Mycobacterium avium subsp. paratuberculosis ELISA Responses in Milk Samples from Vaccinated and Nonvaccinated Dairy Goat Herds in The Netherlands
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Design
2.2. Test
2.3. Data Analysis
3. Results
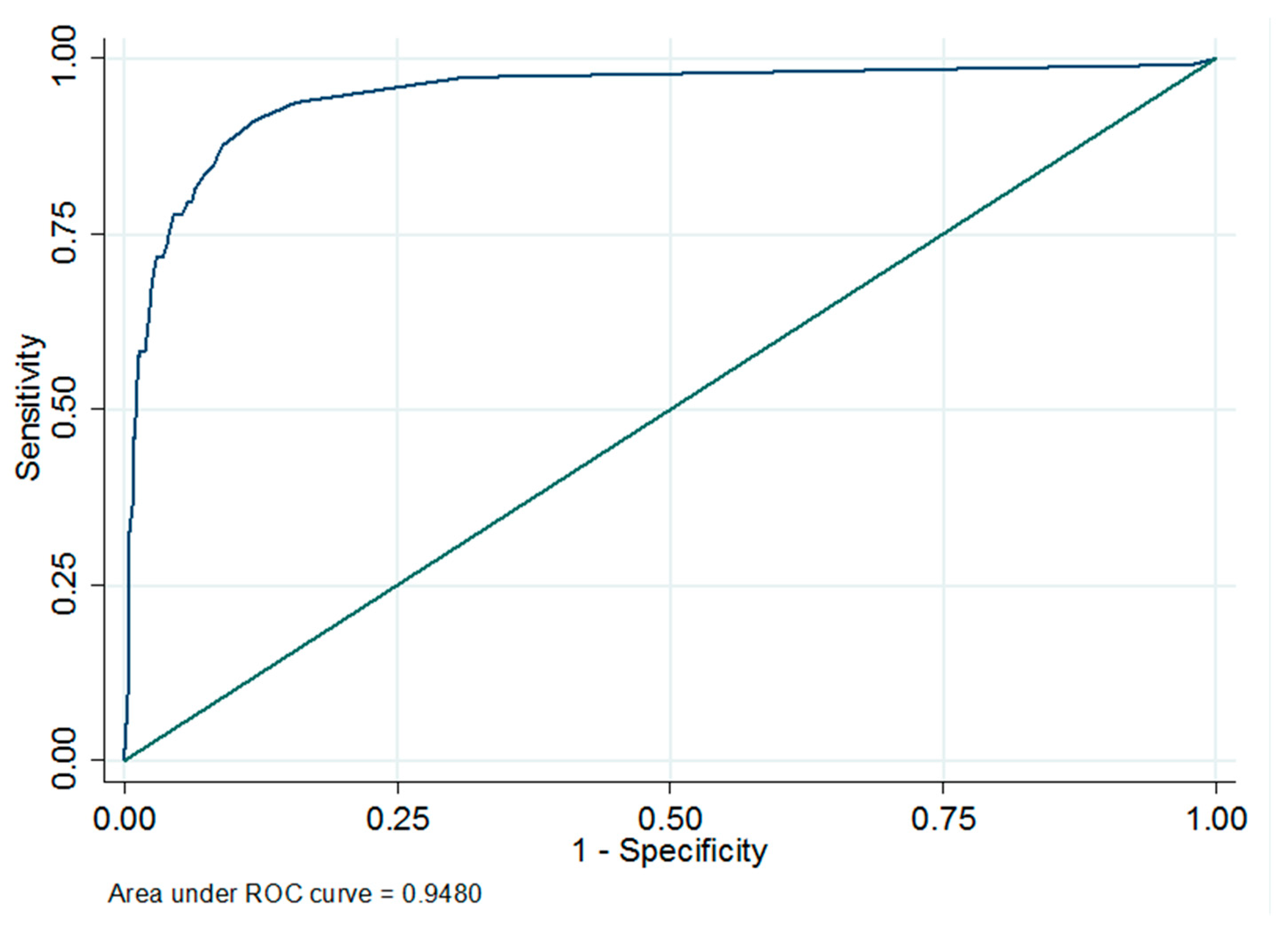
3.1. Cut-Off Calculation of Milk ELISA
3.2. Description of the Study Herds
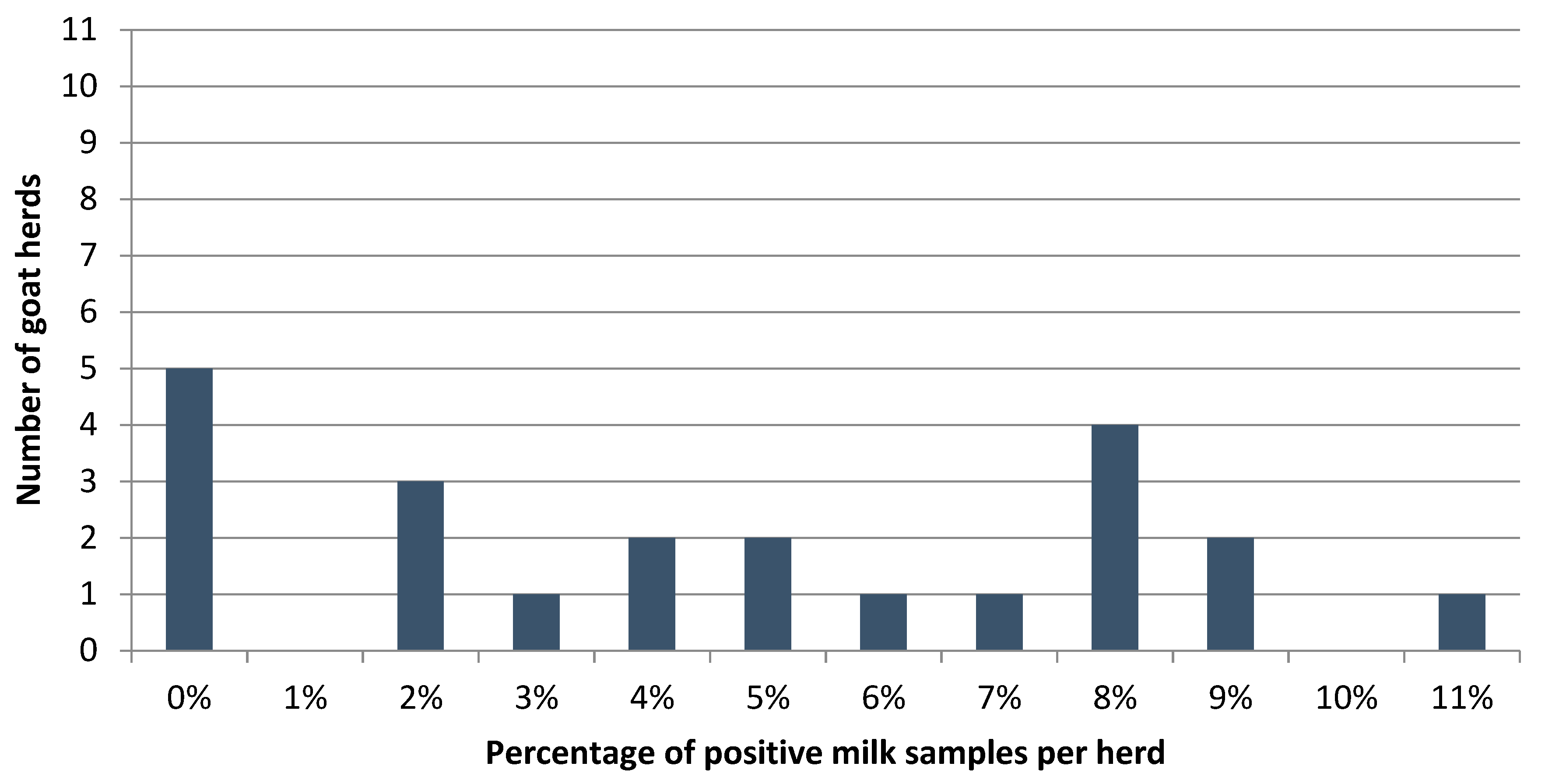
3.3. MAP ELISA Responses on Herd Level
3.4. MAP ELISA Responses on Animal Level
4. Discussion and Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thorel, M.-F.; Krichevsky, M.; Lévy-Frébault, V.V. Numerical taxonomy of mycobactin-dependent mycobacteria, emended description of Mycobacterium avium, and description of Mycobacterium avium subsp. avium subsp. nov., Mycobacterium avium subsp. paratuberculosis subsp. nov., and Mycobacterium avium subsp. silvaticum subsp. nov. Int. J. Syst. Evol. Microbiol. 1990, 40, 254–260. [Google Scholar]
- Benedictus, G.; Dijkhuizen, A.; Stelwagen, J. Economic losses due to paratuberculosis in dairy cattle. Vet. Rec. 1987, 121, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.L.; Wells, S.J.; Wagner, B.A. Herd-level economic losses associated with Johne’s disease on US dairy operations. Prev. Vet. Med. 1999, 40, 179–192. [Google Scholar] [CrossRef]
- Stehman, S.M. Paratuberculosis in small ruminants, deer, and South American camelids. Vet. Clin. N. Am. Food Anim. Pract. 1996, 12, 441–455. [Google Scholar] [CrossRef]
- Barkema, H.W.; Orsel, K.; Nielsen, S.; Koets, A.; Rutten, V.P.; Bannantine, J.; Keefe, G.P.; Kelton, D.F.; Wells, S.J.; Whittington, R.J.; et al. Knowledge gaps that hamper prevention and control of Mycobacterium avium subspecies paratuberculosis infection. Transbound. Emerg. Dis. 2018, 65, 125–148. [Google Scholar] [CrossRef]
- Bauman, C.; Jones-Bitton, A.; Jansen, J.; Kelton, D.; Menzies, P. Evaluation of bulk tank milk PCR and bulk tank milk modified ELISA tests for the detection of paratuberculosis at the herd level in goat and sheep dairies in Ontario, Canada. J. Dairy Sci. 2019, 102, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S.; Toft, N. Ante mortem diagnosis of paratuberculosis: A review of accuracies of ELISA, interferon-gamma assay and faecal culture techniques. Vet. Microbiol. 