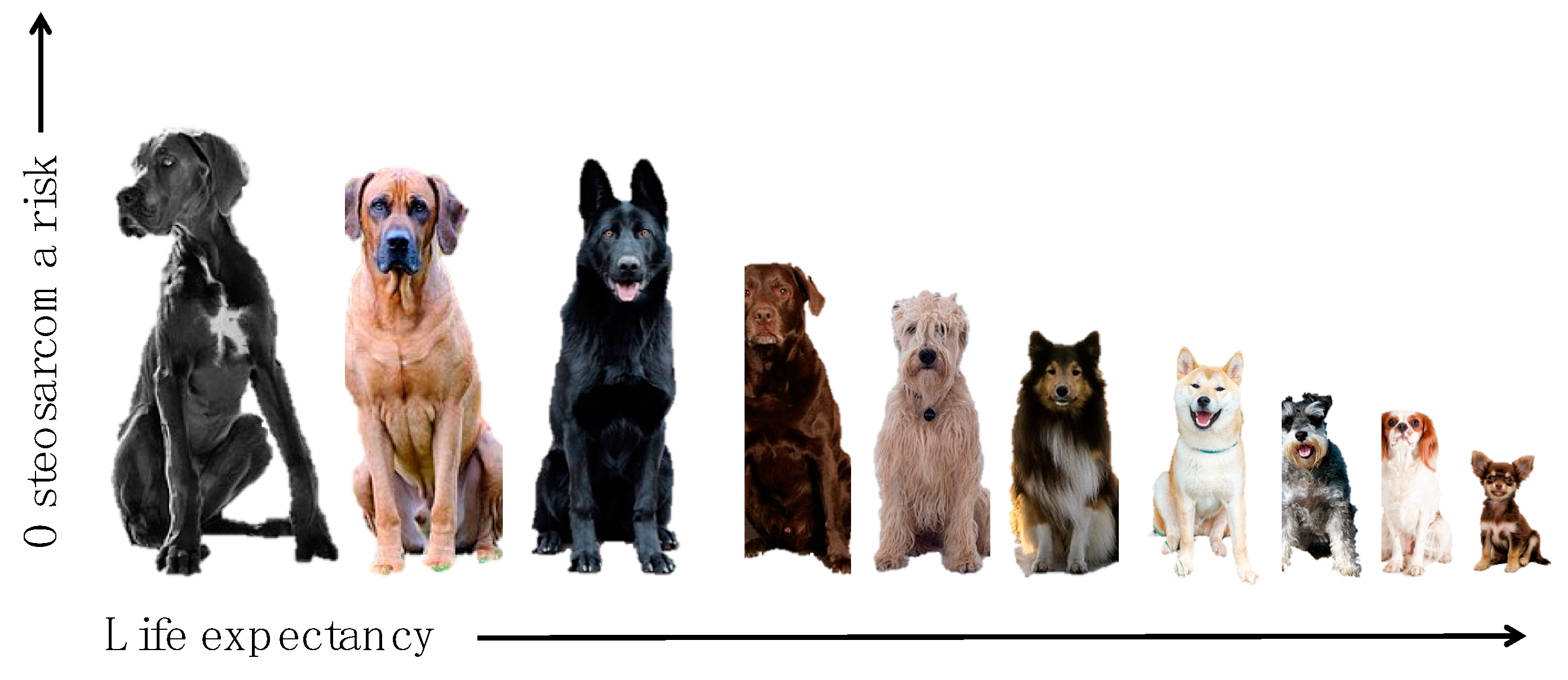
3. Size, Height, and Body Weight
4. Risks from Increased Cell Division/Turnover
5. Germline and Somatic Driver Alterations
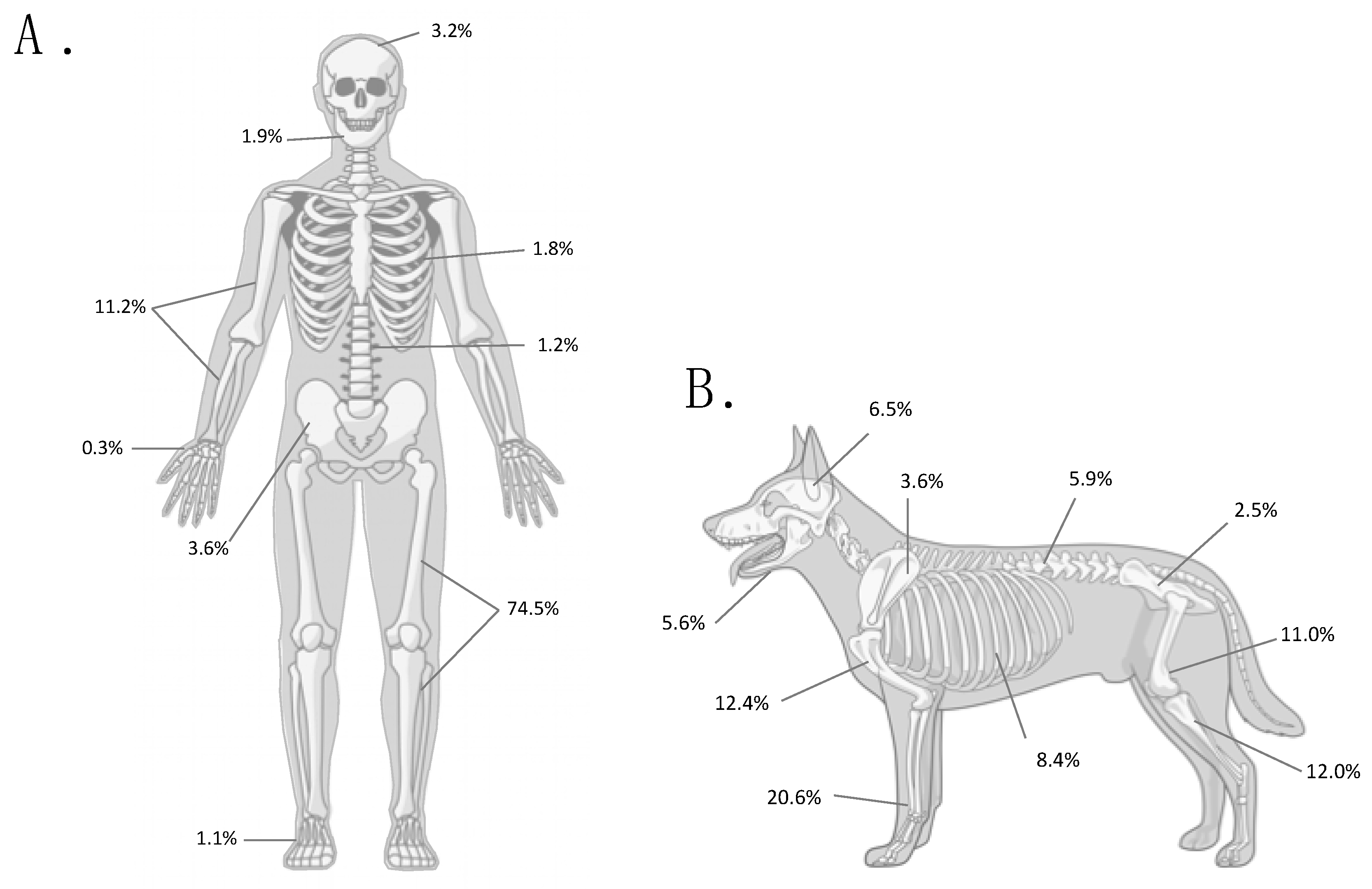
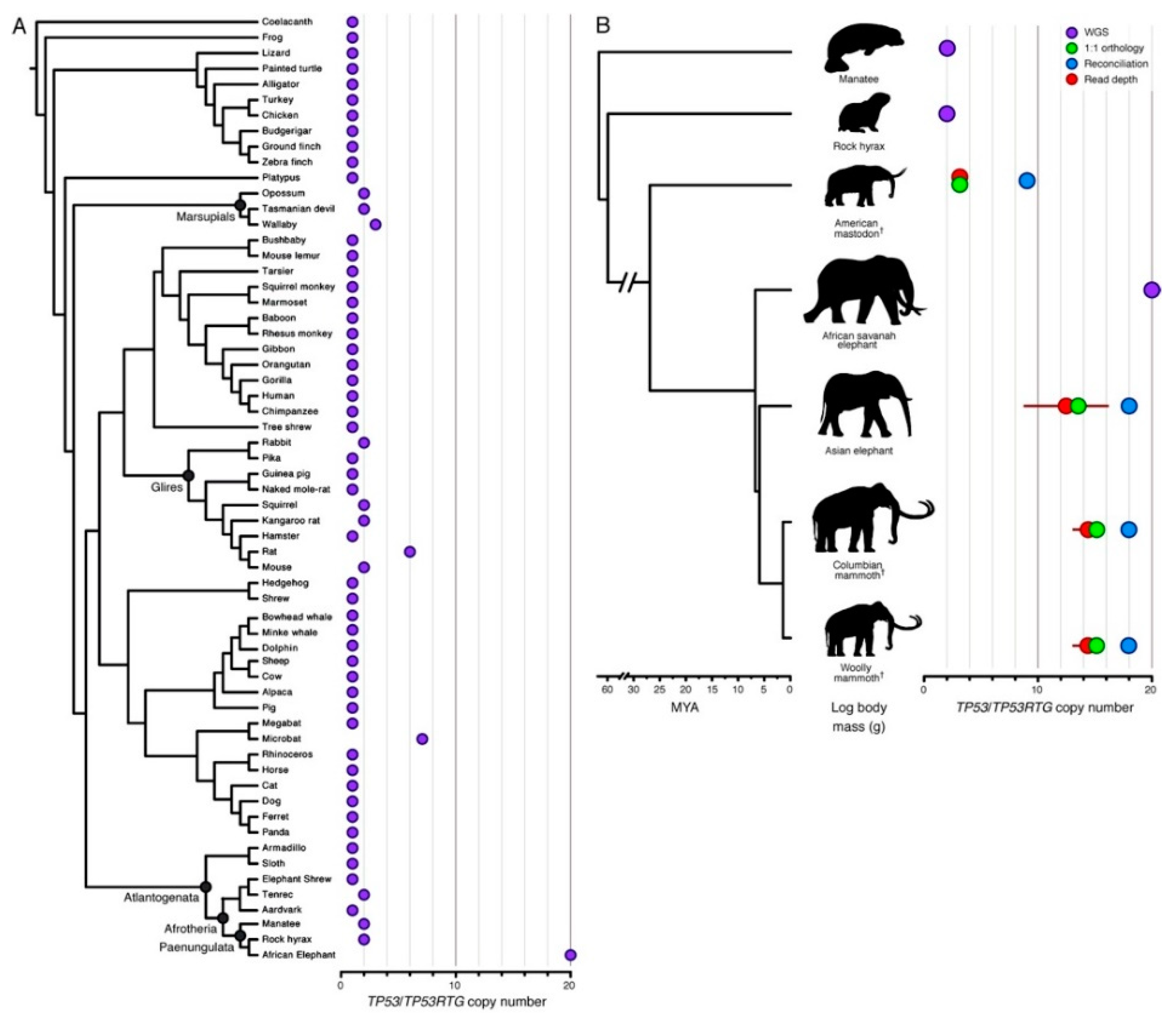
6. Canine Osteosarcoma Provides a Resolution to Peto’s Paradox
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Anfinsen, K.P.; Devesa, S.S.; Bray, F.; Troisi, R.; Jonasdottir, T.J.; Bruland, O.S.; Grotmol, T. Age-period-cohort analysis of primary bone cancer incidence rates in the United States (1976–2005). Cancer Epidemiol. Biomark. Prev. 2011, 20, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Anfinsen, K.P.; Grotmol, T.; Bruland, O.S.; Jonasdottir, T.J. Breed-specific incidence rates of canine primary bone tumors—A population based survey of dogs in Norway. Can. J. Vet. Res. 2011, 75, 209–215. [Google Scholar] [PubMed]
- Simpson, S.; Dunning, M.D.; de Brot, S.; Grau-Roma, L.; Mongan, N.P.; Rutland, C.S. Comparative review of human and canine osteosarcoma: Morphology, epidemiology, prognosis, treatment and genetics. Acta Vet. Scand. 2017, 59, 71. [Google Scholar] [CrossRef]
- Fenger, J.M.; London, C.A.; Kisseberth, W.C. Canine osteosarcoma: A naturally occurring disease to inform pediatric oncology. ILAR J. 2014, 55, 69–85. [Google Scholar] [CrossRef]
- Mueller, F.; Fuchs, B.; Kaser-Hotz, B. Comparative biology of human and canine osteosarcoma. Anticancer Res. 2007, 27, 155–164. [Google Scholar] [PubMed]
- Wycislo, K.L.; Fan, T.M. The immunotherapy of canine osteosarcoma: A historical and systematic review. J. Vet. Intern. Med. 2015, 29, 759–769. [Google Scholar] [CrossRef]
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov). In SEER 18 Research Data: Incidence (2000–2015); DCCPS, Surveillance Research Program, Released April 2018, Based on the November 2017 Submission; National Cancer Institute: Bethesda, MD, USA, 2018. [Google Scholar]
- Al-Khan, A.A.; Gunn, H.J.; Day, M.J.; Tayebi, M.; Ryan, S.D.; Kuntz, C.A.; Saad, E.S.; Richardson, S.J.; Danks, J.A. Immunohistochemical Validation of Spontaneously Arising Canine Osteosarcoma as a Model for Human Osteosarcoma. J. Comp. Pathol. 2017, 157, 256–265. [Google Scholar] [CrossRef]
- Withers, S.S.; Skorupski, K.A.; York, D.; Choi, J.W.; Woolard, K.D.; Laufer-Amorim, R.; Sparger, E.E.; Rodriguez, C.O.; McSorley, S.J.; Monjazeb, A.M.; et al. Association of macrophage and lymphocyte infiltration with outcome in canine osteosarcoma. Vet. Comp. Oncol. 2018. [Google Scholar] [CrossRef]
- Mirabello, L.; Pfeiffer, R.; Murphy, G.; Daw, N.C.; Patino-Garcia, A.; Troisi, R.J.; Hoover, R.N.; Douglass, C.; Schuz, J.; Craft, A.W.; et al. Height at diagnosis and birth-weight as risk factors for osteosarcoma. Cancer Causes Control. 2011, 22, 899–908. [Google Scholar] [CrossRef]
- Longhi, A.; Pasini, A.; Cicognani, A.; Baronio, F.; Pellacani, A.; Baldini, N.; Bacci, G. Height as a risk factor for osteosarcoma. J. Pediatr. Hematol. Oncol. 2005, 27, 314–318. [Google Scholar] [CrossRef]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int. J. Cancer 2009, 125, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.F.; Seton, M.; Merchant, A. Osteosarcoma in Paget’s disease of bone. J. Bone Miner. Res. 2006, 21, P58–P63. [Google Scholar] [CrossRef]
- Price, C.H. The incidence of osteogenic sarcoma in South-West England and its relationship to Paget’s disease of bone. J. Bone Joint Surg. Br. 1962, 44-B, 366–376. [Google Scholar] [CrossRef]
- Egenvall, A.; Nodtvedt, A.; von Euler, H. Bone tumors in a population of 400 000 insured Swedish dogs up to 10 y of age: Incidence and survival. Can. J. Vet. Res. 2007, 71, 292–299. [Google Scholar]
- Hillers, K.R.; Dernell, W.S.; Lafferty, M.H.; Withrow, S.J.; Lana, S.E. Incidence and prognostic importance of lymph node metastases in dogs with appendicular osteosarcoma: 228 cases (1986–2003). J. Am. Vet. Med. Assoc. 2005, 226, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- Sapierzynski, R.; Czopowicz, M. The animal-dependent risk factors in canine osteosarcomas. Pol. J. Vet. Sci. 2017, 20, 293–298. [Google Scholar] [CrossRef]
- Ru, G.; Terracini, B.; Glickman, L.T. Host related risk factors for canine osteosarcoma. Vet. J. 1998, 156, 31–39. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Kirpensteijn, J.; Moens, H.; Kik, M. Histologic prognosticators in feline osteosarcoma: A comparison with phenotypically similar canine osteosarcoma. Vet. Surg. 2008, 37, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, B.; Dickomeit, M.J.; Bali, M.S. Late-onset fracture-associated osteosarcoma in a cat. Vet. Comp. Orthop. Traumatol. 2012, 25, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Taulescu, M.A.; Carlson, C.S.; Amorim, I.F.; De Fatima Gartner, M.; Farcas, L.; Gal, A.F.; Catoi, C. Pathology in practice. Productive osteoblastic osteosarcoma of the left humerus with unilateral eye, cervical muscle, pulmonary, renal, jejunal mesentery, and liver metastases. J. Am. Vet. Med. Assoc. 2014, 245, 1103–1105. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.W.; Dubielzig, R.R.; Riser, W.H.; Jezyk, P. Parosteal osteosarcoma with pulmonary metastases in a cat. Vet. Pathol. 1984, 21, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Groskopf, B.S.; Dubielzig, R.R.; Beaumont, S.L. Orbital extraskeletal osteosarcoma following enucleation in a cat: A case report. Vet. Ophthalmol. 2010, 13, 179–183. [Google Scholar] [CrossRef]
