Competition among Escherichia coli Strains for Space and Resources
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains: Cultures, Media and Culture Conditions
2.2. Competition Experiments
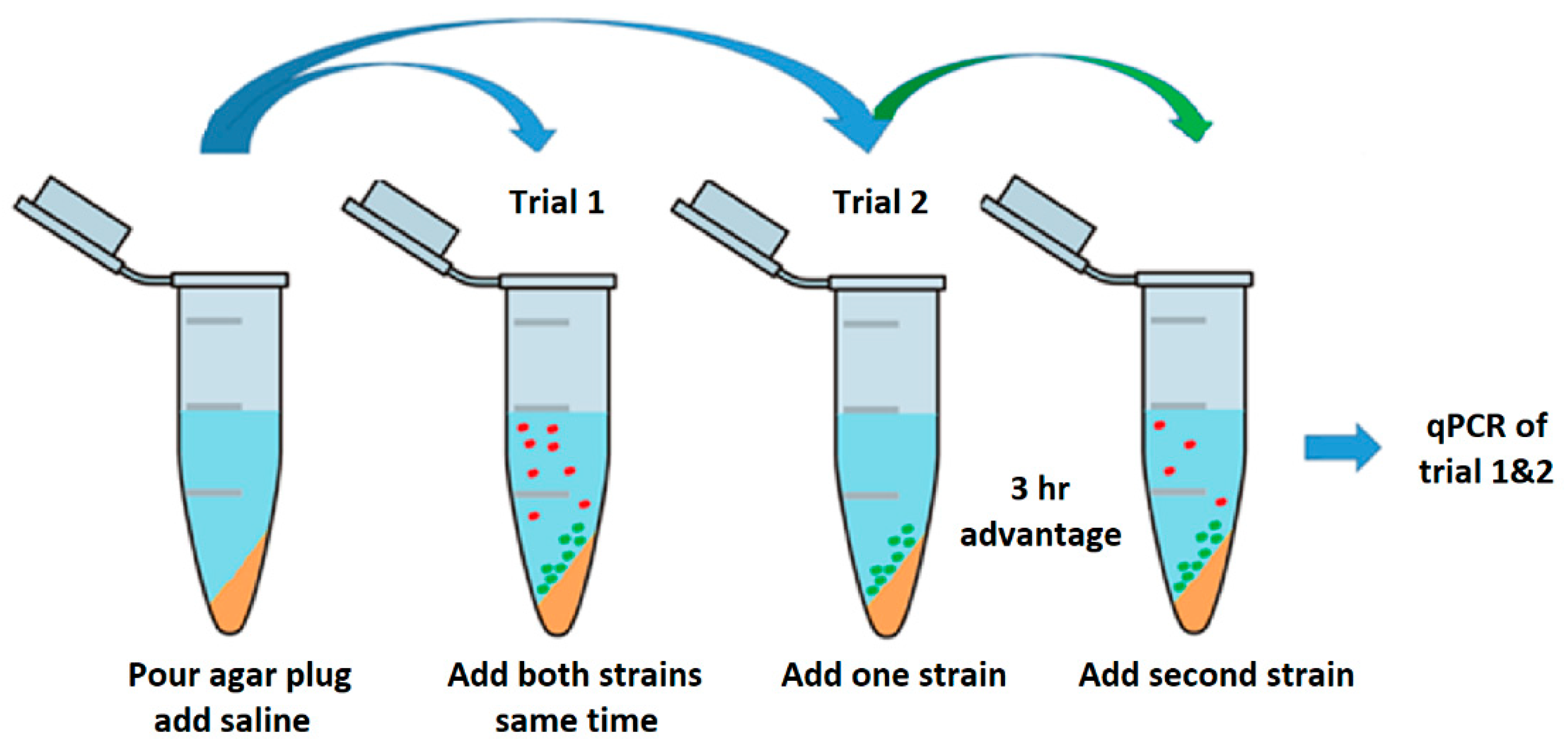
2.2.1. Liquid Competition
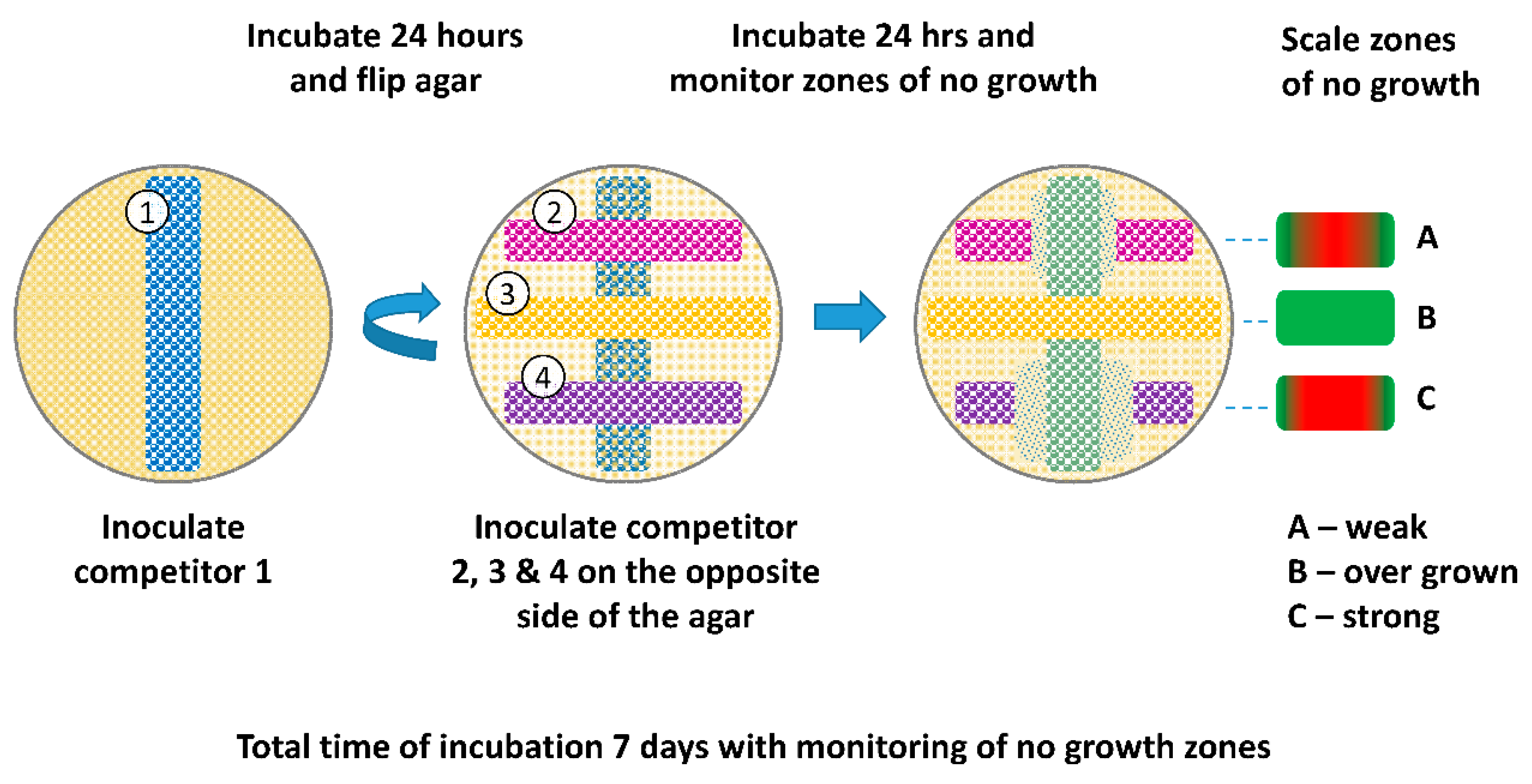
2.2.2. Omelette Method
2.2.3. Plug ‘n’ Prey
2.3. Statistical Analysis
3. Results
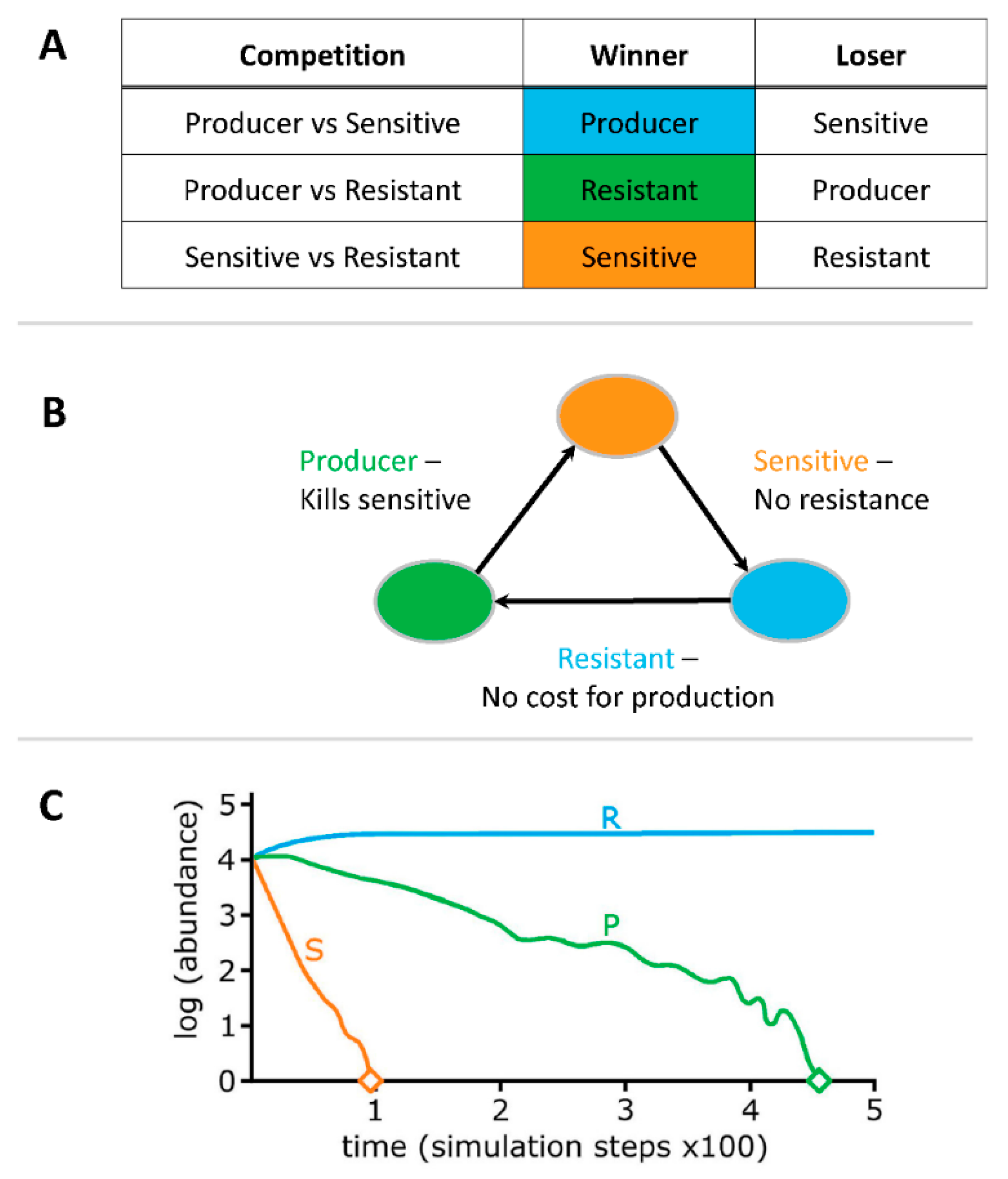
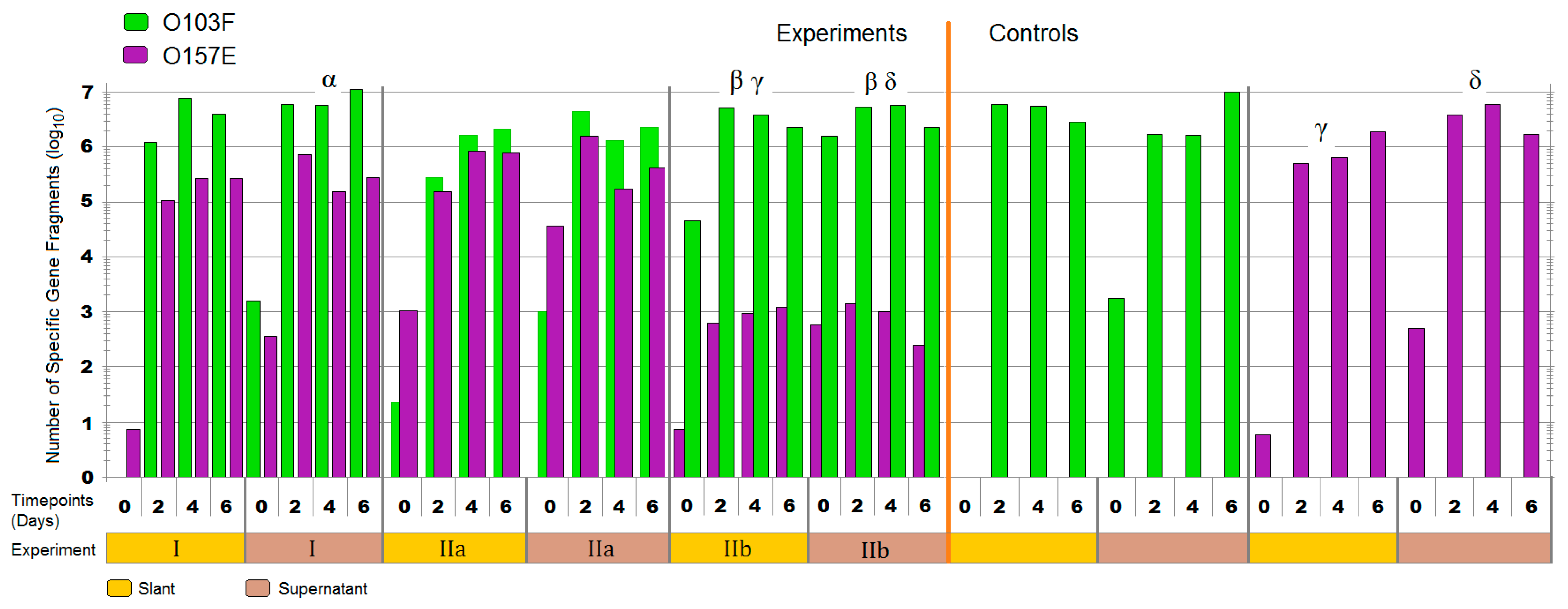
3.1. Liquid Competition
3.2. Omelette Method
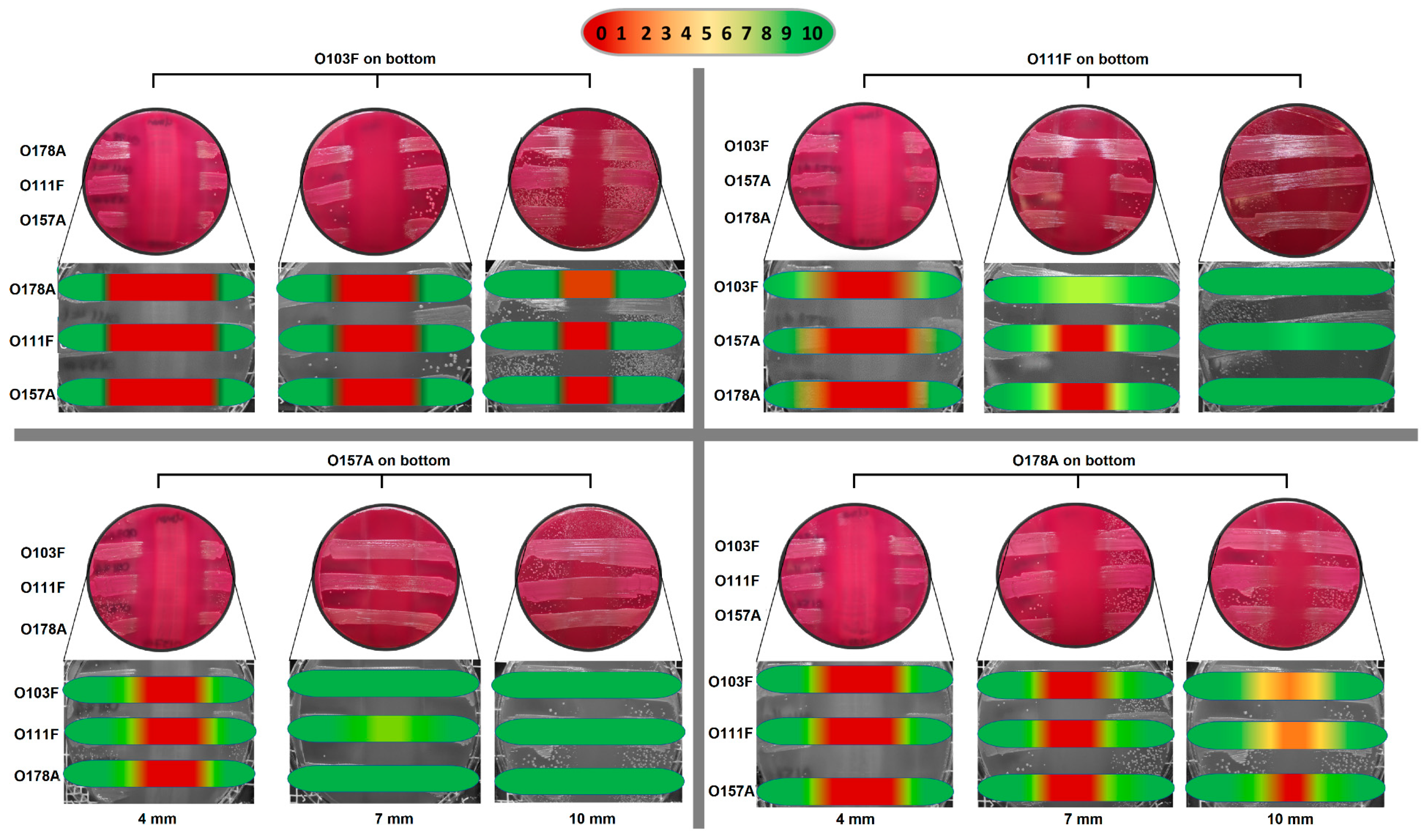
3.3. Plug ‘n’ Prey
4. Discussion
4.1. Liquid Competition
4.2. Omelette Method
4.3. Plug ‘n’ Prey
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gause, G.F. The Struggle for Existence; Dover Publications: Mineola, NY, USA, 2003; pp. 1–163. [Google Scholar]
- Hardin, G. The competitive exclusion principle. Science 1960, 131, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Majeed, H.; Lampert, A.; Ghazaryan, L.; Gillor, O. The weak shall inherit: Bacteriocin-mediated interactions in bacterial populations. PLoS ONE 2013, 8, e63837. [Google Scholar] [CrossRef] [PubMed]
- Stubbendieck, R.M.; Straight, P.D. Multifaceted interfaces of bacterial competition. J. Bacteriol. 2016, 198, 2145–2155. [Google Scholar] [CrossRef] [PubMed]
- De Muinck, E.J.; Stenseth, N.C.; Sachse, D.; vander Roost, J.; Rønningen, K.S.; Rudi, K.; Trosvik, P. context-dependent competition in a model gut bacterial community. PLoS ONE 2013, 8, e67210. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.A.; Wertz, J.E. Bacteriocins: Evolution, ecology, and application. Annu. Rev. Microbiol. 