Coronary Artery Anomalies in Animals
Abstract
:1. Introduction
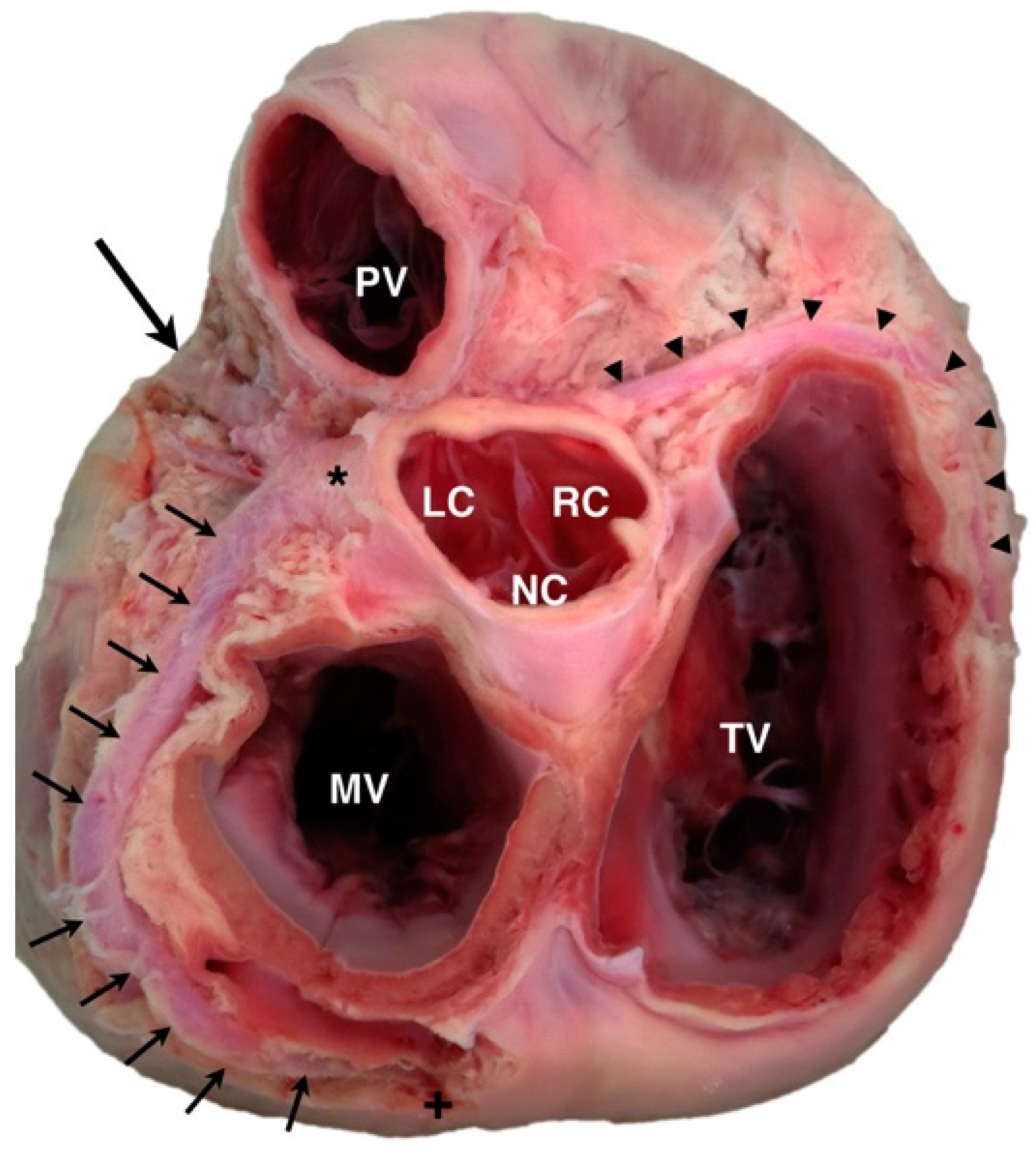
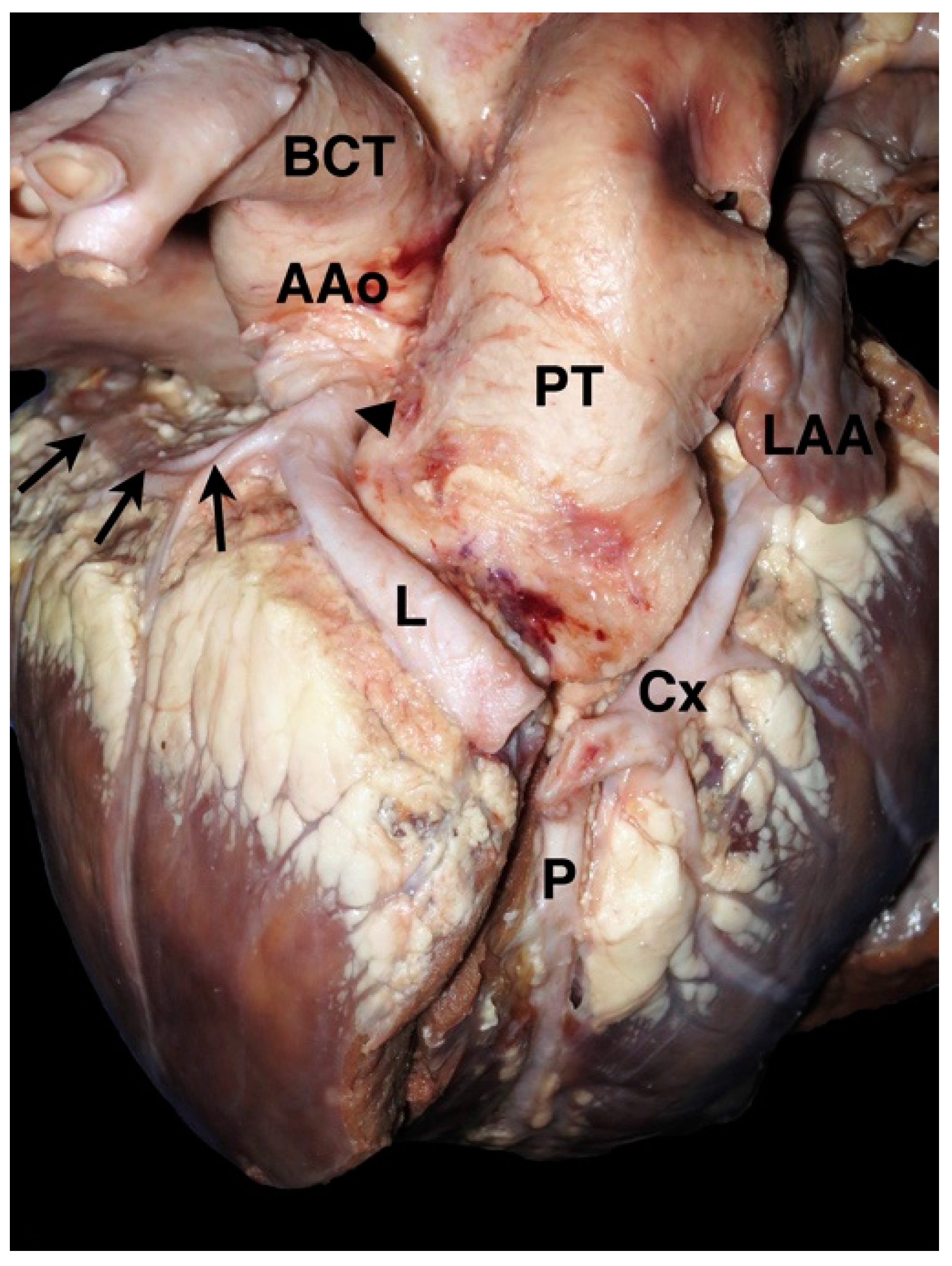
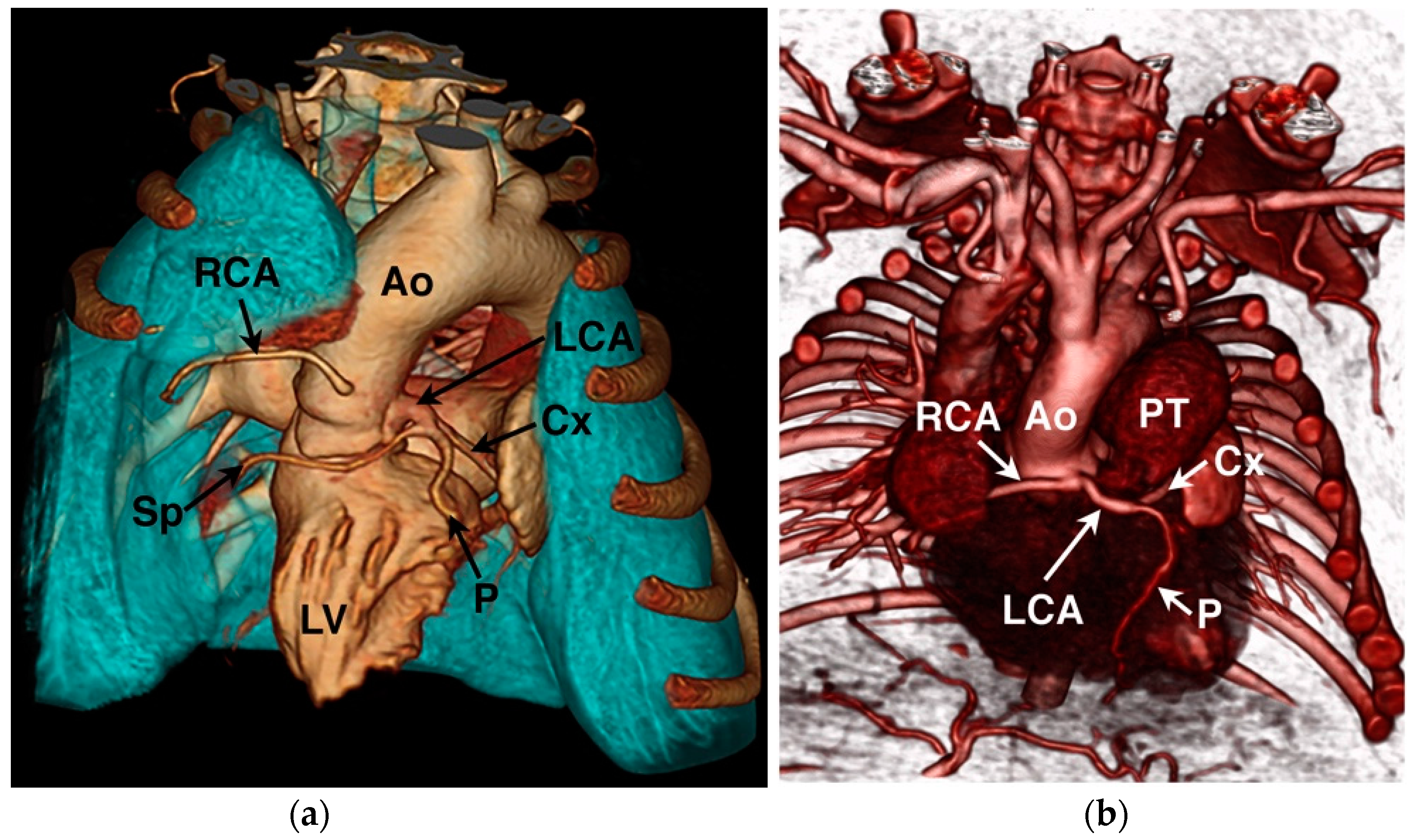
2. Coronary Artery Embryology and Anatomy
3. Prevalence and Categorization of Coronary Artery Anomalies
Veterinary Reports of Coronary Artery Anomalies
4. Anomalies of Coronary Origin and Course
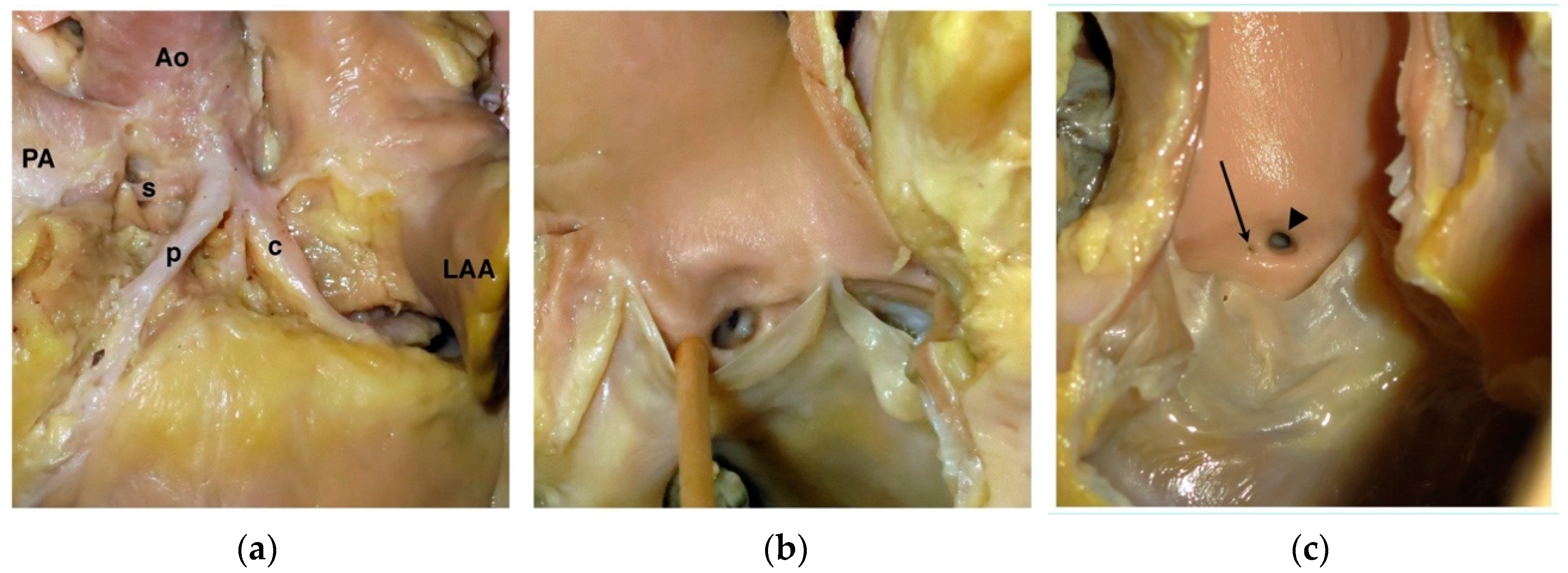
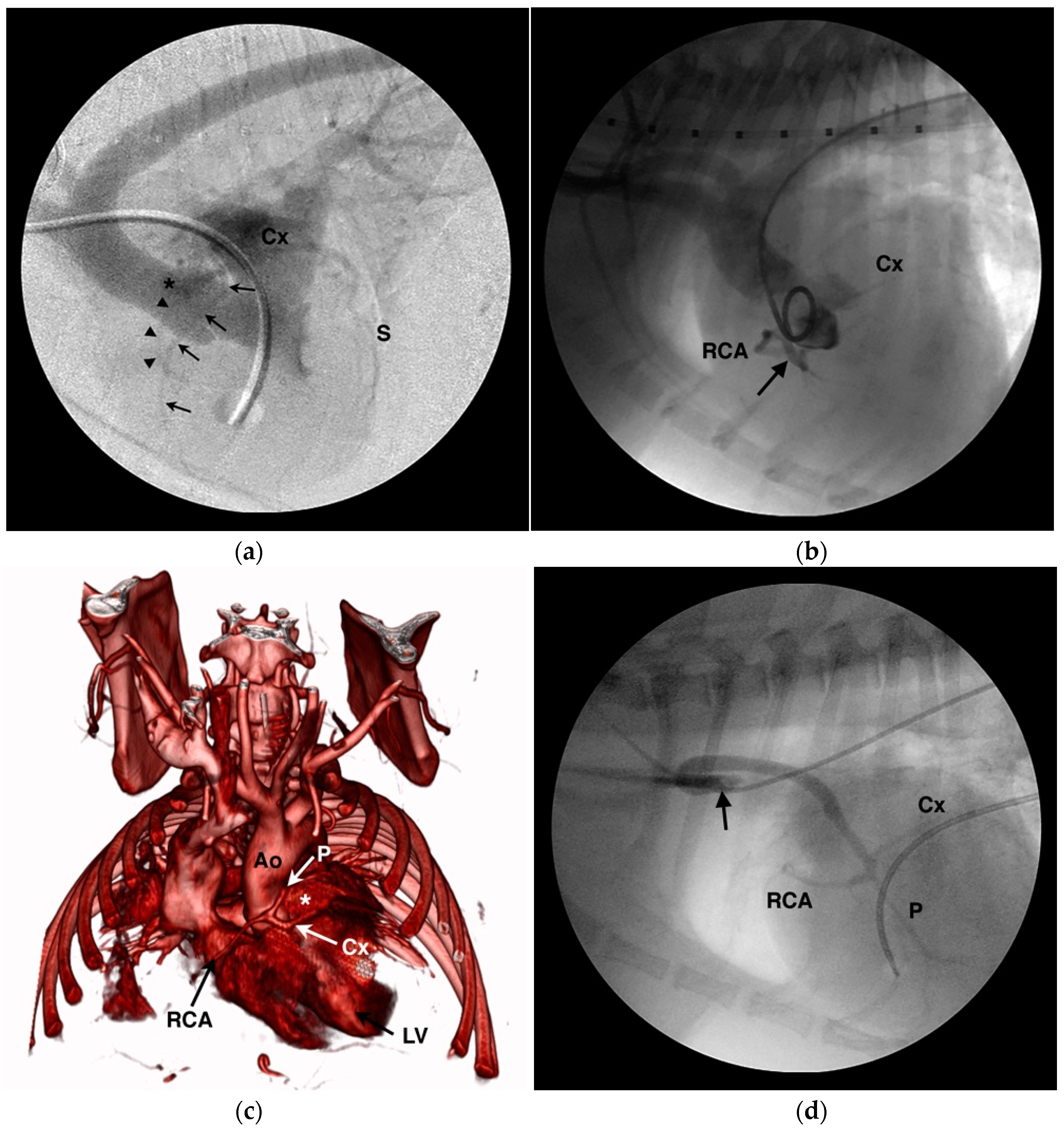
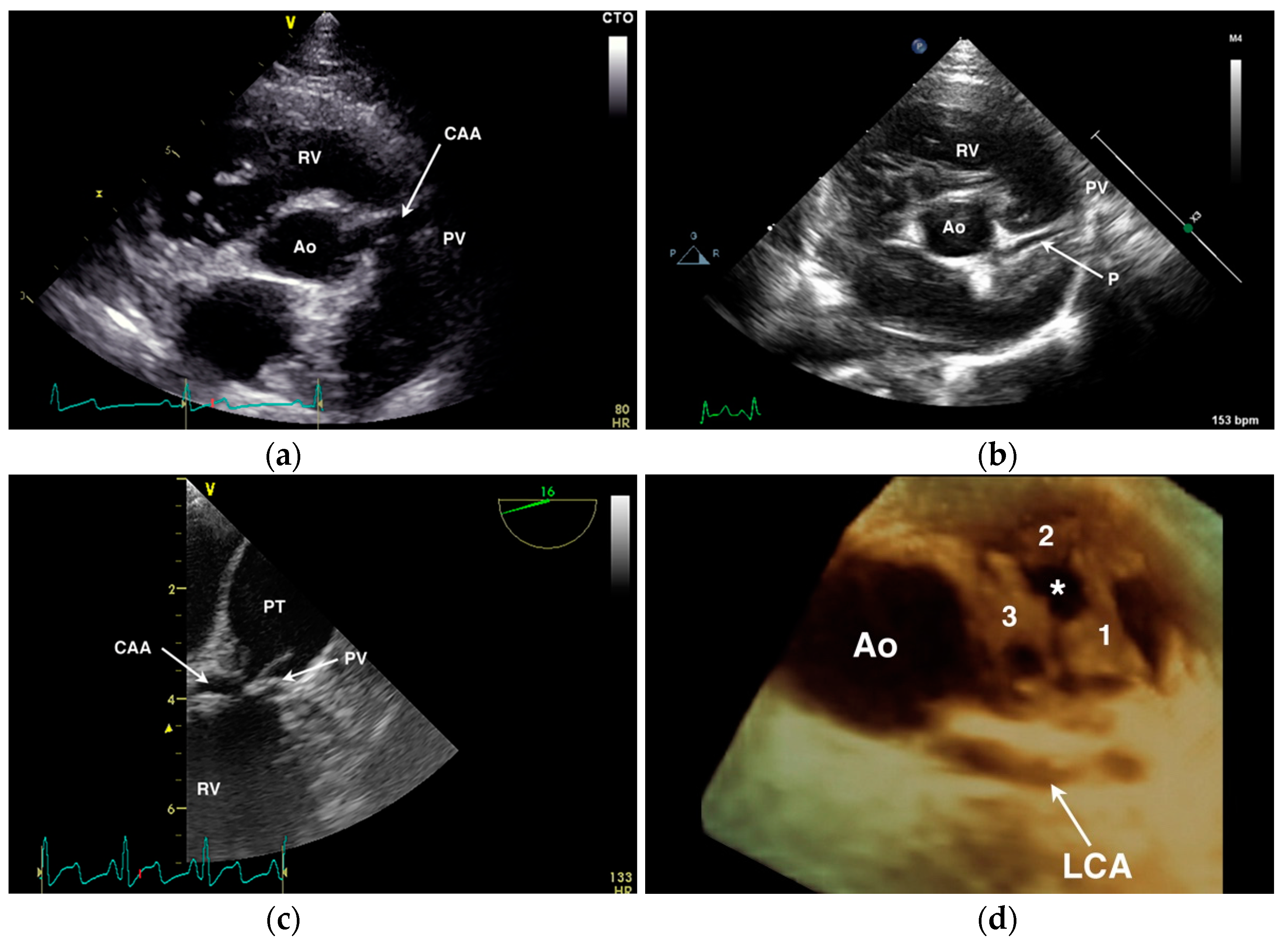
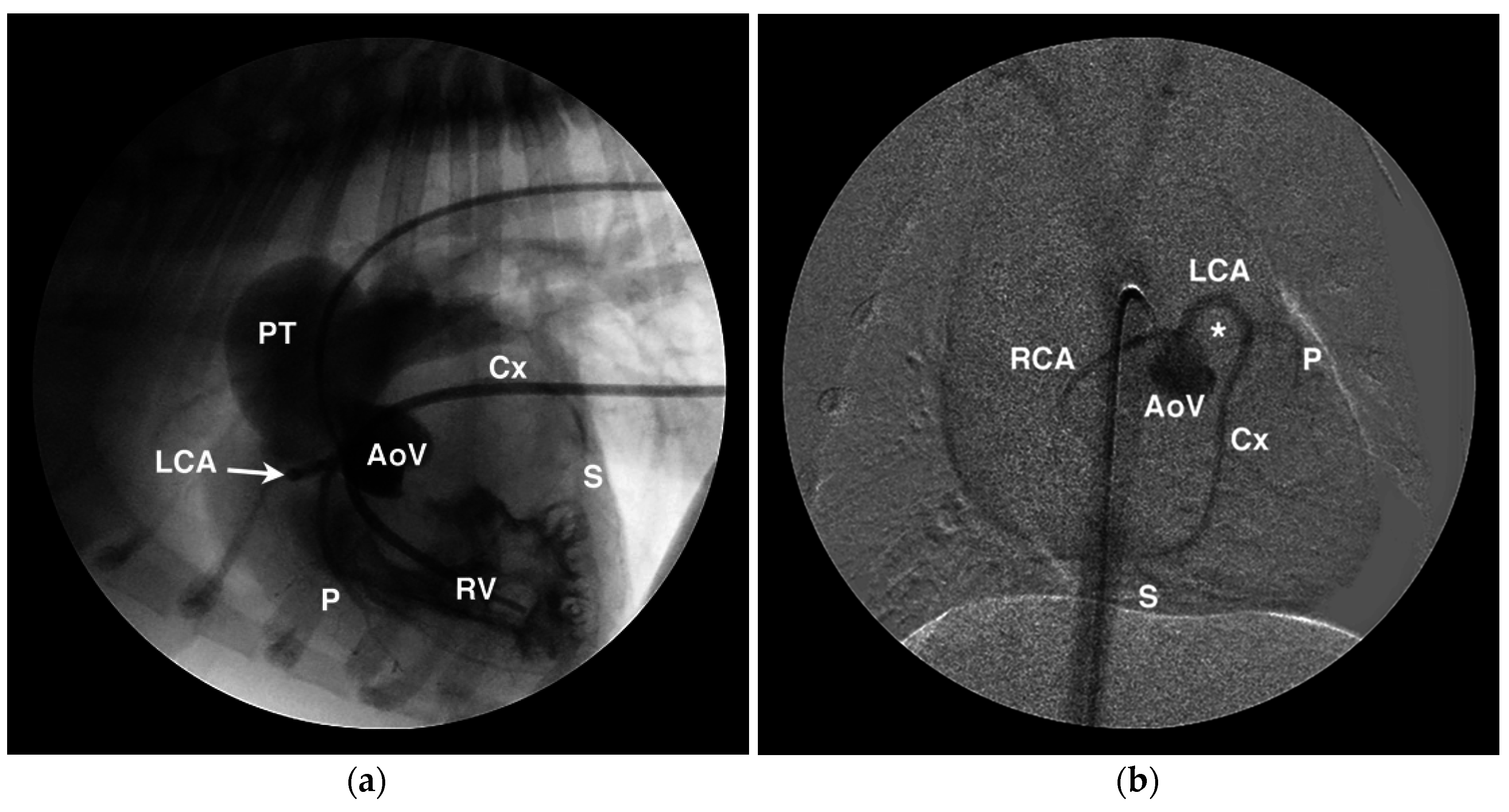
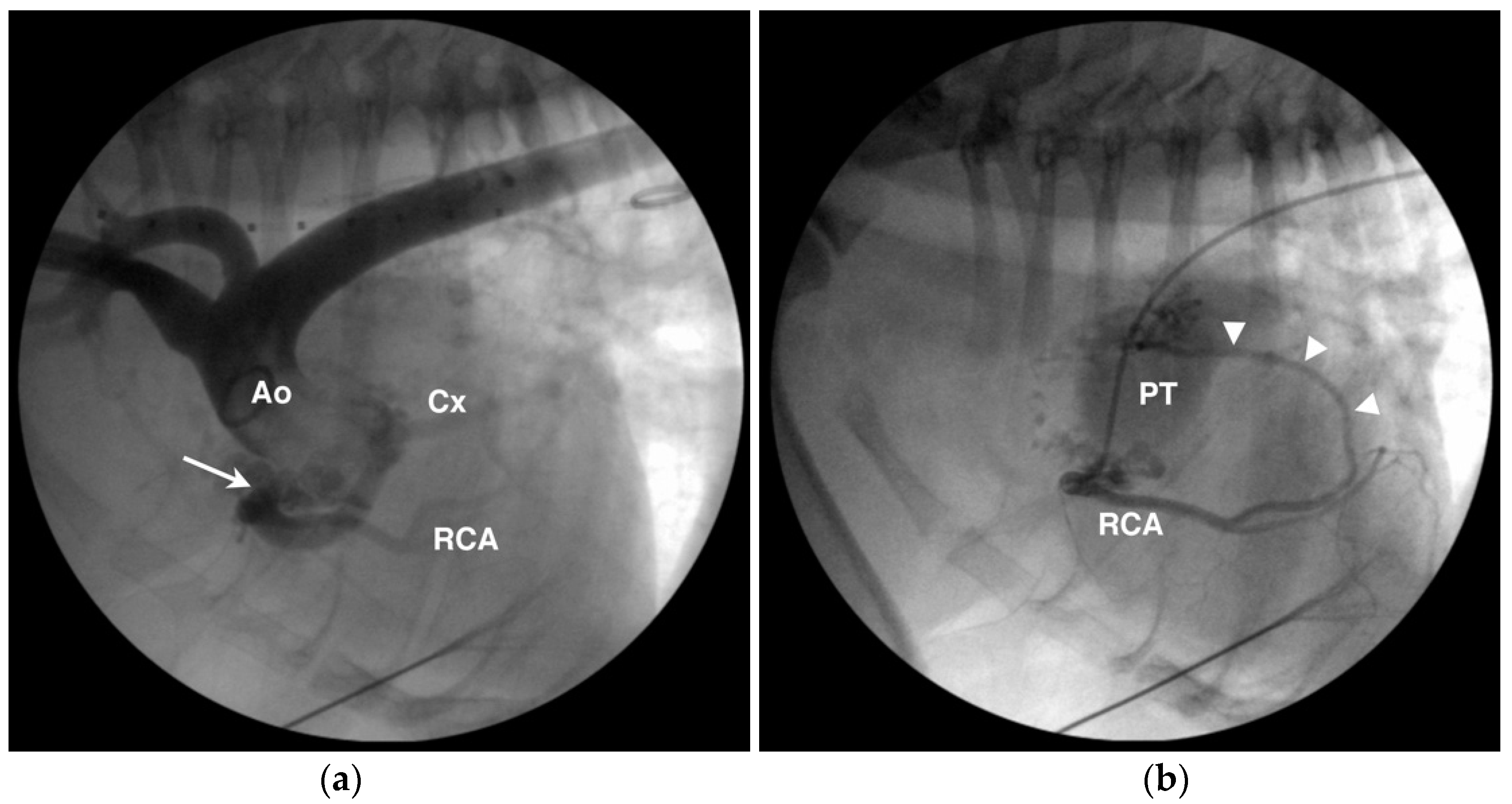
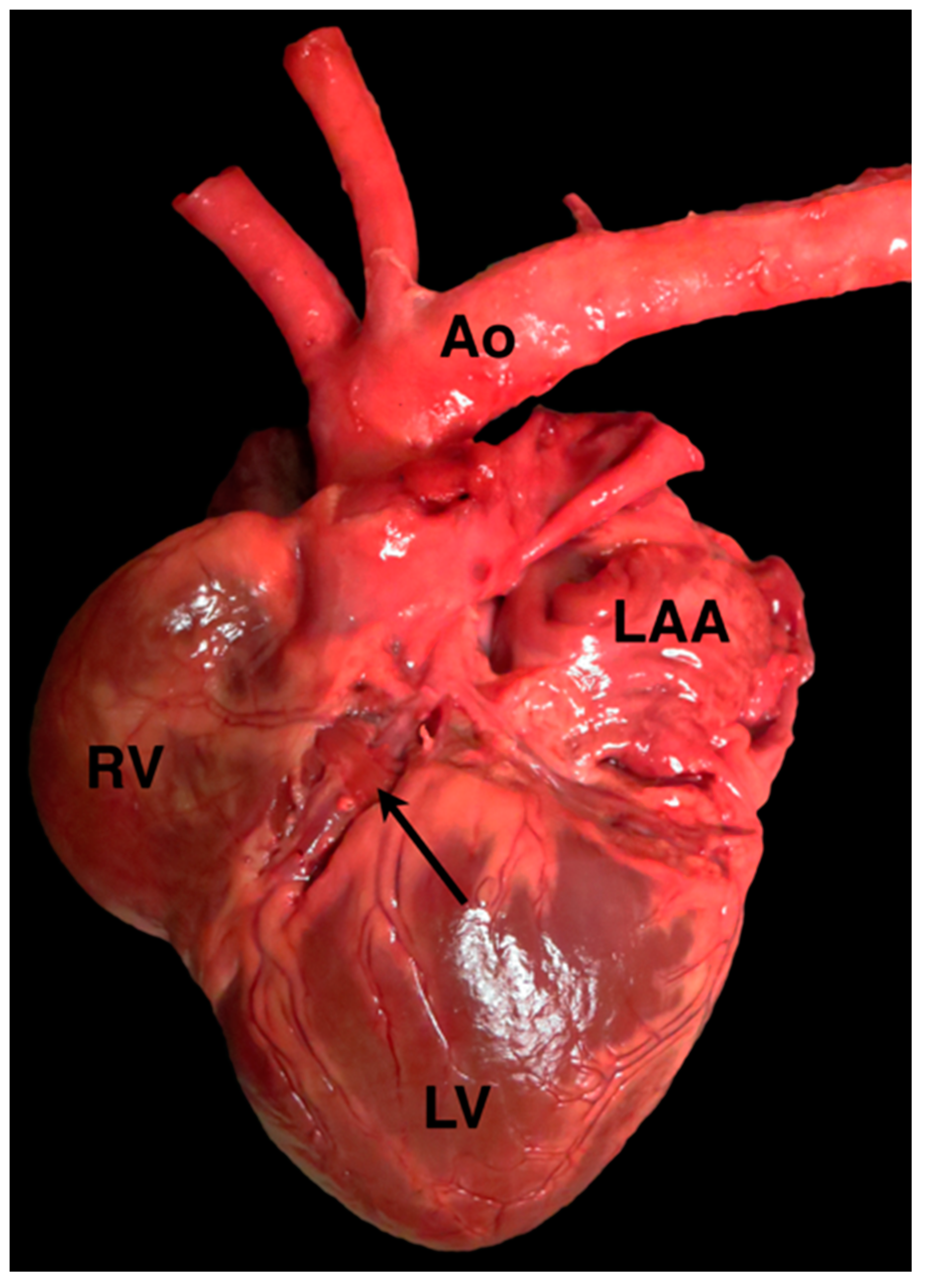
4.1. Anomalous Origin from the Aorta
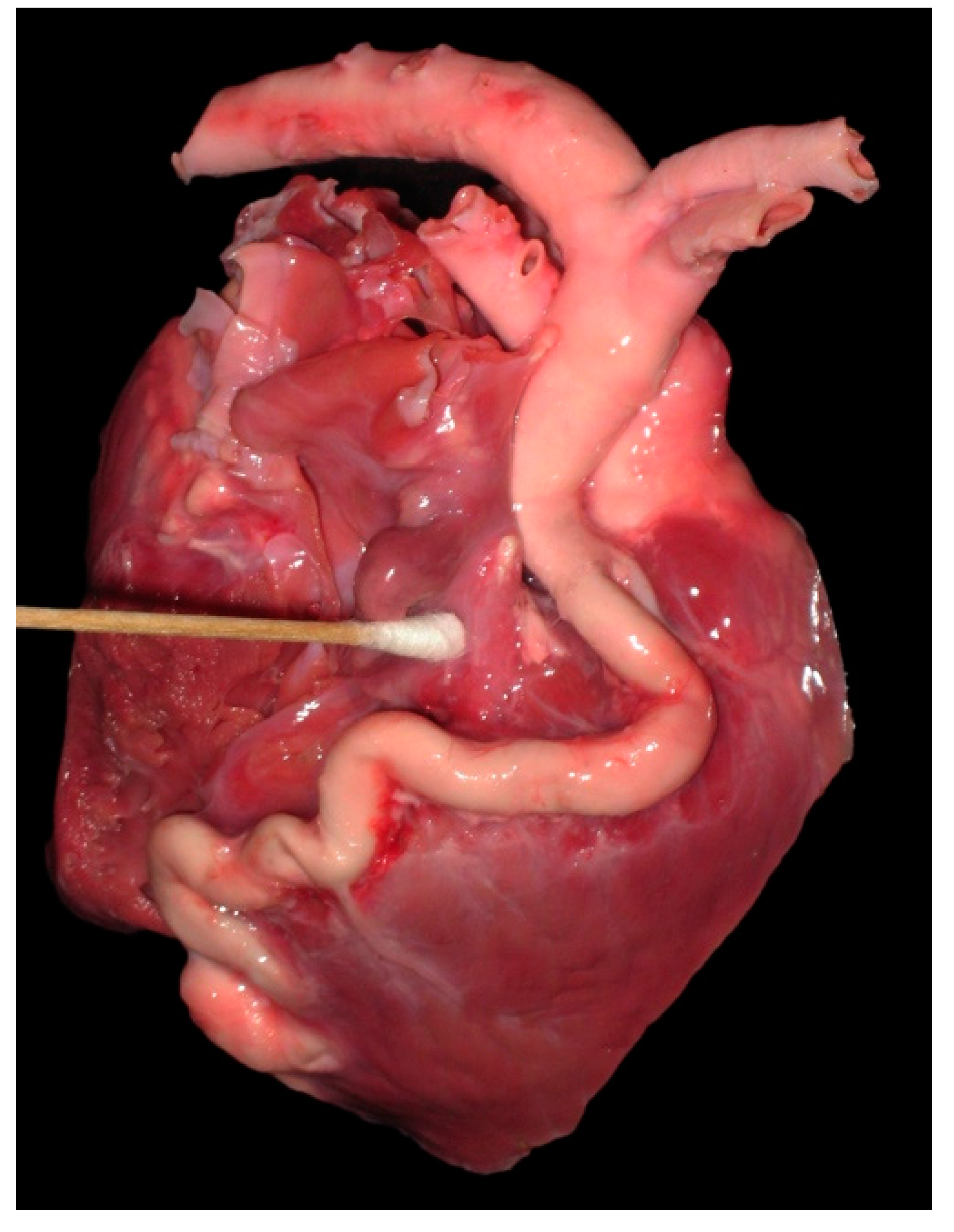
4.2. Anomalous Origin from the Pulmonary Trunk
5. Coronary Arterial Fistulae
6. Coronary Artery Aneurysm
7. Myocardial Bridging of Epicardial Coronary Arteries
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3. [Google Scholar] [CrossRef] [PubMed]
- Driehuys, S.; Van Winkle, T.J.; Sammarco, C.D.; Drobatz, K.J. Myocardial infarction in dogs and cats: 37 cases (1985–1994). J. Am. Vet. Med. Assoc. 1998, 213, 1444–1448. [Google Scholar] [PubMed]
- Falk, T.; Jonsson, L. Ischaemic heart disease in the dog: A review of 65 cases. J. Small Anim. Pract. 2000, 41, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Angelini, P. Coronary artery anomalies: An entity in search of an identity. Circulation 2007, 115, 1296–1305. [Google Scholar] [PubMed]
- Basso, C.; Maron, B.J.; Corrado, D.; Thiene, G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J. Am. Coll. Cardiol. 2000, 35, 1493–1501. [Google Scholar] [CrossRef]
- Buchanan, J.W. Pulmonic stenosis caused by single coronary artery in dogs: Four cases (1965–1984). J. Am. Vet. Med. Assoc. 1990, 196, 115–120. [Google Scholar] [PubMed]
- Fonfara, S.; Martinez Pereira, Y.; Swift, S.; Copeland, H.; Lopez-Alvarez, J.; Summerfield, N.; Cripps, P.; Dukes-McEwan, J. Balloon valvuloplasty for treatment of pulmonic stenosis in english bulldogs with an aberrant coronary artery. J. Vet. Intern. Med. 2010, 24, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Spicer, D.E.; Henderson, D.J.; Chaudhry, B.; Mohun, T.J.; Anderson, R.H. The anatomy and development of normal and abnormal coronary arteries. Cardiol. Young 2015, 25, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Perloff, J.K.; Marelli, A.J. Congenital anomalies of the coronary circulation. In Perloff’s Clinical Recognition of Congenital Heart Disease, 6th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2012; pp. 530–543. [Google Scholar]