- Heldmann, E.; Anderson, M.A.; Wagner-Mann, C. Feline osteosarcoma: 145 cases (1990–1995). J. Am. Anim. Hosp. Assoc. 2000, 36, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Miura, H.; Sakai, H.; Mori, T.; Shibata, S.; Nishida, H.; Maeda, S.; Kamishina, H. Vertebral replacement for the treatment of vertebral osteosarcoma in a cat. J. Vet. Med. Sci. 2017, 79, 999–1002. [Google Scholar] [CrossRef]
- Negrin, A.; Bernardini, M.; Diana, A.; Castagnaro, M. Giant cell osteosarcoma in the calvarium of a cat. Vet. Pathol. 2006, 43, 179–182. [Google Scholar] [CrossRef]
- Stimson, E.L.; Cook, W.T.; Smith, M.M.; Forrester, S.D.; Moon, M.L.; Saunders, G.K. Extraskeletal osteosarcoma in the duodenum of a cat. J. Am. Anim. Hosp. Assoc. 2000, 36, 332–336. [Google Scholar] [CrossRef]
- Yuki, M.; Nitta, M.; Omachi, T. Parathyroid hormone-related protein-induced hypercalcemia due to osteosarcoma in a cat. Vet. Clin. Pathol. 2015, 44, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Bush, J.M.; Fredrickson, R.L.; Ehrhart, E.J. Equine osteosarcoma: A series of 8 cases. Vet. Pathol. 2007, 44, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Cesar, F.B.; Joiner, K.S.; Albanese, V.; Groover, E.S.; Waguespack, R.W. Pathology in Practice. Poorly productive, osteoblastic osteosarcoma of the left paranasal sinuses in a 1-year-old colt. J. Am. Vet. Med. Assoc. 2016, 248, 773–775. [Google Scholar] [CrossRef]
- Jenner, F.; Solano, M.; Gliatto, J.; Lavallee, S.; Kirker-Head, C. Osteosarcoma of the tarsus in a horse. Equine Vet. J. 2003, 35, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Zaruby, J.F.; Williams, J.W.; Lovering, S.L. Periosteal osteosarcoma of the scapula in a horse. Can. Vet. J. 1993, 34, 742–744. [Google Scholar]
- Nagamine, E.; Matsuda, K.; Ishii, C.; Koiwa, M.; Taniyama, H. Primary ischial osteosarcoma occupying the pelvic cavity in a Japanese Black cow. J. Vet. Med. Sci. 2014, 76, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Plumlee, K.H.; Haynes, J.S.; Kersting, K.W.; Thompson, J.R. Osteosarcoma in a cow. J. Am. Vet. Med. Assoc. 1993, 202, 95–96. [Google Scholar] [PubMed]
- Prins, D.G.; Wittek, T.; Barrsett, D.C. Maxillary osteosarcoma in a beef suckler cow. Ir. Vet. J. 2012, 65, 15. [Google Scholar] [CrossRef]
- Benoit-Biancamano, M.O.; D’Anjou, M.A.; Girard, C.; Langlois, I. Rib osteoblastic osteosarcoma in an African hedgehog (Atelerix albiventris). J. Vet. Diagn. Invest. 2006, 18, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Matute, A.; Mendez-Bernal, A.; Ramos-Garduno, L.A. Osteosarcoma in African Hedgehogs (Atelerix albiventris): Five Cases. J. Zoo Wildl. Med. 2017, 48, 453–460. [Google Scholar] [CrossRef]
- Rhody, J.L.; Schiller, C.A. Spinal osteosarcoma in a hedgehog with pedal self-mutilation. Vet. Clin. North Am. Exot. Anim. Pract. 2006, 9, 625–631. [Google Scholar] [CrossRef]
- Mezzles, M.J.; Dick, E.J., Jr.; Owston, M.A.; Bauer, C. Osteosarcoma in Baboons (Papio spp.). Comp. Med. 2015, 65, 144–149. [Google Scholar]
- Russell, S.W.; Jenson, F.C.; Vanderlip, J.E.; Alexander, N.L. Osteosarcoma of the mandible of a baboon (Papio papio): Morphological and virological (oncornavirus) studies, with a review of neoplasms previously described in baboons. J. Comp. Pathol. 1979, 89, 349–360. [Google Scholar] [CrossRef]
- Brunetti, B.; Bo, P.; Sarli, G. Pathology in practice. Productive osteoblastic osteosarcoma with metastases in a guinea pig. J. Am. Vet. Med. Assoc. 2013, 243, 801–803. [Google Scholar] [CrossRef]
- Cojean, O.; Langlois, I.; Begin-Pepin, M.; Helie, P. Chondroblastic osteosarcoma of the middle ear in a guinea pig (Cavia porcellus). Can. Vet. J. 2018, 59, 855–859. [Google Scholar] [PubMed]
- Ishikawa, M.; Kondo, H.; Onuma, M.; Shibuya, H.; Sato, T. Osteoblastic osteosarcoma in a rabbit. Comp. Med. 2012, 62, 124–126. [Google Scholar] [PubMed]
- Kondo, H.; Ishikawa, M.; Maeda, H.; Onuma, M.; Masuda, M.; Shibuya, H.; Koie, H.; Sato, T. Spontaneous osteosarcoma in a rabbit (Oryctolagus cuniculus). Vet. Pathol. 2007, 44, 691–694. [Google Scholar] [CrossRef]
- Dadone, L.I.; Whiteside, D.P.; Black, S.R.; Remedios, A.; Raverty, S. Nasal osteosarcoma and interstitial cell tumor in a Vancouver Island marmot (Marmota vancouverensis). J. Zoo Wildl. Med. 2011, 42, 330–334. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Conrado, F.; Bruno, S.F.; de Alencar, N.X. What is your diagnosis? Scapular mass in a Chinese hamster. Vet. Clin. Pathol. 2013, 42, 533–534. [Google Scholar] [CrossRef]