2002, 56, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Kerr, B.; Riley, M.A.; Feldman, M.W.; Bohannan, B.J.M. Local dispersal promotes biodiversity in a real-life game of rock–paper–scissors. Nature 2002, 418, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Stubbendieck, R.M.; Vargas-Bautista, C.; Straight, P.D. Bacterial communities: Interactions to scale. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.C. Contact-dependent interbacterial toxins deliver a message. Curr. Opin. Microbiol. 2018, 42, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.K.; Diner, E.J.; de Roodenbeke, C.T.; Burgess, B.R.; Poole, S.J.; Braaten, B.A.; Jones, A.M.; Webb, J.S.; Hayes, C.S.; Cotter, P.A.; et al. A widespread family of polymorphic contact-dependent toxin delivery systems in bacteria. Nature 2010, 468, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Majeed, H.; Gillor, O.; Kerr, B.; Riley, M.A. Competitive interactions in Escherichia coli populations: The role of bacteriocins. ISME J. 2011, 5, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Journet, L.; Cascales, E. The type VI secretion system in Escherichia coli and related species. EcoSal Plus 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.A.; Johnson, J.R. Medical and economic impact of extraintestinal infections due to Escherichia coli: Focus on an increasingly important endemic problem. Microbes. Infect. 2003, 5, 449–456. [Google Scholar] [CrossRef]
- Rahal, E.A.; Fadlallah, S.M.; Nassar, F.J.; Kazzi, N.; Matar, G.M. Approaches to treatment of emerging Shiga toxin-producing Escherichia coli infections highlighting the O104:H4 serotype. Front. Cell. Infect. Microbiol. 