Immune Responses of Dendritic Cells to Zoonotic DNA and RNA Viruses
Simple Summary
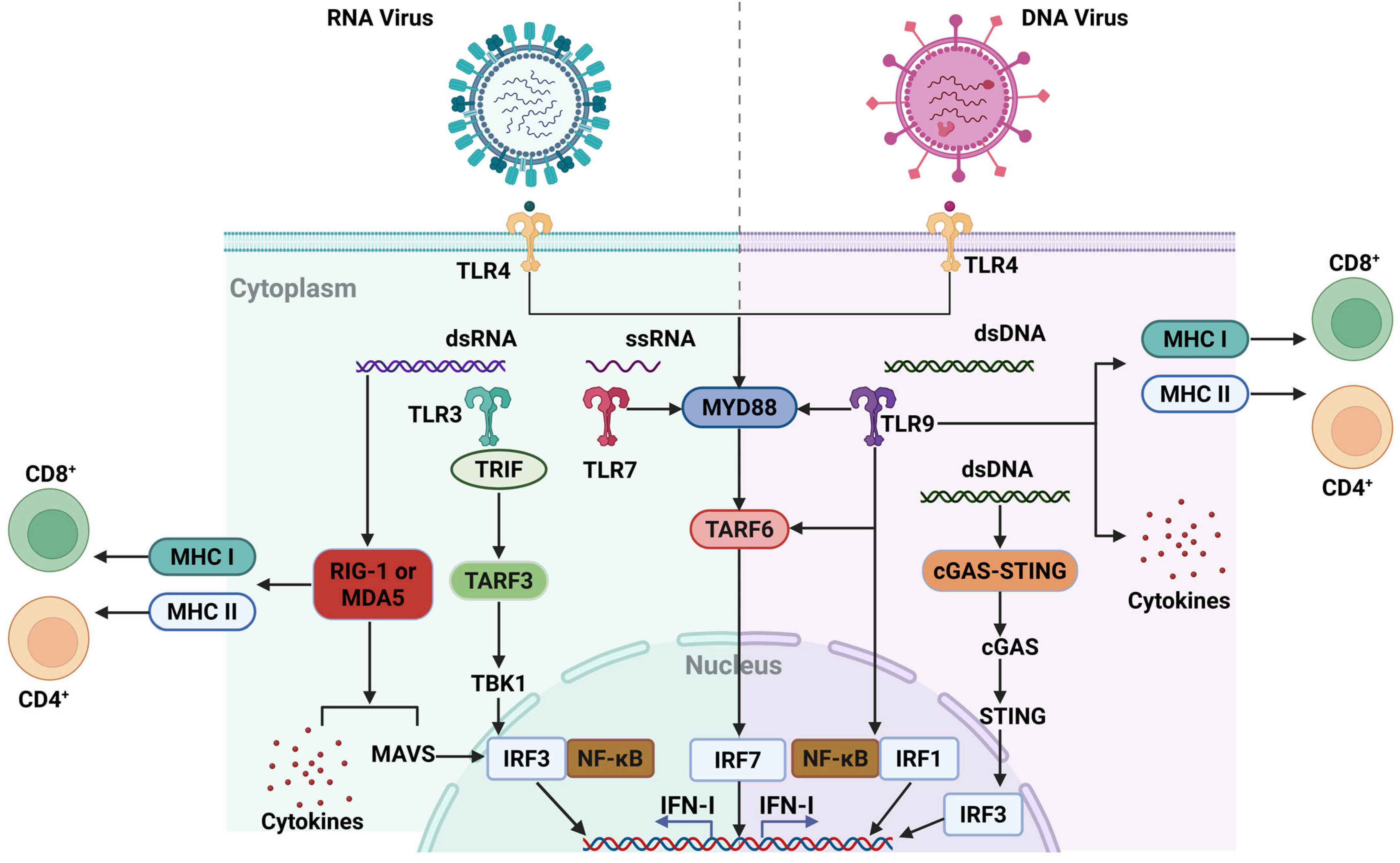
Abstract
1. Introduction
2. Immunological Basis of DCs
| DC Subset | Phenotype in Humans | Phenotype in Mice | Function | Reference |
|---|---|---|---|---|
| cDC1 | Lin-CD64-HLA-DR+CD141+ | Lin-MHC II+CD11c+CD8+/CD103+ | Cross-present soluble or cell-associated antigen; activate CD8+, Th1, and NK cells. | [15,17,18,22] |
| cDC2 | Lin-HLA-DR+CD1c+SIRPα+ | Lin-MHC-II+CD11c+CD11b+SIRPα+/CD103 | Express various PRRs; activate CD4+ T cells. | [19,20,21,74] |
| Inflammatory cDC2 | CD1c+CD5−CD14+/-CD163+ | CD64/MAR-1/Ly6C/CD14 | CD4+ T-cell priming; antigen cross-presentation; IFN-I stimulation. | [23,75,76] |
| Mo-DCs | HLA-DR+CD11c+CD14intCD206+CD1c+ | MHC-II+CD11c+CD11b+CD64intLy6CintCCR2+CD209+ | Produce cytokines to shape differentiation of CD4+ T cell subsets. | [16,77,78] |
| pDCs | HLA-DR+CD11c-CD4+BDCA2+BDCA4+CD123 + | MHC-IIintCD11cintB220+Ly6C+BST2+SiglecH+ | Induce T-cell proliferation after stimulation; produce IFN-I; regulate NK cell, DC, and Mφ survival; expand CD4+ T cells and CD8+ T cells. | [24,79,80,81] |
3. Application of DC Immune Responses for Prevention and Control of Zoonoses
| Type of Virus | Virus | PAMPs | PRRs | Reference |
|---|---|---|---|---|
| DNA viruses | CPXV | HA glycoprotein, A52, B15, K7 | TLR3 | [110,111,112] |
| MPXV | A47, F14L, F3 | TLR4, TLR9, PKR, MDA-5, RIG-I | [113,114,115,116] | |
| RNA viruses | SARS-CoV | S protein | DC-SIGN | [117,118] |
| MERS-CoV | S protein | DPP4 | [119,120] | |
| IAV | HA, NA, NS1, NP, PA-X | TLR3, TLR4, TLR7, RLRs, DC-SIGN, SIGN-R1 | [59,102,105,121,122,123,124,125] |
| Application | Core Strategy | Key Mechanism | Advantage | Challenge |
|---|---|---|---|---|
| RNA virus vaccines | mRNA electroporation | Viral antigen expression → cross-presentation → CD8+ T-cell activation | Direct DC targeting; cellular immunity | In vivo delivery efficiency |
| DNA virus vaccines | DNA sensing activation | Viral DNA detection → DC maturation → T-cell response | Enhanced targeting via engineering | Antigen design complexity |
| Adjuvant systems | TLR agonists/nanoparticles | Boosts DC maturation; stabilizes antigens | Overcomes immune tolerance | Weaker response in humans |
| Mucosal immunization | DC recruiters (CpG/CS-IONzyme) | TED formation → transepithelial antigen capture → mucosal IgA | Enhances barrier immunity; preserves integrity | Significant species differences |
| Immune regulation | tolDC induction | tolDCs suppress inflammation; pDCs modulate tissue damage | Controls chronic disease inflammation | Immunosuppressive microenvironments |
| Combo therapies | DCs + immunomodulators/MSCs | Synergistic effects (e.g., TGF-β + chondrocyte regeneration) | Integrated immune repair | Mechanism complexity |
| Future development | Multi-adjuvant vaccines | Overcoming species gaps; breaking immunosuppression; durable immune memory | Broad-spectrum prevention potential | Requires humanized models |
4. Immune Recognition and Response to DNA Viruses
4.1. Cowpox Virus (CPXV)
4.2. Monkeypox Virus (MPXV)
5. Immune Recognition and Response to RNA Viruses
5.1. Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV)
5.2. Middle East Respiratory Syndrome Coronavirus (MERS-CoV)
5.3. Influenza A Virus (IAV)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, D.; Batra, L.; Malik, M.T. Insights of Novel Coronavirus (SARS-CoV-2) disease outbreak, management and treatment. AIMS Microbiol. 2020, 6, 183–203. [Google Scholar] [CrossRef]
- Morens, D.M.; Fauci, A.S. Emerging Pandemic Diseases: How We Got to COVID-19. Cell 2020, 183, 837. [Google Scholar] [CrossRef]
- García-Sastre, A. Ten Strategies of Interferon Evasion by Viruses. Cell Host Microbe 2017, 22, 176–184. [Google Scholar] [CrossRef]
- Knipe, D.M.; Lieberman, P.M.; Jung, J.U.; McBride, A.A.; Morris, K.V.; Ott, M.; Margolis, D.; Nieto, A.; Nevels, M.; Parks, R.J.; et al. Snapshots: Chromatin control of viral infection. Virology 2013, 435, 141–156. [Google Scholar] [CrossRef]
- Cargnin Faccin, F.; Perez, D.R. Pandemic preparedness through vaccine development for avian influenza viruses. Hum. Vaccines Immunother. 2024, 20, 2347019. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Bourdely, P.; Anselmi, G.; Vaivode, K.; Ramos, R.N.; Missolo-Koussou, Y.; Hidalgo, S.; Tosselo, J.; Nuñez, N.; Richer, W.; Vincent-Salomon, A.; et al. Transcriptional and Functional Analysis of CD1c+ Human Dendritic Cells Identifies a CD163+ Subset Priming CD8+CD103+ T Cells. Immunity 2020, 53, 335–352.e8. [Google Scholar] [CrossRef]
- Briseño, C.G.; Satpathy, A.T.; Davidson, J.T.T.; Ferris, S.T.; Durai, V.; Bagadia, P.; O’Connor, K.W.; Theisen, D.J.; Murphy, T.L.; Murphy, K.M. Notch2-dependent DC2s mediate splenic germinal center responses. Proc. Natl. Acad. Sci. USA 2018, 115, 10726–10731. [Google Scholar] [CrossRef]