- Johnson, J.G., 3rd; Kim, K.; Serio, J.; Paulsen, D.; Rademacher, N.; Pirie, G. Mandibular osteosarcoma in a nutria (Myocastor coypus). J. Zoo Wildl. Med. 2014, 45, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ramos Fernandez, J.; Thomas, N.J.; Dubielzig, R.R.; Drees, R. Osteosarcoma of the maxilla with concurrent osteoma in a southern sea otter (Enhydra lutris nereis). J. Comp. Pathol. 2012, 147, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Churgin, S.M.; Steinberg, H.; Ravi, M.; Hartup, B.K. Sternal osteosarcoma in a blue crane (Anthropoides paradiseus). J. Zoo Wildl. Med. 2013, 44, 1075–1078. [Google Scholar] [CrossRef]
- De Luca Bossa, L.M.; Mennonna, G.; Meomartino, L.; Paciello, O.; Ciccarelli, F.; De Biase, D.; Raia, P.; Caputo, V.; Fioretti, A.; Dipineto, L. Polyostotic Chondroblastic Osteosarcoma in a Kestrel (Falco tinnunculus). J. Avian Med. Surg. 2015, 29, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Lamb, S.; Reavill, D.; Wojcieszyn, J.; Sitinas, N. Osteosarcoma of the tibiotarsus with possible pulmonary metastasis in a ring-necked dove (Streptopelia risoria). J. Avian Med. Surg. 2014, 28, 50–56. [Google Scholar] [CrossRef]
- Sladakovic, I.; Sangster, C.R.; Allan, G.S.; Portas, T.J.; Howlett, C.R.; Blas-Machado, U. Calvarial Osteosarcoma with Cerebral Compression in a Free-Ranging Powerful Owl (Ninox strenua). J. Zoo Wildl. Med. 2017, 48, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Manera, M.; Biavati, S. Branchial osteogenetic neoplasm in barbel Barbus barbus plebejus. Dis Aquat Organ. 1999, 37, 231–236. [Google Scholar] [CrossRef]
- Rahmati-Holasoo, H.; Shokrpoor, S.; Masoudifard, M.; Davudypoor, S.; Vaseghi, M. Telangiectatic osteosarcoma and renal adenocarcinoma in an Oscar (Astronotus ocellatus, Agassiz): Diagnostic imaging and immunohistochemical study. J. Fish. Dis. 2018, 41, 1165–1172. [Google Scholar] [CrossRef]
- Cowan, M.L.; Monks, D.J.; Raidal, S.R. Osteosarcoma in a woma python (Aspidites ramsayi). Aust. Vet. J. 2011, 89, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Needle, D.M.; McKnight, C.A.; Kiupel, M. Chondroblastic osteosarcoma in two related spiny-tailed monitor lizards (Varanus acanthurus). J. Exotic Pet. Med. 2013, 22, 265–269. [Google Scholar] [CrossRef]
- Rothschild, B.M.; Tanke, D.H.; Helbling, M., 2nd; Martin, L.D. Epidemiologic study of tumors in dinosaurs. Naturwissenschaften 2003, 90, 495–500. [Google Scholar] [CrossRef]
- Haridy, Y.; Witzmann, F.; Asbach, P.; Schoch, R.R.; Frobisch, N.; Rothschild, B.M. Triassic Cancer-Osteosarcoma in a 240-Million-Year-Old Stem-Turtle. JAMA Oncol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gubin Iu, M.; Petrovichev, N.N.; Solov’ev Iu, N.; Kochergina, N.V.; Luk’ianchenko, A.B.; Markov, S.M. Cranial bone neoplasm in early triassic amphibia. Vopr. Onkol. 2001, 47, 449–455. [Google Scholar] [PubMed]
- Leeper, H.; Viall, A.; Ruaux, C.; Bracha, S. Preliminary evaluation of serum total cholesterol concentrations in dogs with osteosarcoma. J. Small Anim. Pract. 2017, 58, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Aljubran, A.H.; Griffin, A.; Pintilie, M.; Blackstein, M. Osteosarcoma in adolescents and adults: Survival analysis with and without lung metastases. Ann. Oncol. 2009, 20, 1136–1141. [Google Scholar] [CrossRef]
- Kirpensteijn, J.; Kik, M.; Rutteman, G.R.; Teske, E. Prognostic significance of a new histologic grading system for canine osteosarcoma. Vet. Path. 2002, 39, 240–246. [Google Scholar] [CrossRef]
- Wolke, R.E.; Nielsen, S.W. Site incidence of canine osteosarcoma. J. Small Anim. Pract. 1966, 7, 489–492. [Google Scholar] [CrossRef]
- Duffy, D.; Selmic, L.E.; Kendall, A.R.; Powers, B.E. Outcome following treatment of soft tissue and visceral extraskeletal osteosarcoma in 33 dogs: 2008–2013. Vet. Comp. Oncol. 2017, 15, 46–54. [Google Scholar] [CrossRef]
- Fiegen, A.P.; Tjarks, B.J.; Jassim, A.D. Primary Cutaneous Osteosarcoma. SD Med 2018, 71, 164–166. [Google Scholar]
- Kuntz, C.A.; Dernell, W.S.; Powers, B.E.; Withrow, S. Extraskeletal osteosarcomas in dogs: 14 cases. J. Am. Anim. Hosp. Assoc. 1998, 34, 26–30. [Google Scholar] [CrossRef]
- Langenbach, A.; Anderson, M.A.; Dambach, D.M.; Sorenmo, K.U.; Shofer, F.D. Extraskeletal osteosarcomas in dogs: A retrospective study of 169 cases (1986–1996). J. Am. Anim. Hosp. Assoc. 1998, 34, 113–120. [Google Scholar] [CrossRef]
- Llamas-Velasco, M.; Rutten, A.; Requena, L.; Mentzel, T. Primary cutaneous osteosarcoma of the skin: A report of 2 cases with emphasis on the differential diagnoses. Am. J. Dermatopathol. 2013, 35, e106–e113. [Google Scholar] [CrossRef]
- Jiang, L.; Luan, L.; Yun, H.; Hou, Y.; Shi, H. Extraosseous Osteosarcoma of the Liver Demonstrated on 18F-FDG PET/CT Imaging. Clin. Nucl. Med. 2016, 41, 650–653. [Google Scholar] [CrossRef]
- Tamang, T.G.; Shuster, M.; Chandra, A.B. Primary Hepatic Osteosarcoma: A Rare Cause of Primary Liver Tumor. Clin. Med. Insights Case Rep. 2016, 9, 31–33. [Google Scholar] [CrossRef]
- Dhumeaux, M.P.; Haudiquet, P.R. Primary pulmonary osteosarcoma treated by thoracoscopy-assisted lung resection in a dog. Can. Vet. J. 2009, 50, 755–758. [Google Scholar] [PubMed]