2015, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Cleary, T.G. The role of Shiga-toxin-producing Escherichia coli in hemorrhagic colitis and hemolytic uremic syndrome. Semin. Pediatr. Infect. Dis. 2004, 15, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Brusa, V.; Aliverti, V.; Aliverti, F.; Ortega, E.E.; de la Torre, J.H.; Linares, L.H.; Sanz, M.E.; Etcheverría, A.I.; Padola, N.L.; Galli, L.; et al. Shiga toxin-producing Escherichia coli in beef retail markets from Argentina. Front. Cell. Infect. Microbiol. 2012, 2, 171. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.; Hannon, S.; Booker, C.W.; Jim, G.K. Variable efficacy of a vaccine and direct-fed microbial for controlling Escherichia coli O157:H7 in feces and on hides of feedlot cattle. Foodborne Pathog. Dis. 2014, 11, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Stephens, T.P.; Stanford, K.; Rode, L.M.; Booker, C.W.; Vogstad, A.R.; Schunicht, O.C.; Jim, G.K.; Wildman, B.K.; Perrett, T.; McAllister, T.A. Effect of a direct-fed microbial on animal performance, carcass characteristics and the shedding of Escherichia coli O157 by feedlot cattle. Anim. Feed Sci. Technol. 2010, 158, 65–72. [Google Scholar] [CrossRef]
- Jin, L.; Wang, Y.; Iwaasa, A.D.; Li, Y.; Xu, Z.; Schellenberg, M.P.; Liu, X.L.; McAllister, T.A.; Stanford, K. Purple prairie clover (Dalea purpurea Vent) reduces fecal shedding of Escherichia coli in pastured cattle. J. Food Prot. 2015, 78, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.; Sperandio, V. Shiga toxin in enterohemorrhagic E. coli: Regulation and novel anti-virulence strategies. Front. Cell. Infect. Microbiol. 2012, 2, 81. [Google Scholar] [CrossRef] [PubMed]