- Bachem, A.; Güttler, S.; Hartung, E.; Ebstein, F.; Schaefer, M.; Tannert, A.; Salama, A.; Movassaghi, K.; Opitz, C.; Mages, H.W.; et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J. Exp. Med. 2010, 207, 1273–1281. [Google Scholar] [CrossRef]
- Swiecki, M.; Colonna, M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 2015, 15, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Hale, B.G.; Albrecht, R.A.; García-Sastre, A. Innate immune evasion strategies of influenza viruses. Future Microbiol. 2010, 5, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Paludan, S.R.; Bowie, A.G. Immune sensing of DNA. Immunity 2013, 38, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Edelson, B.T.; Kc, W.; Juang, R.; Kohyama, M.; Benoit, L.A.; Klekotka, P.A.; Moon, C.; Albring, J.C.; Ise, W.; Michael, D.G.; et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J. Exp. Med. 2010, 207, 823–836. [Google Scholar] [CrossRef]
- Schlitzer, A.; McGovern, N.; Ginhoux, F. Dendritic cells and monocyte-derived cells: Two complementary and integrated functional systems. Semin. Cell Dev. Biol. 2015, 41, 9–22. [Google Scholar] [CrossRef]
- Guilliams, M.; Dutertre, C.-A.; Scott, C.L.; McGovern, N.; Sichien, D.; Chakarov, S.; Van Gassen, S.; Chen, J.; Poidinger, M.; De Prijck, S. Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species. Immunity 2016, 45, 669–684. [Google Scholar] [CrossRef]
- Dudziak, D.; Kamphorst, A.O.; Heidkamp, G.F.; Buchholz, V.R.; Trumpfheller, C.; Yamazaki, S.; Cheong, C.; Liu, K.; Lee, H.-W.; Park, C.G. Differential antigen processing by dendritic cell subsets in vivo. Science 2007, 315, 107–111. [Google Scholar] [CrossRef]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013, 31, 563–604. [Google Scholar] [CrossRef]
- Alcántara-Hernández, M.; Leylek, R.; Wagar, L.E.; Engleman, E.G.; Keler, T.; Marinkovich, M.P.; Davis, M.M.; Nolan, G.P.; Idoyaga, J. High-dimensional phenotypic mapping of human dendritic cells reveals interindividual variation and tissue specialization. Immunity 2017, 47, 1037–1050.e6. [Google Scholar] [CrossRef]
- Brown, C.C.; Gudjonson, H.; Pritykin, Y.; Deep, D.; Lavallée, V.-P.; Mendoza, A.; Fromme, R.; Mazutis, L.; Ariyan, C.; Leslie, C. Transcriptional basis of mouse and human dendritic cell heterogeneity. Cell 2019, 179, 846–863.e24. [Google Scholar] [CrossRef] [PubMed]
- Poulin, L.F.; Reyal, Y.; Uronen-Hansson, H.; Schraml, B.U.; Sancho, D.; Murphy, K.M.; Håkansson, U.K.; Ferreira Moita, L.; Agace, W.W.; Bonnet, D. DNGR-1 is a specific and universal marker of mouse and human Batf3-dependent dendritic cells in lymphoid and nonlymphoid tissues. Blood J. Am. Soc. Hematol. 2012, 119, 6052–6062. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Yang, D.; Kim, M.; Haam, K.; Yoo, A.; Choi, J.-H.; Schraml, B.U.; Kim, Y.S.; Kim, D.; Kang, S.-J. Inflammation induces two types of inflammatory dendritic cells in inflamed lymph nodes. Exp. Mol. Med. 2018, 50, e458. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Chen, S.; Eisenbarth, S.C. Dendritic cell regulation of T helper cells. Annu. Rev. Immunol. 2021, 39, 759–790. [Google Scholar] [CrossRef]
- Bevan, M.J. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J. Exp. Med. 1976, 143, 1283–1288. [Google Scholar] [CrossRef]
- Rock, K.L. The ins and outs of cross-presentation. Nat. Immunol. 2003, 4, 941–943. [Google Scholar] [CrossRef]
- Paludan, C.; Schmid, D.; Landthaler, M.; Vockerodt, M.; Kube, D.; Tuschl, T.; Münz, C. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science 2005, 307, 593–596. [Google Scholar] [CrossRef]
- Villani, A.C.; Satija, R.; Reynolds, G.; Sarkizova, S.; Shekhar, K.; Fletcher, J.; Griesbeck, M.; Butler, A.; Zheng, S.; Lazo, S.; et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017, 356, eaah4573. [Google Scholar] [CrossRef]
- Schlitzer, A.; Sivakamasundari, V.; Chen, J.; Sumatoh, H.R.B.; Schreuder, J.; Lum, J.; Malleret, B.; Zhang, S.; Larbi, A.; Zolezzi, F. Identification of cDC1-and cDC2-committed DC progenitors reveals early lineage priming at the common DC progenitor stage in the bone marrow. Nat. Immunol. 2015, 16, 718–728. [Google Scholar] [CrossRef]
- Elahi, Z.; Angel, P.W.; Butcher, S.K.; Rajab, N.; Choi, J.; Deng, Y.; Mintern, J.D.; Radford, K.; Wells, C.A. The Human Dendritic Cell Atlas: An Integrated Transcriptional Tool to Study Human Dendritic Cell Biology. J. Immunol. 2022, 209, 2352–2361. [Google Scholar] [CrossRef]
- Haniffa, M.; Shin, A.; Bigley, V.; McGovern, N.; Teo, P.; See, P.; Wasan, P.S.; Wang, X.N.; Malinarich, F.; Malleret, B.; et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity 2012, 37, 60–73. [Google Scholar] [CrossRef] [PubMed]