- Bezuidenhout, A.J. The heart and arteries. In Miller’s Anatomy of the Dog, 4th ed.; Evans, H.E., De Lahunta, A., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2013; pp. 428–504. [Google Scholar]
- Noestelthaller, A.; Probst, A.; Konig, H.E. Branching patterns of the left main coronary artery in the dog demonstrated by the use of corrosion casting technique. Anat. Histol. Embryol. 2007, 36, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Donald, D.E.; Essex, H.E. The canine septal coronary artery; an anatomic and electrocardiographic study. Am. J. Physiol. 1954, 176, 143–154. [Google Scholar] [PubMed]
- Ozgel, O.; Dursun, N. The arterial vascularization of septum interventriculare in donkeys (Equus asinus L.). Anat. Histol. Embryol. 2005, 34, 80–84. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.L.S.; David, G.S.; Carvalho, M.; Dornelas, D.; Araujo, S.; da Silva, N.C.; Ruiz, C.R.; Fernandes, J.R.; Wafae, N. Anatomical indicators of dominance between the coronary arteries of dogs. Int. J. Morphol. 2011, 29, 845–849. [Google Scholar] [CrossRef]
- Corr, P.B.; Pearle, D.L.; Hinton, J.R.; Roberts, W.C.; Gillis, R.A. Site of myocardial infarction. A determinant of the cardiovascular changes induced in the cat by coronary occlusion. Circ. Res. 1976, 39, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Day, S.B.; Johnson, J.A. The distribution of the coronary arteries of the rabbit. Anat. Rec. 1958, 132, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Pinto Neto, J.L.; Leão, C.E.S.; Vieira, T.H.M.; Lopes, A.K.M.S.; Vieira, S.R.C.; Silva, N.C.D.; Wafae, G.C.; Ruiz, C.R.; Wafae, N. Anatomical indicators of dominance among the coronary arteries in goats. Braz. J. Vet. Res. Anim. Sci. 2009, 46, 48–53. [Google Scholar] [CrossRef]