- Sonnenborn, U. Escherichia coli strain Nissle 1917-from bench to bedside and back: History of a special Escherichia coli strain with probiotic properties. FEMS Microbiol. Lett. 2016, 363, 212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Doyle, M.; Harmon, B.G.; Brown, C.A.; Mueller, P.O.E.; Parks, A.H. Reduction of carriage of enterohemorrhagic Escherichia coli O157:H7 in cattle by inoculation with probiotic bacteria. J. Clin. Microbiol. 1998, 36, 641–647. [Google Scholar] [PubMed]
- Stanford, K.; Johnson, R.P.; Alexander, T.W.; McAllister, T.A.; Reuter, T. Influence of season and feedlot location on prevalence and virulence factors of seven serogroups of Escherichia coli in feces of Western-Canadian slaughter cattle. PLoS ONE 2016, 11, e0159866. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.C.; Stanford, K.; McAllister, T.A.; Thomas, J.; Reuter, T. Competition during enrichment of pathogenic Escherichia coli may result in culture bias. FACETS 2016, 1, 114–126. [Google Scholar] [CrossRef]
- Zhao, T.; Tkalcic, S.; Doyle, M.P.; Harmon, B.G.; Brown, C.A.; Zhao, P. Pathogenicity of enterohemorrhagic Escherichia coli in neonatal calves and evaluation of fecal shedding by treatment with probiotic Escherichia coli. J. Food Prot. 2003, 66, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Majeed, H.; Ghazaryan, L.; Herzberg, M.; Gillor, O. Bacteriocin expression in sessile and planktonic populations of Escherichia coli. J. Antibiot. 2015, 68, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.K.; Pamma, R.; Hernday, A.D.; Bickham, J.E.; Braaten, B.A.; Low, D.A. Contact-dependent inhibition of growth in Escherichia coli. Science 2005, 309, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- Hood, R.D.; Singh, P.; Hsu, F.; Güvener, T.; Carl, M.A.; Trinidad, R.R.; Silverman, J.M.; Ohlson, B.B.; Hicks, K.G.; Plemel, R.L.; et al. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 2010, 7, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Budič, M.; Rijavec, M.; Petkovšek, Ž.; Žgur-Bertok, D. Escherichia coli bacteriocins: Antimicrobial efficacy and prevalence among isolates from patients with bacteraemia. PLoS ONE 2011, 6, e28769. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.M. The Natural History of Bacteriocins. In The Bacteriocins: Current Knowledge and Future Prospects; Dorit, R.L., Roy, S.M., Riley, M.A., Eds.; Caister Academic Press: Norfolk, UK, 2016; pp. 1–10. ISBN 9781910190371. [Google Scholar]
- Bayramoglu, B.; Toubiana, D.; van Vliet, S.; Inglis, R.F.; Shnerb, N.; Gillor, O. Bet-hedging in bacteriocin producing Escherichia coli populations: The single cell perspective. Sci. Rep. 2017, 7, 42068. [Google Scholar] [CrossRef] [PubMed]
- Mavridou, D.A.I.; Gonzalez, D.; Kim, W.; West, S.A.; Foster, K.R. Bacteria use collective behavior to generate diverse combat strategies. Curr. Biol. 2018, 28, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Chikindas, M.L.; Weeks, R.; Drider, D.; Chistyakov, V.A.; Dicks, L.M. Functions and emerging applications of bacteriocins. Curr. Opin. Biotechnol. 2018, 49, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Suganthi, V.; Mohanasrinivasan, V. Optimization studies for enhanced bacteriocin production by Pediococcus pentosaceus KC692718 using response surface methodology. J. Food Sci. Technol. 2015, 52, 3773–3783. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.B.; Peterson, S.B.; Mougous, J.D. Type VI secretion system effectors: Poisons with a purpose. Nat. Rev. Microbiol. 2014, 12, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Fratamico, P.M.; Gunther, N.W. Chapter three—Shiga toxin-producing Escherichia coli. Adv. Appl. Microbiol. 2014, 86, 145–197. [Google Scholar] [CrossRef] [PubMed]