- den Haan, J.D.; Lehar, S.; Bevan, M. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 2000, 192, 1685–1696. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Baena, A.; Yu, K.O.; Saini, N.K.; Kharkwal, S.S.; Goldberg, M.F.; Kunnath-Velayudhan, S.; Carreño, L.J.; Venkataswamy, M.M.; Kim, J.; et al. A single subset of dendritic cells controls the cytokine bias of natural killer T cell responses to diverse glycolipid antigens. Immunity 2014, 40, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.L.; Caton, M.L.; Bogunovic, M.; Greter, M.; Grajkowska, L.T.; Ng, D.; Klinakis, A.; Charo, I.F.; Jung, S.; Gommerman, J.L.; et al. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity 2011, 35, 780–791. [Google Scholar] [CrossRef]
- Persson, E.K.; Uronen-Hansson, H.; Semmrich, M.; Rivollier, A.; Hägerbrand, K.; Marsal, J.; Gudjonsson, S.; Håkansson, U.; Reizis, B.; Kotarsky, K.; et al. IRF4 transcription-factor-dependent CD103+CD11b+ dendritic cells drive mucosal T helper 17 cell differentiation. Immunity 2013, 38, 958–969. [Google Scholar] [CrossRef]
- Schlitzer, A.; McGovern, N.; Teo, P.; Zelante, T.; Atarashi, K.; Low, D.; Ho, A.W.; See, P.; Shin, A.; Wasan, P.S.; et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 2013, 38, 970–983. [Google Scholar] [CrossRef]
- Honda, K.; Yanai, H.; Negishi, H.; Asagiri, M.; Sato, M.; Mizutani, T.; Shimada, N.; Ohba, Y.; Takaoka, A.; Yoshida, N.; et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 2005, 434, 772–777. [Google Scholar] [CrossRef]
- Nakano, H.; Yanagita, M.; Gunn, M.D. CD11c+B220+Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J. Exp. Med. 2001, 194, 1171–1178. [Google Scholar] [CrossRef]
- Mori, M.; Yoneyama, M.; Ito, T.; Takahashi, K.; Inagaki, F.; Fujita, T. Identification of Ser-386 of interferon regulatory factor 3 as critical target for inducible phosphorylation that determines activation. J. Biol. Chem. 2004, 279, 9698–9702. [Google Scholar] [CrossRef]
- Piccioli, D.; Tavarini, S.; Borgogni, E.; Steri, V.; Nuti, S.; Sammicheli, C.; Bardelli, M.; Montagna, D.; Locatelli, F.; Wack, A. Functional specialization of human circulating CD16 and CD1c myeloid dendritic-cell subsets. Blood 2007, 109, 5371–5379. [Google Scholar] [CrossRef]
- Guilliams, M.; Scott, C.L. Liver macrophages in health and disease. Immunity 2022, 55, 1515–1529. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Li, Z.; Dress, R.J.; Zhu, Y.; Zhang, S.; De Feo, D.; Kong, W.T.; Cai, P.; Shin, A.; et al. Dendritic cell type 3 arises from Ly6C+ monocyte-dendritic cell progenitors. Immunity 2023, 56, 1761–1777.e6. [Google Scholar] [CrossRef] [PubMed]
- Fromm, P.D.; Silveira, P.A.; Hsu, J.L.; Papadimitrious, M.S.; Lo, T.H.; Ju, X.; Kupresanin, F.; Romano, A.; Hsu, W.H.; Bryant, C.E.; et al. Distinguishing human peripheral blood CD16+ myeloid cells based on phenotypic characteristics. J. Leukoc. Biol. 2020, 107, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Torres, C.; García-Romo, G.S.; Cornejo-Cortés, M.A.; Rivas-Carvalho, A.; Sánchez-Schmitz, G. CD16+ and CD16- human blood monocyte subsets differentiate in vitro to dendritic cells with different abilities to stimulate CD4+ T cells. Int. Immunol. 2001, 13, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Cattin, A.; Wacleche, V.S.; Fonseca Do Rosario, N.; Marchand, L.R.; Dias, J.; Gosselin, A.; Cohen, E.A.; Estaquier, J.; Chomont, N.; Routy, J.P.; et al. RALDH Activity Induced by Bacterial/Fungal Pathogens in CD16+ Monocyte-Derived Dendritic Cells Boosts HIV Infection and Outgrowth in CD4+ T Cells. J. Immunol. 2021, 206, 2638–2651. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Marongiu, L.; Valache, M.; Facchini, F.A.; Granucci, F. How dendritic cells sense and respond to viral infections. Clin. Sci. 2021, 135, 2217–2242. [Google Scholar] [CrossRef]
- Liu, P.; Hong, Y.; Yang, B.; Shrestha, P.; Sajjad, N.; Chen, J.L. Induction of the Antiviral Immune Response and Its Circumvention by Coronaviruses. Viruses 2020, 12, 1039. [Google Scholar] [CrossRef]
- Pishesha, N.; Harmand, T.J.; Ploegh, H.L. A guide to antigen processing and presentation. Nat. Rev. Immunol. 2022, 22, 751–764. [Google Scholar] [CrossRef]
- Blum, J.S.; Wearsch, P.A.; Cresswell, P. Pathways of Antigen Processing. Annu. Rev. Immunol. 2013, 31, 443–473. [Google Scholar] [CrossRef]
- Joffre, O.P.; Segura, E.; Savina, A.; Amigorena, S. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 2012, 12, 557–569. [Google Scholar] [CrossRef]
- Rock, K.L.; Reits, E.; Neefjes, J. Present Yourself! By MHC Class I and MHC Class II Molecules. Trends Immunol. 2016, 37, 724–737. [Google Scholar] [CrossRef]
- Cruz, F.M.; Chan, A.; Rock, K.L. Pathways of MHC I cross-presentation of exogenous antigens. Semin. Immunol. 2023, 66, 101729. [Google Scholar] [CrossRef]
- Li, K.; Ye, Y.; Liu, L.; Sha, Q.; Wang, X.; Jiao, T.; Zhang, L.; Wang, J. The lipid platform increases the activity of STING agonists to synergize checkpoint blockade therapy against melanoma. Biomater. Sci. 2021, 9, 765–773. [Google Scholar] [CrossRef]