2008, 129, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Maity, M.; Sharma, S.; Gupta, V.K. Comparative evaluation of different diagnostic techniques for detection of naturally occurring paratuberculosis in Gaddi goats. Small Rumin. Res. 2019. [Google Scholar] [CrossRef]
- Ikonomopoulos, J.; Balaskas, C.; Kantzoura, B.; Fragiadaki, E.; Pavlik, I.; Bartos, M.; Lukas, J.C.; Gazouli, M. Comparative evaluation of positive tests to Mycobacterium avium subsp. paratuberculosis in clinically healthy sheep and goats in south-west Greece using molecular techniques, serology, and culture. Vet. J. 2007, 174, 337–343. [Google Scholar] [CrossRef]
- Windsor, P. Paratuberculosis in sheep and goats. Vet. Microbiol. 2015, 181, 161–169. [Google Scholar] [CrossRef]
- Stabel, J.R. Transitions in immune responses to Mycobacterium paratuberculosis. Vet. Microbiol. 2000, 77, 465–473. [Google Scholar] [CrossRef]
- Stabel, J. Host responses to Mycobacterium avium subsp. paratuberculosis: A complex arsenal. Anim. Health Res. Rev. 2006, 7, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Salgado, M.; Manning, E.J.; Collins, M.T. Performance of a Johne’s disease enzyme-linked immunosorbent assay adapted for milk samples from goats. J. Vet. Diagn. Investig. 2005, 17, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Bastida, F.; Juste, R.A. Paratuberculosis control: A review with a focus on vaccination. J. Immune Based Ther. Vaccines 2011, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Corpa, J.M.; Perez, V.; Sanchez, M.A.; Marin, J.F. Control of paratuberculosis (Johne’s disease) in goats by vaccination of adult animals. Vet. Rec. 2000, 146, 195–196. [Google Scholar] [CrossRef]
- Saxegaard, F.; Fodstad, F.H. Control of paratuberculosis (Johne’s disease) in goats by vaccination. Vet. Rec. 1985, 116, 439–441. [Google Scholar] [CrossRef]
- Fernandez-Silva, J.A.; Correa-Valencia, N.M.; Ramirez, N.F. Systematic review of the prevalence of paratuberculosis in cattle, sheep, and goats in Latin America and the Caribbean. Trop. Anim. Health Prod. 2014, 46, 1321–1340. [Google Scholar] [CrossRef]