- Vieira, T.H.M.; Moura, P.C., Jr.; Vieira, S.R.C.; Moura, P.R.; Silva, N.C.; Wafae, G.C.; Ruiz, C.R.; Wafae, N. Anatomical indicators of dominance between the coronary arteries in swine. Morphologie 2008, 92, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Ozgel, O.; Haligur, A.C.; Dursun, N.; Karakurum, E. The macroanatomy of coronary arteries in donkeys (Equus asinus L.). Anat. Histol. Embryol. 2004, 33, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.A.; Abel-Magied, E.M. The coronary arteries of the dromedary camel (Camelus dromedarius). Anat. Histol. Embryol. 1996, 25, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Gebhard, C.; Fuchs, T.A.; Stehli, J.; Gransar, H.; Berman, D.S.; Budoff, M.J.; Achenbach, S.; Al-Mallah, M.; Andreini, D.; Cademartiri, F.; et al. Coronary dominance and prognosis in patients undergoing coronary computed tomographic angiography: Results from the confirm (coronary ct angiography evaluation for clinical outcomes: An international multicenter) registry. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Yoldas, A.; Ozmen, E.; Ozdemir, V. Macroscopic description of the coronary arteries in swiss albino mice (Mus musculus). J. S. Afr. Vet. Assoc. 2010, 81, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Bahar, S.; Ozdemir, V.; Eken, E.; Tipirdamaz, S. The distribution of the coronary arteries in the angora rabbit. Anat. Histol. Embryol. 2007, 36, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, V.; Cevik-Demirkan, A.; Turkmenoglu, I. The right coronary artery is absent in the chinchilla (Chinchilla lanigera). Anat. Histol. Embryol. 2008, 37, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Baraona, F.; Valente, A.M.; Porayette, P.; Pluchinotta, F.R.; Sanders, S.P. Coronary arteries in childhood heart disease: Implications for management of young adults. J. Clin. Exp. Cardiol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Freire, G.; Miller, M.S. Echocardiographic evaluation of coronary arteries in congenital heart disease. Cardiol. Young 2015, 25, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Lorber, R.; Srivastava, S.; Wilder, T.J.; McIntyre, S.; DeCampli, W.M.; Williams, W.G.; Frommelt, P.C.; Parness, I.A.; Blackstone, E.H.; Jacobs, M.L.; et al. Anomalous aortic origin of coronary arteries in the young: Echocardiographic evaluation with surgical correlation. J. Am. Coll. Cardiol. Cardiovasc. Imaging 2015, 8, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.D.; Sammut, E.; Nair, A.; Rajani, R.; Bonamini, R.; Chiribiri, A. Coronary artery anomalies overview: The normal and the abnormal. World J. Radiol. 2016, 8, 537–555. [Google Scholar] [CrossRef] [PubMed]