- Karfis, E.A.; Karaiskos, T.; Cheva, A.; Drossos, G.E. Primary extraosseous osteosarcoma of the lung. Acta Oncol. 2010, 49, 114–116. [Google Scholar] [CrossRef]
- Wajstaub, S.; Bezjak, A.; Howarth, D.; King, M.H.; Catton, C.N. A radio-sensitive primary osteosarcoma in the lung. Int. J. Clin. Oncol. 2011, 16, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.K. Canine extraskeletal osteosarcoma and chondrosarcoma: A clinicopathologic study of 14 cases. Vet. Pathol. 1990, 27, 46–55. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Tomasetti, C.; Vogelstein, B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 2015, 347, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, C.; Li, L.; Vogelstein, B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science 2017, 355, 1330–1334. [Google Scholar] [CrossRef]
- Troisi, R.; Masters, M.N.; Joshipura, K.; Douglass, C.; Cole, B.F.; Hoover, R.N. Perinatal factors, growth and development, and osteosarcoma risk. Br. J. Cancer 2006, 95, 1603–1607. [Google Scholar] [CrossRef]
- Clarke, B.L.; Khosla, S. Androgens and bone. Steroids 2009, 74, 296–305. [Google Scholar] [CrossRef]
- Emons, J.; Chagin, A.S.; Savendahl, L.; Karperien, M.; Wit, J.M. Mechanisms of growth plate maturation and epiphyseal fusion. Horm Res. Paediatr. 2011, 75, 383–391. [Google Scholar] [CrossRef]
- Kent, M.S.; Burton, J.H.; Dank, G.; Bannasch, D.L.; Rebhun, R.B. Association of cancer-related mortality, age and gonadectomy in golden retriever dogs at a veterinary academic center (1989–2016). PLoS ONE 2018, 13, e0192578. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.M.; Creevy, K.E.; Promislow, D.E. Reproductive capability is associated with lifespan and cause of death in companion dogs. PLoS ONE 2013, 8, e61082. [Google Scholar] [CrossRef]
- Cooley, D.M.; Beranek, B.C.; Schlittler, D.L.; Glickman, N.W.; Glickman, L.T.; Waters, D.J. Endogenous gonadal hormone exposure and bone sarcoma risk. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1434–1440. [Google Scholar]
- Lefebvre, S.L.; Yang, M.; Wang, M.; Elliott, D.A.; Buff, P.R.; Lund, E.M. Effect of age at gonadectomy on the probability of dogs becoming overweight. J. Am. Vet. Med. Assoc. 2013, 243, 236–243. [Google Scholar] [CrossRef]
- Salmeri, K.R.; Bloomberg, M.S.; Scruggs, S.L.; Shille, V. Gonadectomy in immature dogs: Effects on skeletal, physical, and behavioral development. J. Am. Vet. Med. Assoc. 1991, 198, 1193–1203. [Google Scholar] [PubMed]
- Root, M.V.; Johnston, S.D.; Olson, P.N. The effect of prepuberal and postpuberal gonadectomy on radial physeal closure in male and female domestic cats. Vet. Radiol. Ultrasound 1997, 38, 42–47. [Google Scholar] [CrossRef]
- Tjalma, R.A. Canine bone sarcoma: Estimation of relative risk as a function of body size. J. Natl. Cancer Inst. 1966, 36, 1137–1150. [Google Scholar]
- Gruntzig, K.; Graf, R.; Boo, G.; Guscetti, F.; Hassig, M.; Axhausen, K.W.; Fabrikant, S.; Welle, M.; Meier, D.; Folkers, G.; et al. Swiss Canine Cancer Registry 1955–2008: Occurrence of the Most Common Tumour Diagnoses and Influence of Age, Breed, Body Size, Sex and Neutering Status on Tumour Development. J. Comp. Pathol. 2016, 155, 156–170. [Google Scholar] [CrossRef]
- Withrow, S.J.; Powers, B.E.; Straw, R.C.; Wilkins, R.M. Comparative aspects of osteosarcoma. Dog versus man. Clin. Orthop. Relat. Res. 1991, 270, 159–168. [Google Scholar]
- Endicott, A.A.; Morimoto, L.M.; Kline, C.N.; Wiemels, J.L.; Metayer, C.; Walsh, K.M. Perinatal factors associated with clinical presentation of osteosarcoma in children and adolescents. Pediatr. Blood Cancer 2017, 64. [Google Scholar] [CrossRef]
- Diessner, B.J.; Spector, L.G. Birthweight and site of osteosarcoma development. Pediatr. Blood Cancer 2017, 64. [Google Scholar] [CrossRef]
- Arora, R.S.; Kontopantelis, E.; Alston, R.D.; Eden, T.O.; Geraci, M.; Birch, J.M. Relationship between height at diagnosis and bone tumours in young people: A meta-analysis. Cancer Causes Control. 2011, 22, 681–688. [Google Scholar] [CrossRef]
- Jesus-Garcia, R.; Seixas, M.T.; Costa, S.R.; Petrilli, A.S.; Laredo Filho, J. Epiphyseal plate involvement in osteosarcoma. Clin. Orthop. Relat. Res. 2000, 373, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Kirpensteijn, J.; Timmermans-Sprang, E.P.; van Garderen, E.; Rutteman, G.R.; Lantinga-van Leeuwen, I.S.; Mol, J.A. Growth hormone gene expression in canine normal growth plates and spontaneous osteosarcoma. Mol. Cell. Endocrinol. 2002, 197, 179–185. [Google Scholar] [CrossRef]
- Larsen, J. Feeding large-breed puppies. Compend. Contin. Educ. Vet. 2010, 32, E1–E4. [Google Scholar]
- Gilley, R.S.; Hiebert, E.; Clapp, K.; Bartl-Wilson, L.; Nappier, M.; Werre, S.; Barnes, K. Long-term Formation of Aggressive Bony Lesions in Dogs with Mid-Diaphyseal Fractures Stabilized with Metallic Plates: Incidence in a Tertiary Referral Hospital Population. Front. Vet. Sci. 2017, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Arthur, E.G.; Arthur, G.L.; Keeler, M.R.; Bryan, J.N. Risk of Osteosarcoma in Dogs After Open Fracture Fixation. Vet. Surg. 2016, 45, 30–35. [Google Scholar] [CrossRef]