- Khol, J.L.; Stein, B.; Dreier, S.; Baumgartner, W. Paratuberculosis (Johne’s disease) in small ruminants in Austria. Slov. Vet. Res. 2006, 43 (Suppl. 10), 129–130. [Google Scholar]
- Mørk, T.; Heier, B.T.; Alvseike, K.R.; Lund, A. Overvåkings-og kontrollprogrammer for landdyr, fisk og skjell i Norge med vekt pa BSE-, salmonella-og paratuberkuloseprogrammene. Norsk Veter. 2003, 115, 707–717. [Google Scholar]
- Cvetnic, Z. Proširenost paratuberkuloze goveda u Republici Hrvatskoj u 2010. godini. Praxis Veterinaria Zagreb. 2002, 50, 255–260. [Google Scholar]
- Djønne, B.; Jensen, M.; Grant, I.; Holstad, G. Detection by immunomagnetic PCR of Mycobacterium avium subsp. paratuberculosis in milk from dairy goats in Norway. Vet. Microbiol. 2003, 92, 135–143. [Google Scholar] [CrossRef]
- Mercier, P.; Baudry, C.; Beaudeau, F.; Seegers, H.; Malher, X. Estimated prevalence of Mycobacterium avium subspecies paratuberculosis infection in herds of dairy goats in France. Vet. Rec. 2010, 167, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Pithua, P.; Kollias, N.S. Estimated prevalence of caprine paratuberculosis in boer goat herds in missouri, USA. Vet. Med. Int. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Muskens, J.; Barkema, H.W.; Russchen, E.; van Maanen, K.; Schukken, Y.H.; Bakker, D. Prevalence and regional distribution of paratuberculosis in dairy herds in The Netherlands. Vet. Microbiol. 2000, 77, 253–261. [Google Scholar] [CrossRef]
- Veldhuis, A.M.B.; Van den Brom, R.; Van Schaik, G.; Vellema, P. Data-Analyse Kleine Herkauwers 2013; GD Animal Health: Deventer, the Netherlands, 2014. [Google Scholar]
- Dimareli-Malli, Z.; Sarris, K. Comparison of DNA probe test and cultivation methods for detection of Mycobacterium avium subsp paratuberculosis in caprine and ovine faeces. Aust. Vet. J. 2001, 79, 47–50. [Google Scholar] [CrossRef]
- Arrighi, H.M.; Hertz-Picciotto, I. The evolving concept of the healthy worker survivor effect. Epidemiology 1994, 5, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S.; Toft, N. A review of prevalences of paratuberculosis in farmed animals in Europe. Prev. Vet. Med. 2009, 88, 1–14. [Google Scholar] [CrossRef]