- Persad, A.K.; LeJeune, J.T. Animal reservoirs of Shiga toxin-producing Escherichia coli. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef]
- Rivas, M.; Chinen, I.; Miliwebsky, E.; Masana, M. Risk factors for Shiga toxin-producing Escherichia coli—Associated human diseases. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Tozzoli, R.; Grande, L.; Michelacci, V.; Ranieri, P.; Maugliani, A.; Caprioli, A.; Morabito, S. Shiga toxin-converting phages and the emergence of new pathogenic Escherichia coli: A world in motion. Front. Cell. Infect. Microbiol. 2014, 4, 80. [Google Scholar] [CrossRef] [PubMed]
- Imamovic, L.; Jofre, J.; Schmidt, H.; Serra-Moreno, R.; Muniesa, M. Phage-mediated Shiga toxin 2 gene transfer in food and water. Appl. Environ. Microbiol. 2009, 75, 1764–1768. [Google Scholar] [CrossRef] [PubMed]
- Nyambe, S.; Burgess, C.; Whyte, P.; Bolton, D. An investigation of vtx2 bacteriophage transduction to different Escherichia coli patho-groups in food matrices and nutrient broth. Food Microbiol. 2017, 68, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Cascales, E. Antibacterial weapons: Targeted destruction in the microbiota. Trends. Microbiol. 2018, 26, 329–338. [Google Scholar] [CrossRef] [PubMed]
 symbol denotes the O111B strain that was still present after 14 days. Symbols: α, β, γ, and δ denote a significant difference between: O103F and O157A-F in competition, O157A-F in competition and O157A-F controls, O26E and O111A-F in competition and O111A-F in competition and O111A-F controls, respectively (p < 0.05).