- Atherton, M.J.; Arthurs, G. Osteosarcoma of the tibia 6 years after tibial plateau leveling osteotomy. J. Am. Anim. Hosp. Assoc. 2012, 48, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.G.; Johnson, E.G.; Vernau, W.; Murphy, B.G. Implant-associated neoplasia in dogs: 16 cases (1983–2013). J. Am. Vet. Med. Assoc. 2015, 247, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.T.; Parker, R.B.; Woodard, J.C. Osteosarcoma following total hip arthroplasty in a dog. J. Small Anim. Pract. 1997, 38, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Sartor, A.J.; Ryan, S.D.; Sellmeyer, T.; Withrow, S.J.; Selmic, L.E. Bi-institutional retrospective cohort study evaluating the incidence of osteosarcoma following tibial plateau levelling osteotomy (2000–2009). Vet. Comp. Orthop. Traumatol. 2014, 27, 339–345. [Google Scholar] [CrossRef]
- Selmic, L.E.; Ryan, S.D.; Boston, S.E.; Liptak, J.M.; Culp, W.T.; Sartor, A.J.; Prpich, C.Y.; Withrow, S.J. Osteosarcoma following tibial plateau leveling osteotomy in dogs: 29 cases (1997–2011). J. Am. Vet. Med. Assoc. 2014, 244, 1053–1059. [Google Scholar] [CrossRef]
- Karlsson, E.K.; Sigurdsson, S.; Ivansson, E.; Thomas, R.; Elvers, I.; Wright, J.; Howald, C.; Tonomura, N.; Perloski, M.; Swofford, R.; et al. Genome-wide analyses implicate 33 loci in heritable dog osteosarcoma, including regulatory variants near CDKN2A/B. Genome Biol. 2013, 14, R132. [Google Scholar] [CrossRef]
- Duffy, M.E.; Anderson, C.L.; Choy, K.; Fidel, J.L. Metronomic administration of lomustine following palliative radiation therapy for appendicular osteosarcoma in dogs. Can. Vet. J. 2018, 59, 136–142. [Google Scholar]
- Slovak, J.E.; Kieves, N.R.; Haynes, J. Extraskeletal Osteosarcoma Induced by a Foreign Body Granuloma. J. Am. Anim. Hosp. Assoc. 2015, 51, 315–319. [Google Scholar] [CrossRef]
- Selmic, L.E.; Griffin, L.R.; Rector, M.H.; Lafferty, M.; Pool, R.; Ehrhart, N.P. Treatment of extraskeletal osteosarcoma at a previous injection site resulting in prolonged survival in 1 dog. Can. Vet. J. 2016, 57, 950–954. [Google Scholar]
- Thrasher, J.P.; Barrett, R.B.; Tyler, D.E. Osteogenic carcinoma of the canine esophagus (Spirocerca lupi?). Vet. Med. Small Anim. Clin. 1968, 63, 333–336. [Google Scholar]
- Thrasher, J.P.; Ichinose, H.; Pitot, H.C. Osteogenic sarcoma of the canine esophagus associated with spirocerca lupi infection. Am. J. Vet. Res. 1963, 24, 808–818. [Google Scholar]
- Ranen, E.; Lavy, E.; Aizenberg, I.; Perl, S.; Harrus, S. Spirocercosis-associated esophageal sarcomas in dogs. A retrospective study of 17 cases (1997–2003). Vet. Parasitol. 2004, 119, 209–221. [Google Scholar] [CrossRef]
- Yas, E.; Kelmer, G.; Shipov, A.; Ben-Oz, J.; Segev, G. Successful transendoscopic oesophageal mass ablation in two dogs with Spirocerca lupi associated oesophageal sarcoma. J. Small Anim. Pract. 2013, 54, 495–498. [Google Scholar] [CrossRef]
- Lee, M.A.; Yi, J.; Chae, J.M. Cutaneous osteosarcoma arising from a burn scar. Skelet. Radiol. 2017, 46, 547–551. [Google Scholar] [CrossRef]
- Park, S.G.; Song, J.Y.; Song, I.G.; Kim, M.S.; Shin, B.S. Cutaneous extraskeletal osteosarcoma on the scar of a previous bone graft. Ann. Dermatol. 2011, 23, S160–S164. [Google Scholar] [CrossRef]
- Santos-Juanes, J.; Galache, C.; Miralles, M.; Curto, J.R.; Sanchez del Rio, J.; Soto, J. Primary cutaneous extraskeletal osteosarcoma under a previous electrodessicated actinic keratosis. J. Am. Acad. Dermatol. 2004, 51, S166–S168. [Google Scholar] [CrossRef]
- Savage, S.A.; Mirabello, L. Using epidemiology and genomics to understand osteosarcoma etiology. Sarcoma 2011, 2011, 548151. [Google Scholar] [CrossRef]
- Smida, J.; Xu, H.; Zhang, Y.; Baumhoer, D.; Ribi, S.; Kovac, M.; von Luettichau, I.; Bielack, S.; O’Leary, V.B.; Leib-Mosch, C.; et al. Genome-wide analysis of somatic copy number alterations and chromosomal breakages in osteosarcoma. Int. J. Cancer 2017, 141, 816–828. [Google Scholar] [CrossRef]
- Wedekind, M.F.; Wagner, L.M.; Cripe, T.P. Immunotherapy for osteosarcoma: Where do we go from here? Pediatr. Blood Cancer 2018, 65, e27227. [Google Scholar] [CrossRef]
- Rickel, K.; Fang, F.; Tao, J. Molecular genetics of osteosarcoma. Bone 2017, 102, 69–79. [Google Scholar] [CrossRef]
- Lorenz, S.; Baroy, T.; Sun, J.; Nome, T.; Vodak, D.; Bryne, J.C.; Hakelien, A.M.; Fernandez-Cuesta, L.; Mohlendick, B.; Rieder, H.; et al. Unscrambling the genomic chaos of osteosarcoma reveals extensive transcript fusion, recurrent rearrangements and frequent novel TP53 aberrations. Oncotarget 2016, 7, 5273–5288. [Google Scholar] [CrossRef]
- Ribi, S.; Baumhoer, D.; Lee, K.; Edison; Teo, A.S.; Madan, B.; Zhang, K.; Kohlmann, W.K.; Yao, F.; Lee, W.H.; et al. TP53 intron 1 hotspot rearrangements are specific to sporadic osteosarcoma and can cause Li-Fraumeni syndrome. Oncotarget 2015, 6, 7727–7740. [Google Scholar] [CrossRef]