- da Silveira, M.P.; da Silva Fagundes, K.K.; Bizuti, M.R.; Starck, É.; Rossi, R.C.; de Resende, E.S.D.T. Physical exercise as a tool to help the immune system against COVID-19: An integrative review of the current literature. Clin. Exp. Med. 2021, 21, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Palacio, N.; Dangi, T.; Chung, Y.R.; Wang, Y.; Loredo-Varela, J.L.; Zhang, Z.; Penaloza-MacMaster, P. Early type I IFN blockade improves the efficacy of viral vaccines. J. Exp. Med. 2020, 217, e20191220. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Greber, U.F. Virus interactions with endocytic pathways in macrophages and dendritic cells. Trends Microbiol. 2013, 21, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, I.; Jeon, D.; Moseman, J.E.; Muralidhar, A.; Potluri, H.K.; McNeel, D.G. Role of B cells as antigen presenting cells. Front. Immunol. 2022, 13, 954936. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Acosta, P.L.; Byrne, A.B.; Hijano, D.R.; Talarico, L.B. Human Type I Interferon Antiviral Effects in Respiratory and Reemerging Viral Infections. J. Immunol. Res. 2020, 2020, 1372494. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Cheng, Y.; Cao, X. Dendritic cell migration in inflammation and immunity. Cell. Mol. Immunol. 2021, 18, 2461–2471. [Google Scholar] [CrossRef]
- Li, A.; Yi, M.; Qin, S.; Song, Y.; Chu, Q.; Wu, K. Activating cGAS-STING pathway for the optimal effect of cancer immunotherapy. J. Hematol. Oncol. 2019, 12, 35. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef]
- Aguirre, S.; Luthra, P.; Sanchez-Aparicio, M.T.; Maestre, A.M.; Patel, J.; Lamothe, F.; Fredericks, A.C.; Tripathi, S.; Zhu, T.; Pintado-Silva, J.; et al. Dengue virus NS2B protein targets cGAS for degradation and prevents mitochondrial DNA sensing during infection. Nat. Microbiol. 2017, 2, 17037. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shi, Y.; Pan, X.; Wu, S.; Hou, R.; Zhang, Y.; Zhong, T.; Tang, H.; Du, W.; Wang, L.; et al. SARS-CoV-2 ORF9b inhibits RIG-I-MAVS antiviral signaling by interrupting K63-linked ubiquitination of NEMO. Cell Rep. 2021, 34, 108761. [Google Scholar] [CrossRef] [PubMed]
- West, A.P.; Khoury-Hanold, W.; Staron, M.; Tal, M.C.; Pineda, C.M.; Lang, S.M.; Bestwick, M.; Duguay, B.A.; Raimundo, N.; MacDuff, D.A.; et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 2015, 520, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Yum, S.; Li, M.; Fang, Y.; Chen, Z.J. TBK1 recruitment to STING activates both IRF3 and NF-κB that mediate immune defense against tumors and viral infections. Proc. Natl. Acad. Sci. USA 2021, 118, e2100225118. [Google Scholar] [CrossRef]
- Domizio, J.D.; Gulen, M.F.; Saidoune, F.; Thacker, V.V.; Yatim, A.; Sharma, K.; Nass, T.; Guenova, E.; Schaller, M.; Conrad, C.; et al. The cGAS-STING pathway drives type I IFN immunopathology in COVID-19. Nature 2022, 603, 145–151. [Google Scholar] [CrossRef]
- Kedzierska, K.; Koutsakos, M. The ABC of Major Histocompatibility Complexes and T Cell Receptors in Health and Disease. Viral Immunol. 2020, 33, 160–178. [Google Scholar] [CrossRef]
- Arasa, J.; Collado-Diaz, V.; Halin, C. Structure and Immune Function of Afferent Lymphatics and Their Mechanistic Contribution to Dendritic Cell and T Cell Trafficking. Cells 2021, 10, 1269. [Google Scholar] [CrossRef]
- Ness, S.; Lin, S.; Gordon, J.R. Regulatory Dendritic Cells, T Cell Tolerance, and Dendritic Cell Therapy for Immunologic Disease. Front. Immunol. 2021, 12, 633436. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Kroemer, G. Hallmarks of T cell aging. Nat. Immunol. 2021, 22, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.L.; Murphy, K.M. Dendritic cells in cancer immunology. Cell. Mol. Immunol. 2022, 19, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Nizzoli, G.; Krietsch, J.; Weick, A.; Steinfelder, S.; Facciotti, F.; Gruarin, P.; Bianco, A.; Steckel, B.; Moro, M.; Crosti, M. Human CD1c+ dendritic cells secrete high levels of IL-12 and potently prime cytotoxic T-cell responses. Blood J. Am. Soc. Hematol. 2013, 122, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Bosteels, C.; Neyt, K.; Vanheerswynghels, M.; van Helden, M.J.; Sichien, D.; Debeuf, N.; De Prijck, S.; Bosteels, V.; Vandamme, N.; Martens, L. Inflammatory type 2 cDCs acquire features of cDC1s and macrophages to orchestrate immunity to respiratory virus infection. Immunity 2020, 52, 1039–1056.e9. [Google Scholar] [CrossRef]
- Backer, R.A.; Probst, H.C.; Clausen, B.E. Classical DC2 subsets and monocyte-derived DC: Delineating the developmental and functional relationship. Eur. J. Immunol. 2023, 53, 2149548. [Google Scholar] [CrossRef]
- Menezes, S.; Melandri, D.; Anselmi, G.; Perchet, T.; Loschko, J.; Dubrot, J.; Patel, R.; Gautier, E.L.; Hugues, S.; Longhi, M.P. The heterogeneity of Ly6Chi monocytes controls their differentiation into iNOS+ macrophages or monocyte-derived dendritic cells. Immunity 2016, 45, 1205–1218. [Google Scholar] [CrossRef]