- Bauman, C.; Jones-Bitton, A.; Menzies, P.; Toft, N.; Jansen, J.; Kelton, D. Prevalence of paratuberculosis in the dairy goat and dairy sheep industries in Ontario, Canada. Can. Vet. J. 2016, 57, 169–175. [Google Scholar]
- Dimareli-Malli, Z.; Stevenson, K.; Sarris, K.; Sossidou, K. Study of microbiological and molecular typing aspects of paratuberculosis in sheep and goats in Northern Greece. Transbound. Emerg. Dis. 2009, 56, 285–290. [Google Scholar] [CrossRef]
- Reviriego, F.J.; Moreno, M.A.; Domínguez, L. Soil type as a putative risk factor of ovine and caprine paratuberculosis seropositivity in Spain. Prev. Vet. Med. 2000, 43, 43–51. [Google Scholar] [CrossRef]
- Mühlherr, J.E.; Zweifel, C.; Corti, S.; Blanco, J.; Stephan, R. Microbiological quality of raw goat’s and ewe’s bulk-tank milk in Switzerland. J. Dairy Sci. 2003, 86, 3849–3856. [Google Scholar] [CrossRef]
- Grant, I.; O’Riordan, L.; Ball, H.; Rowe, M. Incidence of Mycobacterium paratuberculosis in raw sheep and goats’ milk in England, Wales and Northern Ireland. Vet. Microbiol. 2001, 79, 123–131. [Google Scholar] [CrossRef]
- Mendes, S.; Boinas, F.; Albuquerque, T.; Fernandes, L.; Afonso, A.; Amado, A. Epidemiological studies on paratuberculosis in small ruminants in Portugal. Epidemiol. Sante Anim. 2004, 45, 61–71. [Google Scholar]
- Ocepek, M.; Krt, B.; Pate, M.; Pogačnik, M. Seroprevalence of paratuberculosis in Slovenia between 1999 and 2001. Slov. Vet. Res. 2002, 39, 179–185. [Google Scholar]
- Storset, A.K.; Hasvold, H.J.; Valheim, M.; Brun-Hansen, H.; Berntsen, G.; Whist, S.K.; Djønne, B.; Press, C.; Holstad, G.; Larsen, H. Subclinical paratuberculosis in goats following experimental infection. An immunological and microbiological study. Vet. Immunol. Immunopathol. 2001, 80, 271–287. [Google Scholar] [CrossRef]
- Corpa, J.M.; Perez, V.; Garcia Marin, J.F. Differences in the immune responses in lambs and kids vaccinated against paratuberculosis, according to the age of vaccination. Vet. Microbiol. 2000, 77, 475–485. [Google Scholar] [CrossRef]
- Köhler, H.; Gyra, H.; Zimmer, K.; Dräger, K.; Burkert, B.; Lemser, B.; Hausleithner, D.; Cußler, K.; Klawonn, W.; He, R.G. Immune reactions in cattle after immunization with a Mycobacterium paratuberculosis vaccine and implications for the diagnosis of M. paratuberculosis and M. bovis infections. Zoonoses Public Health 2001, 48, 185–195. [Google Scholar] [CrossRef]