- Sakthikumar, S.; Elvers, I.; Kim, J.; Arendt, M.L.; Thomas, R.; Turner-Maier, J.; Swofford, R.; Johnson, J.; Schumacher, S.E.; Alfoldi, J.; et al. SETD2 Is Recurrently Mutated in Whole-Exome Sequenced Canine Osteosarcoma. Cancer Res. 2018, 78, 3421–3431. [Google Scholar] [CrossRef]
- Fahey, C.C.; Davis, I.J. SETting the Stage for Cancer Development: SETD2 and the Consequences of Lost Methylation. Cold Spring Harb. Perspect. Med. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.K.; McPherson, J.R.; Tay, S.T.; Das, K.; Tan, I.B.; Ng, C.C.; Chia, N.Y.; Zhang, S.L.; Myint, S.S.; Hu, L.; et al. SETD2 histone modifier loss in aggressive GI stromal tumours. Gut 2016, 65, 1960–1972. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kluiver, J.; Osinga, J.; Westers, H.; van Werkhoven, M.B.; Seelen, M.A.; Sijmons, R.H.; van den Berg, A.; Kok, K. Functional Studies on Primary Tubular Epithelial Cells Indicate a Tumor Suppressor Role of SETD2 in Clear Cell Renal Cell Carcinoma. Neoplasia 2016, 18, 339–346. [Google Scholar] [CrossRef]
- Zhu, X.; He, F.; Zeng, H.; Ling, S.; Chen, A.; Wang, Y.; Yan, X.; Wei, W.; Pang, Y.; Cheng, H.; et al. Identification of functional cooperative mutations of SETD2 in human acute leukemia. Nat. Genet. 2014, 46, 287–293. [Google Scholar] [CrossRef]
- Behjati, S.; Tarpey, P.S.; Haase, K.; Ye, H.; Young, M.D.; Alexandrov, L.B.; Farndon, S.J.; Collord, G.; Wedge, D.C.; Martincorena, I.; et al. Recurrent mutation of IGF signalling genes and distinct patterns of genomic rearrangement in osteosarcoma. Nat. Commun. 2017, 8, 15936. [Google Scholar] [CrossRef]
- Carnevale, A.; Lieberman, E.; Cardenas, R. Li-Fraumeni syndrome in pediatric patients with soft tissue sarcoma or osteosarcoma. Arch. Med. Res. 1997, 28, 383–386. [Google Scholar]
- Garcia Garcia, A.; Barros, F.; Bouzas, M.L.; Penaranda, J.M. Li-Fraumeni syndrome and osteosarcoma of the maxilla. J. Oral. Maxillofac. Surg. 1998, 56, 1106–1109. [Google Scholar] [CrossRef]
- Porter, D.E.; Holden, S.T.; Steel, C.M.; Cohen, B.B.; Wallace, M.R.; Reid, R. A significant proportion of patients with osteosarcoma may belong to Li-Fraumeni cancer families. J. Bone Joint Surg. Br. 1992, 74, 883–886. [Google Scholar] [CrossRef]
- Yoshida, G.J.; Fuchimoto, Y.; Osumi, T.; Shimada, H.; Hosaka, S.; Morioka, H.; Mukai, M.; Masugi, Y.; Sakamoto, M.; Kuroda, T. Li-Fraumeni syndrome with simultaneous osteosarcoma and liver cancer: Increased expression of a CD44 variant isoform after chemotherapy. BMC Cancer 2012, 12, 444. [Google Scholar] [CrossRef]
- Pratt, C.B.; Michalkiewicz, E.N.; Rao, B.N.; Lipson, M.; Cain, A.; Kaste, S. Multifocal osteosarcoma following retinoblastoma. Ophthalmic Genet. 1999, 20, 23–29. [Google Scholar] [CrossRef]
- Potepan, P.; Luksch, R.; Sozzi, G.; Testi, A.; Laffranchi, A.; Danesini, G.M.; Parafioriti, A.; Giardini, R.; Spagnoli, I. Multifocal osteosarcoma as second tumor after childhood retinoblastoma. Skelet. Radiol. 1999, 28, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, G.; Kochar, H.S.; Julka, P.K.; Bahadur, S. Osteosarcoma as a second malignant disease in a case of bilateral retinoblastoma. Indian J. Otolaryngol. Head Neck Surg. 2011, 63, 115–117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chauveinc, L.; Mosseri, V.; Quintana, E.; Desjardins, L.; Schlienger, P.; Doz, F.; Dutrillaux, B. Osteosarcoma following retinoblastoma: Age at onset and latency period. Ophthalmic Genet. 2001, 22, 77–88. [Google Scholar] [CrossRef]
- Fujiwara, T.; Fujiwara, M.; Numoto, K.; Ogura, K.; Yoshida, A.; Yonemoto, T.; Suzuki, S.; Kawai, A. Second primary osteosarcomas in patients with retinoblastoma. Jpn. J. Clin. Oncol. 2015, 45, 1139–1145. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, J.A.; Choi, S.Y.; Kang, H.J.; Lee, J.W.; Kim, H.; Kim, J.H.; Sung, K.W.; Shin, H.Y.; Ahn, H.S.; Park, K.D. Treatment outcome of osteosarcoma after bilateral retinoblastoma: A retrospective study of eight cases. Br. J. Ophthalmol. 2014, 98, 1355–1359. [Google Scholar] [CrossRef]
- MacCarthy, A.; Bayne, A.M.; Brownbill, P.A.; Bunch, K.J.; Diggens, N.L.; Draper, G.J.; Hawkins, M.M.; Jenkinson, H.C.; Kingston, J.E.; Stiller, C.A.; et al. Second and subsequent tumours among 1927 retinoblastoma patients diagnosed in Britain 1951–2004. Br. J. Cancer 2013, 108, 2455–2463. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Hasegawa, T.; Tateishi, U.; Itoi, E. Second primary osteosarcoma with rosette-like structure in a patient with retinoblastoma. Virchows Arch. 2004, 445, 421–424. [Google Scholar] [CrossRef]
- Stine, K.C.; Saylors, R.L.; Saccente, S.; Becton, D.L. Long-term survival in osteosarcoma patients following retinoblastoma using doxorubicin, cisplatin, and methotrexate. Med. Pediatr. Oncol. 2003, 41, 77–78. [Google Scholar] [CrossRef]
- Wong, F.L.; Boice, J.D., Jr.; Abramson, D.H.; Tarone, R.E.; Kleinerman, R.A.; Stovall, M.; Goldman, M.B.; Seddon, J.M.; Tarbell, N.; Fraumeni, J.F., Jr.; et al. Cancer incidence after retinoblastoma. Radiation dose and sarcoma risk. JAMA 1997, 278, 1262–1267. [Google Scholar] [CrossRef]