- Segura, E.; Touzot, M.; Bohineust, A.; Cappuccio, A.; Chiocchia, G.; Hosmalin, A.; Dalod, M.; Soumelis, V.; Amigorena, S. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 2013, 38, 336–348. [Google Scholar] [CrossRef]
- Colonna, M.; Trinchieri, G.; Liu, Y.-J. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 2004, 5, 1219–1226. [Google Scholar] [CrossRef]
- Liu, Y.-J. IPC: Professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005, 23, 275–306. [Google Scholar] [CrossRef]
- Cervantes-Barragan, L.; Lewis, K.L.; Firner, S.; Thiel, V.; Hugues, S.; Reith, W.; Ludewig, B.; Reizis, B. Plasmacytoid dendritic cells control T-cell response to chronic viral infection. Proc. Natl. Acad. Sci. USA 2012, 109, 3012–3017. [Google Scholar] [CrossRef]
- Bai, L.; Li, W.; Zheng, W.; Xu, D.; Chen, N.; Cui, J. Promising targets based on pattern recognition receptors for cancer immunotherapy. Pharmacol. Res. 2020, 159, 105017. [Google Scholar] [CrossRef] [PubMed]
- Ahmed-Hassan, H.; Sisson, B.; Shukla, R.K.; Wijewantha, Y.; Funderburg, N.T.; Li, Z.; Hayes, D., Jr.; Demberg, T.; Liyanage, N.P.M. Innate Immune Responses to Highly Pathogenic Coronaviruses and Other Significant Respiratory Viral Infections. Front. Immunol. 2020, 11, 1979. [Google Scholar] [CrossRef] [PubMed]
- Willemen, Y.; Versteven, M.; Peeters, M.; Berneman, Z.N.; Smits, E.L.J. Ribonucleic Acid Engineering of Dendritic Cells for Therapeutic Vaccination: Ready ‘N Able to Improve Clinical Outcome? Cancers 2020, 12, 299. [Google Scholar] [CrossRef]
- Ohara, R.A.; Murphy, K.M. The evolving biology of cross-presentation. Semin. Immunol. 2023, 66, 101711. [Google Scholar] [CrossRef]
- Fernández-Delgado, I.; Calzada-Fraile, D.; Sánchez-Madrid, F. Immune Regulation by Dendritic Cell Extracellular Vesicles in Cancer Immunotherapy and Vaccines. Cancers 2020, 12, 3558. [Google Scholar] [CrossRef] [PubMed]
- Pittet, M.J.; Di Pilato, M.; Garris, C.; Mempel, T.R. Dendritic cells as shepherds of T cell immunity in cancer. Immunity 2023, 56, 2218–2230. [Google Scholar] [CrossRef]
- Shemesh, C.S.; Hsu, J.C.; Hosseini, I.; Shen, B.Q.; Rotte, A.; Twomey, P.; Girish, S.; Wu, B. Personalized Cancer Vaccines: Clinical Landscape, Challenges, and Opportunities. Mol. Ther. 2021, 29, 555–570. [Google Scholar] [CrossRef]
- Roßmann, L.; Bagola, K.; Stephen, T.; Gerards, A.L.; Walber, B.; Ullrich, A.; Schülke, S.; Kamp, C.; Spreitzer, I.; Hasan, M.; et al. Distinct single-component adjuvants steer human DC-mediated T-cell polarization via Toll-like receptor signaling toward a potent antiviral immune response. Proc. Natl. Acad. Sci. USA 2021, 118, e2103651118. [Google Scholar] [CrossRef]
- Fu, C.; Zhou, L.; Mi, Q.S.; Jiang, A. DC-Based Vaccines for Cancer Immunotherapy. Vaccines 2020, 8, 706. [Google Scholar] [CrossRef]
- Laureano, R.S.; Sprooten, J.; Vanmeerbeerk, I.; Borras, D.M.; Govaerts, J.; Naulaerts, S.; Berneman, Z.N.; Beuselinck, B.; Bol, K.F.; Borst, J.; et al. Trial watch: Dendritic cell (DC)-based immunotherapy for cancer. Oncoimmunology 2022, 11, 2096363. [Google Scholar] [CrossRef]
- Rafieenia, F.; Nikkhah, E.; Nourmohammadi, F.; Hosseini, S.; Abdollahi, A.; Sharifi, N.; Aliakbarian, M.; Forghani Fard, M.M.; Gholamin, M.; Abbaszadegan, M.R. Allogeneic tumor cell line-based vaccines: A good alternative to autologous and cancer stem cell vaccines in colorectal cancer. Iran. J. Basic Med. Sci. 2021, 24, 1231–1239. [Google Scholar] [PubMed]
- Morante-Palacios, O.; Fondelli, F.; Ballestar, E.; Martínez-Cáceres, E.M. Tolerogenic Dendritic Cells in Autoimmunity and Inflammatory Diseases. Trends Immunol. 2021, 42, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Alahdal, M.; Zhang, H.; Huang, R.; Sun, W.; Deng, Z.; Duan, L.; Ouyang, H.; Wang, D. Potential efficacy of dendritic cell immunomodulation in the treatment of osteoarthritis. Rheumatology 2021, 60, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Cao, J.; Li, B.; Nice, E.C.; Mao, H.; Zhang, Y.; Huang, C. Managing the immune microenvironment of osteosarcoma: The outlook for osteosarcoma treatment. Bone Res. 2023, 11, 11. [Google Scholar] [CrossRef]
- Pastor, Y.; Ghazzaui, N.; Hammoudi, A.; Centlivre, M.; Cardinaud, S.; Levy, Y. Refining the DC-targeting vaccination for preventing emerging infectious diseases. Front. Immunol. 2022, 13, 949779. [Google Scholar] [CrossRef]
- Suuring, M.; Moreau, A. Regulatory Macrophages and Tolerogenic Dendritic Cells in Myeloid Regulatory Cell-Based Therapies. Int. J. Mol. Sci. 2021, 22, 7970. [Google Scholar] [CrossRef]
- Abbasi-Kenarsari, H.; Heidari, N.; Baghaei, K.; Amani, D.; Zali, M.R.; Gaffari Khaligh, S.; Shafiee, A.; Hashemi, S.M. Synergistic therapeutic effect of mesenchymal stem cells and tolerogenic dendritic cells in an acute colitis mouse model. Int. Immunopharmacol. 2020, 88, 107006. [Google Scholar] [CrossRef]