symbol denotes the O111B strain that was still present after 14 days. Symbols: α, β, γ, and δ denote a significant difference between: O103F and O157A-F in competition, O157A-F in competition and O157A-F controls, O26E and O111A-F in competition and O111A-F in competition and O111A-F controls, respectively (p < 0.05).
 symbol denotes the O111B strain that was still present after 14 days. Symbols: α, β, γ, and δ denote a significant difference between: O103F and O157A-F in competition, O157A-F in competition and O157A-F controls, O26E and O111A-F in competition and O111A-F in competition and O111A-F controls, respectively (p < 0.05).
symbol denotes the O111B strain that was still present after 14 days. Symbols: α, β, γ, and δ denote a significant difference between: O103F and O157A-F in competition, O157A-F in competition and O157A-F controls, O26E and O111A-F in competition and O111A-F in competition and O111A-F controls, respectively (p < 0.05).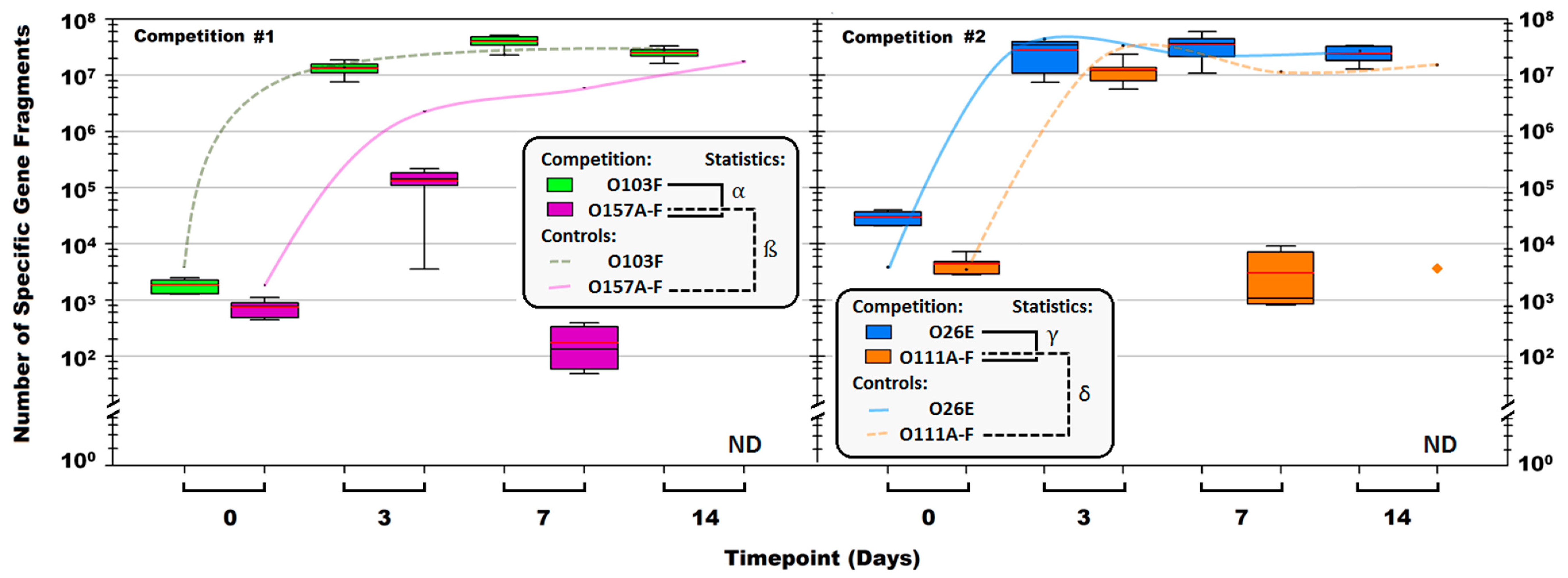
| Rank | Serogroup | H-Type | Toxin | eae | hlyA | Liquid | Omelette | Plug n’ Prey | Discussed in Manuscript | |
|---|---|---|---|---|---|---|---|---|---|---|
| Strongest | O103F | NM | − | − | − | √ | √ | √ | √ | |
| Strong | O26E | H9 | − | − | − | √ | √ | √ | ||
| O178A | H7 | − | NT | NT | √ | √ | ||||
| O178B | NT | − | − | − | √ | |||||
| Weak | O26 | A | NT | − | − | NT | √ | |||
| B | NT | − | + | NT | √ | |||||
| C | H18 | − | − | − | √ | |||||
| D | H11 | stx 1 | + | + | √ | √ | ||||
| F | NM | − | + | − | √ | |||||
| O45 | A | H4 | − | − | − | √ | ||||
| B | NT | stx 1 | + | NT | √ | |||||
| C | NT | − | − | NT | √ | |||||