- Day, S.B. A left coronary artery originating from a single coronary stem in a dog. Anat. Rec. 1959, 134, 55–59. [Google Scholar] [CrossRef]
- Buchanan, J.W. Pathogenesis of single right coronary artery and pulmonic stenosis in english bulldogs. J. Vet. Intern. Med. 2001, 15, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Minami, T.; Wakao, Y.; Buchanan, J.; Muto, M.; Watanabe, T.; Suzuki, T.; Takahashi, M. A case of pulmonic stenosis with single coronary artery in a dog. Jpn. J. Vet. Sci. 1989, 51, 453–456. [Google Scholar] [CrossRef]
- Navalon, I.; Pradelli, D.; Bussadori, C.M. Transesophageal echocardiography to diagnose anomalous right coronary artery type r2a in dogs. J. Vet. Cardiol. 2015, 17, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Visser, L.C.; Scansen, B.A.; Schober, K.E. Single left coronary ostium and an anomalous prepulmonic right coronary artery in 2 dogs with congenital pulmonary valve stenosis. J. Vet. Cardiol. 2013, 15, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Laborda-Vidal, P.; Pedro, B.; Baker, M.; Gelzer, A.R.; Dukes-McEwan, J.; Maddox, T.W. Use of ECG-gated computed tomography, echocardiography and selective angiography in five dogs with pulmonic stenosis and one dog with pulmonic stenosis and aberrant coronary arteries. J. Vet. Cardiol. 2016, 18, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Waterman, M.I.; Abbott, J.A. Novel coronary artery anomaly in an English bulldog with pulmonic stenosis. J. Vet. Intern. Med. 2013, 27, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- Van Mierop, L.H.; Patterson, D.F.; Schnarr, W.R. Pathogenesis of persistent truncus arteriosus in light of observations made in a dog embryo with the anomaly. Am. J. Cardiol. 1978, 41, 755–762. [Google Scholar] [CrossRef]
- Koo, S.T.; LeBlanc, N.L.; Scollan, K.F.; Sisson, D.D. Complete transposition of the great arteries with double outlet right ventricle in a dog. J. Vet. Cardiol. 2016, 18, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Turk, M.A.; Turk, J.R.; Hopkins, M.G.; Wagner, J.A. Unexpected death in an adult dog with anomalous origin of the left coronary artery from the pulmonary trunk. Cornell Vet. 1984, 74, 344–348. [Google Scholar] [PubMed]