- Hansen, M.F.; Nellissery, M.J.; Bhatia, P. Common mechanisms of osteosarcoma and Paget’s disease. J. Bone Miner. Res. 1999, 14, 39–44. [Google Scholar] [CrossRef]
- Dray, M.S.; Miller, M.V. Paget’s osteosarcoma and post-radiation osteosarcoma: Secondary osteosarcoma at Middlemore Hospital, New Zealand. Pathology 2008, 40, 604–610. [Google Scholar] [CrossRef] [PubMed]
- McNairn, J.D.; Damron, T.A.; Landas, S.K.; Ambrose, J.L.; Shrimpton, A.E. Inheritance of osteosarcoma and Paget’s disease of bone: A familial loss of heterozygosity study. J. Mol. Diagn. 2001, 3, 171–177. [Google Scholar] [CrossRef]
- Brenton, D.P.; Isenberg, D.A.; Bertram, J. Osteosarcoma complicating familial Paget’s disease. Postgrad. Med. J. 1980, 56, 238–243. [Google Scholar] [CrossRef]
- Frassica, F.J.; Sim, F.H.; Frassica, D.A.; Wold, L.E. Survival and management considerations in postirradiation osteosarcoma and Paget’s osteosarcoma. Clin. Orthop. Relat. Res. 1991, 270, 120–127. [Google Scholar]
- Olivier, M.; Goldgar, D.E.; Sodha, N.; Ohgaki, H.; Kleihues, P.; Hainaut, P.; Eeles, R.A. Li-Fraumeni and related syndromes: Correlation between tumor type, family structure, and TP53 genotype. Cancer Res. 2003, 63, 6643–6650. [Google Scholar] [PubMed]
- Mai, P.L.; Best, A.F.; Peters, J.A.; DeCastro, R.M.; Khincha, P.P.; Loud, J.T.; Bremer, R.C.; Rosenberg, P.S.; Savage, S.A. Risks of first and subsequent cancers among TP53 mutation carriers in the National Cancer Institute Li-Fraumeni syndrome cohort. Cancer 2016, 122, 3673–3681. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, L.; Yeager, M.; Mai, P.L.; Gastier-Foster, J.M.; Gorlick, R.; Khanna, C.; Patino-Garcia, A.; Sierrasesumaga, L.; Lecanda, F.; Andrulis, I.L.; et al. Germline TP53 variants and susceptibility to osteosarcoma. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef] [PubMed]
- Spector, L.; Lock, I.; Lane, J.; Sarver, A.; Krailo, M.; Nagarajan, R.; Pankratz, N. Abstract A37: De novo and transmitted germline TP53 variation in pediatric osteosarcoma: A report from the Children’s Oncology Group. Cancer Res. 2016, 76, A37. [Google Scholar] [CrossRef]
- Kleinerman, R.A.; Tucker, M.A.; Tarone, R.E.; Abramson, D.H.; Seddon, J.M.; Stovall, M.; Li, F.P.; Fraumeni, J.F., Jr. Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: An extended follow-up. J. Clin. Oncol. 2005, 23, 2272–2279. [Google Scholar] [CrossRef]
- Chen, X.; Bahrami, A.; Pappo, A.; Easton, J.; Dalton, J.; Hedlund, E.; Ellison, D.; Shurtleff, S.; Wu, G.; Wei, L.; et al. Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma. Cell Rep. 2014, 7, 104–112. [Google Scholar] [CrossRef]
- Perry, J.A.; Kiezun, A.; Tonzi, P.; Van Allen, E.M.; Carter, S.L.; Baca, S.C.; Cowley, G.S.; Bhatt, A.S.; Rheinbay, E.; Pedamallu, C.S.; et al. Complementary genomic approaches highlight the PI3K/mTOR pathway as a common vulnerability in osteosarcoma. Proc. Natl. Acad. Sci. USA 2014, 111, E5564–E5573. [Google Scholar] [CrossRef] [PubMed]
- Belchis, D.A.; Meece, C.A.; Benko, F.A.; Rogan, P.K.; Williams, R.A.; Gocke, C.D. Loss of heterozygosity and microsatellite instability at the retinoblastoma locus in osteosarcomas. Diagn. Mol. Pathol. 1996, 5, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Benassi, M.S.; Molendini, L.; Gamberi, G.; Ragazzini, P.; Sollazzo, M.R.; Merli, M.; Asp, J.; Magagnoli, G.; Balladelli, A.; Bertoni, F.; et al. Alteration of pRb/p16/cdk4 regulation in human osteosarcoma. Int. J. Cancer 1999, 84, 489–493. [Google Scholar] [CrossRef]
- Mirabello, L.; Yu, K.; Berndt, S.I.; Burdett, L.; Wang, Z.; Chowdhury, S.; Teshome, K.; Uzoka, A.; Hutchinson, A.; Grotmol, T.; et al. A comprehensive candidate gene approach identifies genetic variation associated with osteosarcoma. BMC Cancer 2011, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- Musselman, J.R.; Bergemann, T.L.; Ross, J.A.; Sklar, C.; Silverstein, K.A.; Langer, E.K.; Savage, S.A.; Nagarajan, R.; Krailo, M.; Malkin, D.; et al. Case-parent analysis of variation in pubertal hormone genes and pediatric osteosarcoma: A Children’s Oncology Group (COG) study. Int. J. Mol. Epidemiol. Genet. 2012, 3, 286–293. [Google Scholar]
- Savage, S.A.; Mirabello, L.; Wang, Z.; Gastier-Foster, J.M.; Gorlick, R.; Khanna, C.; Flanagan, A.M.; Tirabosco, R.; Andrulis, I.L.; Wunder, J.S.; et al. Genome-wide association study identifies two susceptibility loci for osteosarcoma. Nat. Genet. 2013, 45, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, L.; Koster, R.; Moriarity, B.S.; Spector, L.G.; Meltzer, P.S.; Gary, J.; Machiela, M.J.; Pankratz, N.; Panagiotou, O.A.; Largaespada, D.; et al. A Genome-Wide Scan Identifies Variants in NFIB Associated with Metastasis in Patients with Osteosarcoma. Cancer Discov. 2015, 5, 920–931. [Google Scholar] [CrossRef]
- Yang, Y.; Basu, S.; Mirabello, L.; Spector, L.; Zhang, L. A Bayesian Gene-Based Genome-Wide Association Study Analysis of Osteosarcoma Trio Data Using a Hierarchically Structured Prior. Cancer Inform. 2018, 17. [Google Scholar] [CrossRef]