- Qin, T.; Ma, S.; Miao, X.; Tang, Y.; Huangfu, D.; Wang, J.; Jiang, J.; Xu, N.; Yin, Y.; Chen, S.; et al. Mucosal Vaccination for Influenza Protection Enhanced by Catalytic Immune-Adjuvant. Adv. Sci. 2020, 7, 2000771. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, J.; Gao, L. Catalytic antimicrobial therapy using nanozymes. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1769. [Google Scholar] [CrossRef]
- Yin, Y.; Qin, T.; Wang, X.; Lin, J.; Yu, Q.; Yang, Q. CpG DNA assists the whole inactivated H9N2 influenza virus in crossing the intestinal epithelial barriers via transepithelial uptake of dendritic cell dendrites. Mucosal Immunol. 2015, 8, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Yin, Y.; Yu, Q.; Huang, L.; Wang, X.; Lin, J.; Yang, Q. CpG Oligodeoxynucleotides Facilitate Delivery of Whole Inactivated H9N2 Influenza Virus via Transepithelial Dendrites of Dendritic Cells in Nasal Mucosa. J. Virol. 2015, 89, 5904–5918. [Google Scholar] [CrossRef]
- Fitzgerald-Bocarsly, P.; Feng, D. The role of type I interferon production by dendritic cells in host defense. Biochimie 2007, 89, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Taks, E.J.M.; Moorlag, S.; Netea, M.G.; van der Meer, J.W.M. Shifting the Immune Memory Paradigm: Trained Immunity in Viral Infections. Annu. Rev. Virol. 2022, 9, 469–489. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Miao, X.; Huangfu, D.; Zhao, X.; Zhang, M.; Qin, T.; Peng, D.; Liu, X. H5N1 avian influenza virus without 80-84 amino acid deletion at the NS1 protein hijacks the innate immune system of dendritic cells for an enhanced mammalian pathogenicity. Transbound. Emerg. Dis. 2021, 68, 2401–2413. [Google Scholar] [CrossRef]
- Hansen, S.J.; Rushton, J.; Dekonenko, A.; Chand, H.S.; Olson, G.K.; Hutt, J.A.; Pickup, D.; Lyons, C.R.; Lipscomb, M.F. Cowpox virus inhibits human dendritic cell immune function by nonlethal, nonproductive infection. Virology 2011, 412, 411–425. [Google Scholar] [CrossRef]
- Wang, J.X.; Choi, S.Y.C.; Niu, X.; Kang, N.; Xue, H.; Killam, J.; Wang, Y. Lactic Acid and an Acidic Tumor Microenvironment suppress Anticancer Immunity. Int. J. Mol. Sci. 2020, 21, 8363. [Google Scholar] [CrossRef]
- Osterlund, P.; Pirhonen, J.; Ikonen, N.; Rönkkö, E.; Strengell, M.; Mäkelä, S.M.; Broman, M.; Hamming, O.J.; Hartmann, R.; Ziegler, T.; et al. Pandemic H1N1 2009 influenza A virus induces weak cytokine responses in human macrophages and dendritic cells and is highly sensitive to the antiviral actions of interferons. J. Virol. 2010, 84, 1414–1422. [Google Scholar] [CrossRef]
- Martín-Moreno, A.; Muñoz-Fernández, M.A. Dendritic Cells, the Double Agent in the War Against HIV-1. Front. Immunol. 2019, 10, 2485. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Zhao, Y.; Ma, X.; Yi, H. Toll-like receptor 3 (TLR3) regulation mechanisms and roles in antiviral innate immune responses. J. Zhejiang Univ. Sci. B 2021, 22, 609–632. [Google Scholar] [CrossRef]
- Mancini, M.; Vidal, S.M. Mechanisms of Natural Killer Cell Evasion Through Viral Adaptation. Annu. Rev. Immunol. 2020, 38, 511–539. [Google Scholar] [CrossRef]
- Torres, A.A.; Albarnaz, J.D.; Bonjardim, C.A.; Smith, G.L. Multiple Bcl-2 family immunomodulators from vaccinia virus regulate MAPK/AP-1 activation. J. Gen. Virol. 2016, 97, 2346–2351. [Google Scholar] [CrossRef]
- Parnian, R.; Heydarifard, F.; Mousavi, F.S.; Heydarifard, Z.; Zandi, M. Innate immune response to monkeypox virus infection: Mechanisms and immune escape. J. Innate Immun. 2024, 16, 413–424. [Google Scholar] [PubMed]
- Arndt, W.D.; Cotsmire, S.; Trainor, K.; Harrington, H.; Hauns, K.; Kibler, K.V.; Huynh, T.P.; Jacobs, B.L. Evasion of the innate immune type I interferon system by monkeypox virus. J. Virol. 2015, 89, 10489–10499. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Liu, Y.; Dou, D.; Su, B. The unique immune evasion mechanisms of the mpox virus and their implication for developing new vaccines and immunotherapies. Virol. Sin. 2024, 39, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.; Arunagiri, T.; Mani, S.; Kumaran, V.R.; Kannaiah, K.P.; Chanduluru, H.K. From pox to protection: Understanding Monkeypox pathophysiology and immune resilience. Trop. Med. Health 2025, 53, 33. [Google Scholar] [CrossRef]
- Chang, T.; Yang, J.; Deng, H.; Chen, D.; Yang, X.; Tang, Z.H. Depletion and Dysfunction of Dendritic Cells: Understanding SARS-CoV-2 Infection. Front. Immunol. 2022, 13, 843342. [Google Scholar] [CrossRef]