- Hernandez, J.L.; Bélanger, M.-C.; Benoit-Biancamano, M.-O.; Girard, C.; Pibarot, P. Left coronary aneurysmal dilation and subaortic stenosis in a dog. J. Vet. Cardiol. 2008, 10, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, A.; Côté, E.; Eyster, G.E. Congenital coronary-pulmonary arterial shunt in a German shepherd dog: Diagnosis and surgical correction. J. Vet. Cardiol. 2011, 13, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Tangkawattana, P.; Muto, M.; Nakade, T.; Taniyama, H.; Miyata, Y.; Nakayama, T.; Hamlin, R.L. Myocardial bridge muscle on left anterior descending coronary artery differs from subepicardial myocardium of the left ventricle in dogs. Acta Anat. 1996, 157, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Tangkawattana, P.; Muto, M.; Nakayama, T.; Karkoura, A.; Yamano, S.; Yamaguchi, M. Prevalence, vasculature, and innervation of myocardial bridges in dogs. Am. J. Vet. Res. 1997, 58, 1209–1215. [Google Scholar] [PubMed]
- Bartoli, C.R.; Wead, W.B.; Giridharan, G.A.; Prabhu, S.D.; Koenig, S.C.; Dowling, R.D. Mechanism of myocardial ischemia with an anomalous left coronary artery from the right sinus of Valsalva. J. Thorac. Cardiovasc. Surg. 2012, 144, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Dennis, S.M.; Gardiner, M.R. Congenital coronary artery defect in a cow. Vet. Rec. 1972, 90, 281. [Google Scholar] [CrossRef] [PubMed]
- Vitums, A. Dual origin of the left coronary artery in a newborn calf. Anat. Anz. 1973, 133, 73–75. [Google Scholar] [PubMed]
- Bildfell, R.J.; Pringle, J.K.; Miller, L.M. Coronary arterioventricular anomaly in a calf. J. Vet. Diagn. Investig. 1996, 8, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Sandusky, G.E.; Smith, C.W. Anomalous left coronary artery in a calf. J. Am. Vet. Med. Assoc. 1978, 173, 475–477. [Google Scholar] [PubMed]
- Shank, A.M.; Bryant, U.K.; Jackson, C.B.; Williams, N.M.; Janes, J.G. Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) in four calves. Vet. Pathol. 2008, 45, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Yasuda, M.; Murakami, T. Aberrant origin of coronary arteries from pulmonary trunk in cattle. Adv. Anim. Cardiol. 2009, 42, 1–7. [Google Scholar]
- Rooney, J.R.; Franks, W.C. Congenital cardiac anomalies in horses. Pathol. Vet. 1964, 1, 454–464. [Google Scholar] [CrossRef]