- Peto, R.; Roe, F.J.; Lee, P.N.; Levy, L.; Clack, J. Cancer and ageing in mice and men. Br. J. Cancer 1975, 32, 411–426. [Google Scholar] [CrossRef]
- Armitage, P.; Doll, R. The age distribution of cancer and a multi-stage theory of carcinogenesis. Br. J. Cancer 1954, 8, 1–12. [Google Scholar] [CrossRef]
- Abegglen, L.M.; Caulin, A.F.; Chan, A.; Lee, K.; Robinson, R.; Campbell, M.S.; Kiso, W.K.; Schmitt, D.L.; Waddell, P.J.; Bhaskara, S.; et al. Potential Mechanisms for Cancer Resistance in Elephants and Comparative Cellular Response to DNA Damage in Humans. JAMA 2015, 314, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; Robert, L. Cell senescence: Role in aging and age-related diseases. Interdiscip. Top. Gerontol. 2014, 39, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.M.; Ryder, O.A.; Houck, M.L.; Charter, S.J.; Walker, W.; Forsyth, N.R.; Austad, S.N.; Venditti, C.; Pagel, M.; Shay, J.W.; et al. Comparative biology of mammalian telomeres: Hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell 2011, 10, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Valentijn, L.J.; Koster, J.; Zwijnenburg, D.A.; Hasselt, N.E.; van Sluis, P.; Volckmann, R.; van Noesel, M.M.; George, R.E.; Tytgat, G.A.; Molenaar, J.J.; et al. TERT rearrangements are frequent in neuroblastoma and identify aggressive tumors. Nat. Genet. 2015, 47, 1411–1414. [Google Scholar] [CrossRef]
- Telomeres Mendelian Randomization, C.; Haycock, P.C.; Burgess, S.; Nounu, A.; Zheng, J.; Okoli, G.N.; Bowden, J.; Wade, K.H.; Timpson, N.J.; Evans, D.M.; et al. Association Between Telomere Length and Risk of Cancer and Non-Neoplastic Diseases: A Mendelian Randomization Study. JAMA Oncol. 2017, 3, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.M.; Whitehead, T.P.; de Smith, A.J.; Smirnov, I.V.; Park, M.; Endicott, A.A.; Francis, S.S.; Codd, V.; Group, E.C.T.; Samani, N.J.; et al. Common genetic variants associated with telomere length confer risk for neuroblastoma and other childhood cancers. Carcinogenesis 2016, 37, 576–582. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Calabrese, P.; Shibata, D. A simple algebraic cancer equation: Calculating how cancers may arise with normal mutation rates. BMC Cancer 2010, 10, 3. [Google Scholar] [CrossRef]
- Sulak, M.; Fong, L.; Mika, K.; Chigurupati, S.; Yon, L.; Mongan, N.P.; Emes, R.D.; Lynch, V.J. TP53 copy number expansion is associated with the evolution of increased body size and an enhanced DNA damage response in elephants. eLife 2016, 5. [Google Scholar] [CrossRef]
- Vazquez, J.M.; Sulak, M.; Chigurupati, S.; Lynch, V.J. A Zombie LIF Gene in Elephants Is Upregulated by TP53 to Induce Apoptosis in Response to DNA Damage. Cell Rep. 2018, 24, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Keane, M.; Semeiks, J.; Webb, A.E.; Li, Y.I.; Quesada, V.; Craig, T.; Madsen, L.B.; van Dam, S.; Brawand, D.; Marques, P.I.; et al. Insights into the evolution of longevity from the bowhead whale genome. Cell Rep. 2015, 10, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Seluanov, A.; Chen, Z.; Hine, C.; Sasahara, T.H.; Ribeiro, A.A.; Catania, K.C.; Presgraves, D.C.; Gorbunova, V. Telomerase activity coevolves with body mass not lifespan. Aging Cell 2007, 6, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Seluanov, A.; Hine, C.; Azpurua, J.; Feigenson, M.; Bozzella, M.; Mao, Z.; Catania, K.C.; Gorbunova, V. Hypersensitivity to contact inhibition provides a clue to cancer resistance of naked mole-rat. Proc. Natl. Acad. Sci. USA 2009, 106, 19352–19357. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, A.; Hong, S.J.; Gifford, A.; Weinberg, R.A. Species- and cell type-specific requirements for cellular transformation. Cancer Cell 2004, 6, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.C.; Stephenson, B.; Hauck, M.; Dillberger, J. Heritability and segregation analysis of osteosarcoma in the Scottish deerhound. Genomics 2007, 90, 354–363. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makielski, K.M.; Mills, L.J.; Sarver, A.L.; Henson, M.S.; Spector, L.G.; Naik, S.; Modiano, J.F. Risk Factors for Development of Canine and Human Osteosarcoma: A Comparative Review. Vet. Sci. 2019, 6, 48. https://doi.org/10.3390/vetsci6020048
Makielski KM, Mills LJ, Sarver AL, Henson MS, Spector LG, Naik S, Modiano JF. Risk Factors for Development of Canine and Human Osteosarcoma: A Comparative Review. Veterinary Sciences. 2019; 6(2):48. https://doi.org/10.3390/vetsci6020048
Chicago/Turabian StyleMakielski, Kelly M., Lauren J. Mills, Aaron L. Sarver, Michael S. Henson, Logan G. Spector, Shruthi Naik, and Jaime F. Modiano. 2019. "Risk Factors for Development of Canine and Human Osteosarcoma: A Comparative Review" Veterinary Sciences 6, no. 2: 48. https://doi.org/10.3390/vetsci6020048
APA StyleMakielski, K. M., Mills, L. J., Sarver, A. L., Henson, M. S., Spector, L. G., Naik, S., & Modiano, J. F. (2019). Risk Factors for Development of Canine and Human Osteosarcoma: A Comparative Review. Veterinary Sciences, 6(2), 48. https://doi.org/10.3390/vetsci6020048