- Yang, D.; Chu, H.; Hou, Y.; Chai, Y.; Shuai, H.; Lee, A.C.; Zhang, X.; Wang, Y.; Hu, B.; Huang, X.; et al. Attenuated Interferon and Proinflammatory Response in SARS-CoV-2-Infected Human Dendritic Cells Is Associated With Viral Antagonism of STAT1 Phosphorylation. J. Infect. Dis. 2020, 222, 734–745. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, M.L.; Chien, C.S.; Yarmishyn, A.A.; Yang, Y.P.; Lai, W.Y.; Luo, Y.H.; Lin, Y.T.; Chen, Y.J.; Chang, P.C.; et al. Highlight of Immune Pathogenic Response and Hematopathologic Effect in SARS-CoV, MERS-CoV, and SARS-Cov-2 Infection. Front. Immunol. 2020, 11, 1022. [Google Scholar] [CrossRef]
- Zhou, J.; Chu, H.; Li, C.; Wong, B.H.; Cheng, Z.S.; Poon, V.K.; Sun, T.; Lau, C.C.; Wong, K.K.; Chan, J.Y.; et al. Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: Implications for pathogenesis. J. Infect. Dis. 2014, 209, 1331–1342. [Google Scholar] [CrossRef]
- Fu, C.; Ma, T.; Zhou, L.; Mi, Q.S.; Jiang, A. Dendritic Cell-Based Vaccines Against Cancer: Challenges, Advances and Future Opportunities. Immunol. Investig. 2022, 51, 2133–2158. [Google Scholar] [CrossRef]
- Goff, P.H.; Hayashi, T.; Martínez-Gil, L.; Corr, M.; Crain, B.; Yao, S.; Cottam, H.B.; Chan, M.; Ramos, I.; Eggink, D.; et al. Synthetic Toll-like receptor 4 (TLR4) and TLR7 ligands as influenza virus vaccine adjuvants induce rapid, sustained, and broadly protective responses. J. Virol. 2015, 89, 3221–3235. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, H.; Sun, Y.; Chen, F. Antiviral role of Toll-like receptors and cytokines against the new 2009 H1N1 virus infection. Mol. Biol. Rep. 2012, 39, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Chen, Y.; Huangfu, D.; Yin, Y.; Miao, X.; Yin, Y.; Chen, S.; Peng, D.; Liu, X. PA-X protein assists H9N2 subtype avian influenza virus in escaping immune response of mucosal dendritic cells. Transbound. Emerg. Dis. 2022, 69, e3088–e3100. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Chen, Y.; Huangfu, D.; Yin, Y.; Miao, X.; Yin, Y.; Chen, S.; Peng, D.; Liu, X. PA-X protein of H1N1 subtype influenza virus disables the nasal mucosal dendritic cells for strengthening virulence. Virulence 2022, 13, 1928–1942. [Google Scholar] [CrossRef]
- Kervevan, J.; Chakrabarti, L.A. Role of CD4+ T Cells in the Control of Viral Infections: Recent Advances and Open Questions. Int. J. Mol. Sci. 2021, 22, 523. [Google Scholar] [CrossRef]
- Galati, D.; Zanotta, S.; Bocchino, M.; De Filippi, R.; Pinto, A. The subtle interplay between gamma delta T lymphocytes and dendritic cells: Is there a role for a therapeutic cancer vaccine in the era of combinatorial strategies? Cancer Immunol. Immunother. 2021, 70, 1797–1809. [Google Scholar] [CrossRef]
- Mirlekar, B.; Pylayeva-Gupta, Y. IL-12 Family Cytokines in Cancer and Immunotherapy. Cancers 2021, 13, 167. [Google Scholar] [CrossRef]
- Elgebaly, S.A.; Todd, R.; Kreutzer, D.L.; Christenson, R.; El-Khazragy, N.; Arafa, R.K.; Rabie, M.A.; Mohamed, A.F.; Ahmed, L.A.; El Sayed, N.S. Nourin-Associated miRNAs: Novel Inflammatory Monitoring Markers for Cyclocreatine Phosphate Therapy in Heart Failure. Int. J. Mol. Sci. 2021, 22, 3575. [Google Scholar] [CrossRef]
- Prasit, K.K. Harnessing iNKT Cells to Improve In Situ Vaccination for Cancer Therapy. Ph.D. Thesis, Victoria University of Wellington, Wellington, New Zealand, 2020. [Google Scholar]
- Lawler, C.; Brady, G. Poxviral Targeting of Interferon Regulatory Factor Activation. Viruses 2020, 12, 1191. [Google Scholar] [CrossRef]
- Oda, S.; Schröder, M.; Khan, A.R. Structural basis for targeting of human RNA helicase DDX3 by poxvirus protein K7. Structure 2009, 17, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Cánova, D.; Moens, U.L.; Brinkmann, A.; Nitsche, A.; Okeke, M.I. Genomic Sequencing and Analysis of a Novel Human Cowpox Virus With Mosaic Sequences From North America and Old World Orthopoxvirus. Front. Microbiol. 2022, 13, 868887. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan Rajsri, K.; Rao, M. Poxvirus-driven human diseases and emerging therapeutics. Ther. Adv. Infect. Dis. 2022, 9, 20499361221136751. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.A.; Gálvez, N.M.S.; Andrade, C.A.; Pacheco, G.A.; Bohmwald, K.; Berrios, R.V.; Bueno, S.M.; Kalergis, A.M. The Role of Dendritic Cells During Infections Caused by Highly Prevalent Viruses. Front. Immunol. 2020, 11, 1513. [Google Scholar] [CrossRef]
- Alzhanova, D.; Früh, K. Modulation of the host immune response by cowpox virus. Microbes Infect. 2010, 12, 900–909. [Google Scholar] [CrossRef]
- Lin, L.C.W.; Croft, S.N.; Croft, N.P.; Wong, Y.C.; Smith, S.A.; Tang, S.S.; Purcell, A.W.; Tscharke, D.C. Direct Priming of CD8+ T Cells Persists in the Face of Cowpox Virus Inhibitors of Antigen Presentation. J. Virol. 2021, 95, 10. [Google Scholar] [CrossRef]
- Hsu, J.; Kim, S.; Anandasabapathy, N. Vaccinia Virus: Mechanisms Supporting Immune Evasion and Successful Long-Term Protective Immunity. Viruses 2024, 16, 870. [Google Scholar] [CrossRef]
- Perdiguero, B.; Esteban, M. The interferon system and vaccinia virus evasion mechanisms. J. Interferon Cytokine Res. 2009, 29, 581–598. [Google Scholar] [CrossRef]