- Karlstam, E.; Ho, S.Y.; Shokrai, A.; Agren, E.; Michaelsson, M. Anomalous aortic origin of the left coronary artery in a horse. Equine Vet. J. 1999, 31, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.A.; Gogas, B.D.; Sumida, A.; Nagai, H.; King Iii, S.B.; Chronos, N.; Hou, D. Anomalous right coronary artery originating from the left sinus of Valsalva in a Yucatan minipig. Comp. Med. 2012, 62, 127–130. [Google Scholar] [PubMed]
- Fernandez, M.C.; Duran, A.C.; Real, R.; Lopez, D.; Fernandez, B.; de Andres, A.V.; Arque, J.M.; Gallego, A.; Sans-Coma, V. Coronary artery anomalies and aortic valve morphology in the syrian hamster. Lab. Anim. 2000, 34, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Duran, A.C.; Fernandez-Gallego, T.; Fernandez, B.; Fernandez, M.C.; Arque, J.M.; Sans-Coma, V. Solitary coronary ostium in the aorta in Syrian hamsters. A morphological study of 130 cases. Cardiovasc. Pathol. 2005, 14, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Sans-Coma, V.; Arque, J.M.; Duran, A.C.; Cardo, M. Origin of the left main coronary artery from the pulmonary trunk in the syrian hamster. Am. J. Cardiol. 1988, 62, 159–161. [Google Scholar] [CrossRef]
- Duran, A.C.; Arque, J.M.; Fernandez, B.; Fernandez, M.C.; Fernandez-Gallego, T.; Rodriguez, C.; Sans-Coma, V. Rudimentary coronary artery in Syrian hamsters (Mesocricetus auratus). Anat. Histol. Embryol. 2009, 38, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Dodge-Khatami, A.; Mavroudis, C.; Backer, C.L. Congenital heart surgery nomenclature and database project: Anomalies of the coronary arteries. Ann. Thorac. Surg. 2000, 69, S270–297. [Google Scholar] [CrossRef]
- Lipton, M.J.; Barry, W.H.; Obrez, I.; Silverman, J.F.; Wexler, L. Isolated single coronary artery: Diagnosis, angiographic classification, and clinical significance. Radiology 1979, 130, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Tadros, S.S.; Soni, S.; Madan, S. Single coronary artery anomaly: Classification and evaluation using multidetector computed tomography and magnetic resonance angiography. Pediatr. Cardiol. 2014, 35, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Angelini, P. Is echocardiography adequate to identify the severity of anomalous coronary arteries? J. Am. Coll. Cardiol. Cardiovasc. Imaging 2016, 9, 898–899. [Google Scholar] [CrossRef] [PubMed]
- Kittleson, M.; Thomas, W.; Loyer, C.; Kienle, R. Single coronary artery (type R2A). J. Vet. Intern. Med. 1992, 6, 250–251. [Google Scholar] [PubMed]