- Reddacliff, L. Field Evaluation of OJD Control Using Gudair; NSW Department of Primary Industries &Meat & Livestock Australia Limited: North Sydney, Australia, 2005; No. OJD.009. [Google Scholar]
- Angelidou, E.; Kostoulas, P.; Leontides, L. Bayesian validation of a serum and milk ELISA for antibodies against Mycobacterium avium subspecies paratuberculosis in Greek dairy goats across lactation. J. Dairy Sci. 2013, 97, 819–828. [Google Scholar] [CrossRef]
| Number | Percentage Positive | 95% Confidence Interval | |
|---|---|---|---|
| Vaccinated | 1518 | 6.7% | 5.5–8.1% |
| More than year ago | 1470 | 6.7% | 5.5–8.1% |
| Less than year ago | 48 | 6.3% | 1.3–17.2% |
| Nonvaccinated | 900 | 1.1% | 0.5–2.0% |
| Total | 2418 | 4.6% | 3.8–5.5% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luttikholt, S.; Lievaart-Peterson, K.; Gonggrijp, M.; Aalberts, M.; van Schaik, G.; Vellema, P. Mycobacterium avium subsp. paratuberculosis ELISA Responses in Milk Samples from Vaccinated and Nonvaccinated Dairy Goat Herds in The Netherlands. Vet. Sci. 2019, 6, 58. https://doi.org/10.3390/vetsci6020058
Luttikholt S, Lievaart-Peterson K, Gonggrijp M, Aalberts M, van Schaik G, Vellema P. Mycobacterium avium subsp. paratuberculosis ELISA Responses in Milk Samples from Vaccinated and Nonvaccinated Dairy Goat Herds in The Netherlands. Veterinary Sciences. 2019; 6(2):58. https://doi.org/10.3390/vetsci6020058
Chicago/Turabian StyleLuttikholt, Saskia, Karianne Lievaart-Peterson, Maaike Gonggrijp, Marian Aalberts, Gerdien van Schaik, and Piet Vellema. 2019. "Mycobacterium avium subsp. paratuberculosis ELISA Responses in Milk Samples from Vaccinated and Nonvaccinated Dairy Goat Herds in The Netherlands" Veterinary Sciences 6, no. 2: 58. https://doi.org/10.3390/vetsci6020058
APA StyleLuttikholt, S., Lievaart-Peterson, K., Gonggrijp, M., Aalberts, M., van Schaik, G., & Vellema, P. (2019). Mycobacterium avium subsp. paratuberculosis ELISA Responses in Milk Samples from Vaccinated and Nonvaccinated Dairy Goat Herds in The Netherlands. Veterinary Sciences, 6(2), 58. https://doi.org/10.3390/vetsci6020058