| O51A | NM | − | − | − | √ | |||||
| O103 | A | NM | − | + | + | √ | ||||
| B | NM | − | + | + | √ | |||||
| C | NM | − | + | + | √ | |||||
| D | H38 | − | − | − | √ | |||||
| E | NM | − | + | + | √ | √ | ||||
| O111 | A | NM | stx 1 | + | + | √ | √ | |||
| B | NM | stx 1 | + | + | √ | |||||
| C | H8 | stx 1 | + | + | √ | |||||
| D | H8 | stx 1 | + | + | √ | |||||
| E | NM | stx 1 | + | + | √ | |||||
| F | NM | stx 1 | + | + | √ | √ | √ | |||
| O145 | A | NM | stx 1 | + | + | √ | ||||
| B | H25 | − | + | + | √ | |||||
| O157 | A | H7 | stx 1 & 2 | + | + | √ | √ | √ | ||
| B | NT | stx 1 & 2 | + | NT | √ | |||||
| C | NT | stx 1 & 2 | + | NT | √ | |||||
| D | H7 | stx 1 & 2 | + | + | √ | |||||
| E | NT | stx 2 | + | NT | √ | √ | √ | √ | ||
| F | NT | stx 1 & 2 | + | NT | √ | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paquette, S.-J.; Zaheer, R.; Stanford, K.; Thomas, J.; Reuter, T. Competition among Escherichia coli Strains for Space and Resources. Vet. Sci. 2018, 5, 93. https://doi.org/10.3390/vetsci5040093
Paquette S-J, Zaheer R, Stanford K, Thomas J, Reuter T. Competition among Escherichia coli Strains for Space and Resources. Veterinary Sciences. 2018; 5(4):93. https://doi.org/10.3390/vetsci5040093
Chicago/Turabian StylePaquette, Sarah-Jo, Rahat Zaheer, Kim Stanford, James Thomas, and Tim Reuter. 2018. "Competition among Escherichia coli Strains for Space and Resources" Veterinary Sciences 5, no. 4: 93. https://doi.org/10.3390/vetsci5040093
APA StylePaquette, S.-J., Zaheer, R., Stanford, K., Thomas, J., & Reuter, T. (2018). Competition among Escherichia coli Strains for Space and Resources. Veterinary Sciences, 5(4), 93. https://doi.org/10.3390/vetsci5040093