- Buchanan, J.W.; Patterson, D.F. Selective angiography and angiocardiography in dogs with congenital cardiovascular disease. J. Am. Vet. Radiol. Soc. 1965, 6, 21–39. [Google Scholar] [CrossRef]
- Loukas, M.; Groat, C.; Khangura, R.; Owens, D.G.; Anderson, R.H. The normal and abnormal anatomy of the coronary arteries. Clin. Anat. 2009, 22, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Hlavacek, A.; Loukas, M.; Spicer, D.; Anderson, R.H. Anomalous origin and course of the coronary arteries. Cardiol. Young 2010, 20 (Suppl. 3), 20–25. [Google Scholar] [CrossRef] [PubMed]
- Scansen, B.A.; Chiappone, G.A.; Layman, R.; White, R.D. Computed tomography angiography of the coronary arterial circulation of bulldogs under conscious sedation is feasible and defines anomalous anatomy. (abstract). J. Vet. Intern. Med. 2014, 28, 1353. [Google Scholar]
- Buchanan, J.W. Causes and prevalence of cardiovascular diseases. In Current Veterinary Therapy XI: Small Animal Practice; Kirk, R.W., Bonagura, J.D., Eds.; WB Saunders Co.: Philadelphia, PA, USA, 1992; pp. 647–654. [Google Scholar]
- Marr, C.M.; Reef, V.B.; Brazil, T.J.; Thomas, W.P.; Knottenbelt, D.C.; Kelly, D.F.; Baker, J.R.; Reimer, J.M.; Maxson, A.D.; Crowhurst, J.S. Aorto-cardiac fistulas in seven horses. Vet. Radiol. Ultrasound 1998, 39, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Mangukia, C.V. Coronary artery fistula. Ann. Thorac. Surg. 2012, 93, 2084–2092. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, S.A. Coronary arterial fistulas. Orphanet J. Rare Dis. 2006, 1. [Google Scholar] [CrossRef] [PubMed]
- Perloff, J.K.; Marelli, A.J. Pulmonary atresia with intact ventricular septum. In Perloff’s Clinical Recognition of Congenital Heart Disease, 6th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2012; pp. 429–438. [Google Scholar]
- Culshaw, G.J.; Wagner, T.; Luis Fuentes, V.; Schwarz, T.; Yool, D.A.; French, A.T.; Brockman, D.J. Identification and surgical ligation of aortopulmonic vascular malformation causing left heart volume overload in 4 dogs. J. Vet. Intern. Med. 2013, 27, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Pahlavan, P.S.; Niroomand, F. Coronary artery aneurysm: A review. Clin. Cardiol. 2006, 29, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, G.; Migliore, F.; Cademartiri, F.; Fraccaro, C.; Iliceto, S. Left anterior descending artery myocardial bridging: A clinical approach. J. Am. Coll. Cardiol. 2016, 68, 2887–2899. [Google Scholar] [CrossRef] [PubMed]
- Kosinski, A.; Grzybiak, M.; Kozlowski, D. Distribution of myocardial bridges in domestic pig. Pol. J. Vet. Sci. 2010, 13, 689–693. [Google Scholar] [CrossRef] [PubMed]
| Major Coronary Artery Anomalies |
| Anomalous origin from the pulmonary artery |
| Left coronary artery from PA a,b |
| Right coronary artery from PA |
| Both coronary arteries from PA |
| Coronary cameral fistula |
| Draining to RV b,c |
| Draining to RA |
| Draining to LV b |
| Draining to LA |
| Coronary to pulmonary arterial shunt a,b |
| Congenital atresia of the LCA |
| Minor Coronary Artery Anomalies |
| Anomalous origin from the aorta |
| Origin of LCA from right aortic sinus a,b,d |
| Origin of RCA from left aortic sinus a,e |
| Origin of paraconal branch from right aortic sinus a |
| Origin of paraconal branch from RCA |
| Origin of circumflex branch from right aortic sinus a |
| Origin of circumflex branch from RCA |
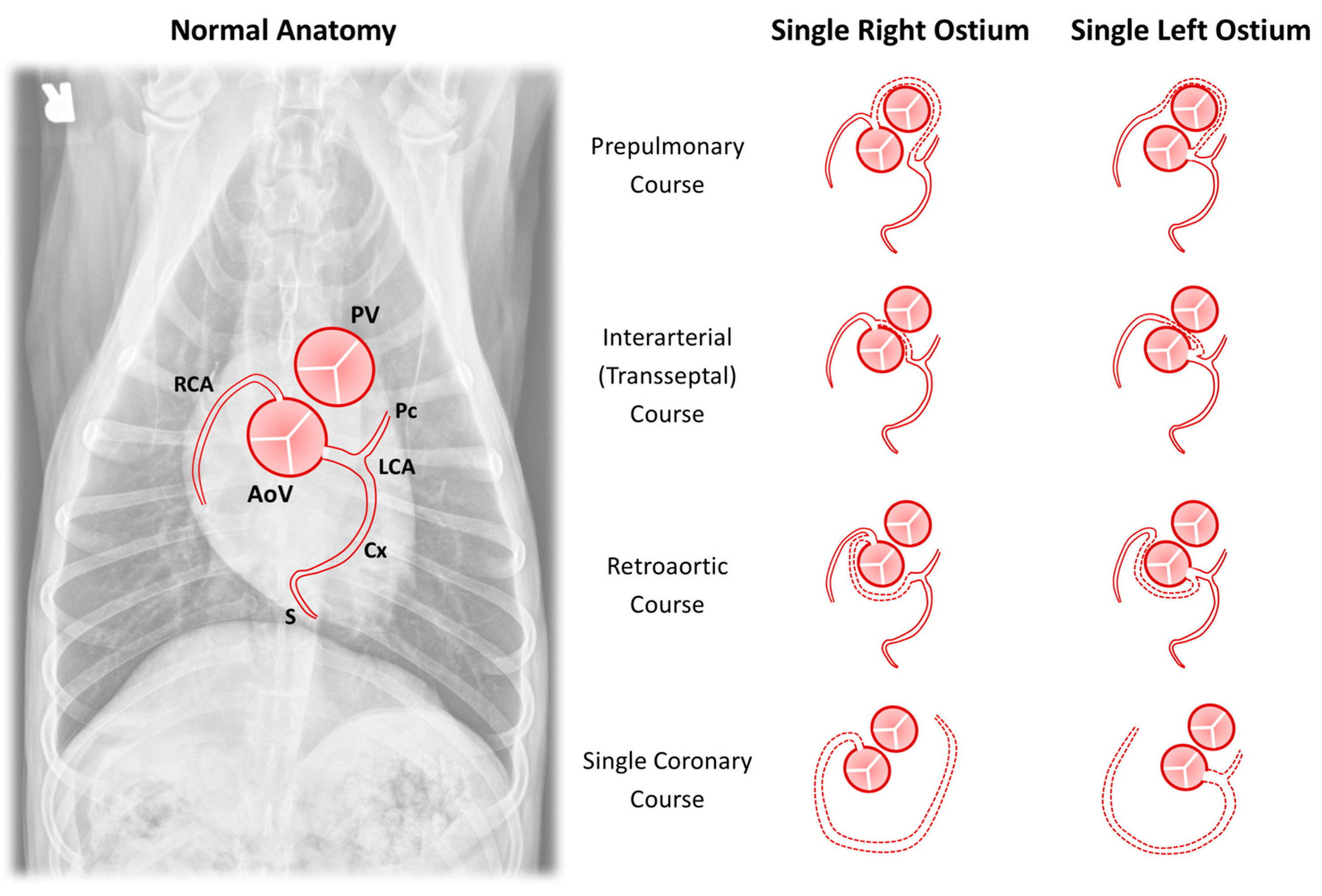
| Single coronary artery |
| Single left coronary artery |
| Single right coronary artery |
| Inverted coronary arteries |
| Other anomalous aortic origin a |
| Anomalous epicardial course |
| Prepulmonary course a,b,d,* |
| Interarterial course a,b,d |
| Transseptal course a |
| Retroaortic course a |
| Duplication of a coronary artery |
| Duplication of paraconal branch |
| Duplication of circumflex branch |
| Duplication of RCA |
| Multiple ostia within left aortic sinus a |
| Multiple ostia within right aortic sinus a |
| High take-off coronary artery a |
| Coronary artery aneurysm a |
| Myocardial bridging of an epicardial coronary artery a,e |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scansen, B.A. Coronary Artery Anomalies in Animals. Vet. Sci. 2017, 4, 20. https://doi.org/10.3390/vetsci4020020
Scansen BA. Coronary Artery Anomalies in Animals. Veterinary Sciences. 2017; 4(2):20. https://doi.org/10.3390/vetsci4020020
Chicago/Turabian StyleScansen, Brian A. 2017. "Coronary Artery Anomalies in Animals" Veterinary Sciences 4, no. 2: 20. https://doi.org/10.3390/vetsci4020020
APA StyleScansen, B. A. (2017). Coronary Artery Anomalies in Animals. Veterinary Sciences, 4(2), 20. https://doi.org/10.3390/vetsci4020020