- Saghazadeh, A.; Rezaei, N. Poxviruses and the immune system: Implications for monkeypox virus. Int. Immunopharmacol. 2022, 113 Pt A, 109364. [Google Scholar] [CrossRef]
- McCollum, A.M.; Damon, I.K. Human monkeypox. Clin. Infect. Dis. 2014, 58, 260–267. [Google Scholar] [CrossRef]
- Abebaw, D.; Akelew, Y.; Adugna, A.; Teffera, Z.H.; Tegegne, B.A.; Fenta, A.; Amare, G.A.; Jemal, M.; Baylie, T.; Atnaf, A. Antigen recognition and immune response to monkeypox virus infection: Implications for Mpox vaccine design–a narrative review. Infez. Med. 2025, 33, 151–162. [Google Scholar] [PubMed]
- Zaucha, G.M.; Jahrling, P.B.; Geisbert, T.W.; Swearengen, J.R.; Hensley, L. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis). Lab. Investig. 2001, 81, 1581–1600. [Google Scholar] [CrossRef] [PubMed]
- Lum, F.-M.; Torres-Ruesta, A.; Tay, M.Z.; Lin, R.T.; Lye, D.C.; Rénia, L.; Ng, L.F. Monkeypox: Disease epidemiology, host immunity and clinical interventions. Nat. Rev. Immunol. 2022, 22, 597–613. [Google Scholar] [CrossRef]
- Arndt, W.D.; White, S.D.; Johnson, B.P.; Huynh, T.; Liao, J.; Harrington, H.; Cotsmire, S.; Kibler, K.V.; Langland, J.; Jacobs, B.L. Monkeypox virus induces the synthesis of less dsRNA than vaccinia virus, and is more resistant to the anti-poxvirus drug, IBT, than vaccinia virus. Virology 2016, 497, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.; Ma, J.; Zhao, Y.; Jia, X.; Zhang, H.; Fan, W.; Jia, X.; Bai, X.; Zhao, Y.; Lu, Y.; et al. The nuclear localization signal of monkeypox virus protein P2 orthologue is critical for inhibition of IRF3-mediated innate immunity. Emerg. Microbes Infect. 2024, 13, 2372344. [Google Scholar] [CrossRef]
- Alakunle, E.; Kolawole, D.; Diaz-Canova, D.; Alele, F.; Adegboye, O.; Moens, U.; Okeke, M.I. A comprehensive review of monkeypox virus and mpox characteristics. Front. Cell. Infect. Microbiol. 2024, 14, 1360586. [Google Scholar] [CrossRef]
- Hammarlund, E.; Dasgupta, A.; Pinilla, C.; Norori, P.; Früh, K.; Slifka, M.K. Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation. Proc. Natl. Acad. Sci. USA 2008, 105, 14567–14572. [Google Scholar] [CrossRef]
- Chen, J.L.; Wang, B.; Lu, Y.; Antoun, E.; Bird, O.; Drennan, P.G.; Yin, Z.; Liu, G.; Yao, X.; Pidoux, M.; et al. T cell memory response to MPXV infection exhibits greater effector function and migratory potential compared to MVA-BN vaccination. Nat. Commun. 2025, 16, 4362. [Google Scholar] [CrossRef]
- Motwani, M.; Pesiridis, S.; Fitzgerald, K.A. DNA sensing by the cGAS–STING pathway in health and disease. Nat. Rev. Genet. 2019, 20, 657–674. [Google Scholar] [CrossRef]
- Albarnaz, J.D.; Ren, H.; Torres, A.A.; Shmeleva, E.V.; Melo, C.A.; Bannister, A.J.; Brember, M.P.; Chung, B.Y.; Smith, G.L. Molecular mimicry of NF-κB by vaccinia virus protein enables selective inhibition of antiviral responses. Nat. Microbiol. 2022, 7, 154–168. [Google Scholar] [CrossRef]
- Minkoff, J.M.; tenOever, B. Innate immune evasion strategies of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 178–194. [Google Scholar] [CrossRef]
- Choudhury, S.M.; Ma, X.; Abdullah, S.W.; Zheng, H. Activation and Inhibition of the NLRP3 Inflammasome by RNA Viruses. J. Inflamm. Res. 2021, 14, 1145–1163. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Cervantes, J.; Ramírez-Jarquín, J.O.; Vaca, L. Interaction Between Virus-Like Particles (VLPs) and Pattern Recognition Receptors (PRRs) From Dendritic Cells (DCs): Toward Better Engineering of VLPs. Front. Immunol. 2020, 11, 1100. [Google Scholar] [CrossRef] [PubMed]
- Onomoto, K.; Onoguchi, K.; Yoneyama, M. Regulation of RIG-I-like receptor-mediated signaling: Interaction between host and viral factors. Cell. Mol. Immunol. 2021, 18, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Rehwinkel, J.; Gack, M.U. RIG-I-like receptors: Their regulation and roles in RNA sensing. Nat. Rev. Immunol. 2020, 20, 537–551. [Google Scholar] [CrossRef]
- Rojas, J.M.; Alejo, A.; Martín, V.; Sevilla, N. Viral pathogen-induced mechanisms to antagonize mammalian interferon (IFN) signaling pathway. Cell. Mol. Life Sci. 2021, 78, 1423–1444. [Google Scholar] [CrossRef]
- Del Prete, A.; Salvi, V.; Soriani, A.; Laffranchi, M.; Sozio, F.; Bosisio, D.; Sozzani, S. Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cell. Mol. Immunol. 2023, 20, 432–447. [Google Scholar] [CrossRef]
- An, T.Q.; Li, J.N.; Su, C.M.; Yoo, D. Molecular and Cellular Mechanisms for PRRSV Pathogenesis and Host Response to Infection. Virus Res. 2020, 286, 197980. [Google Scholar] [CrossRef]
- Girkin, J.L.N.; Maltby, S.; Bartlett, N.W. Toll-like receptor-agonist-based therapies for respiratory viral diseases: Thinking outside the cell. Eur. Respir. Rev. 2022, 31, 210274. [Google Scholar] [CrossRef]
- Ye, Y.; Gaugler, B.; Mohty, M.; Malard, F. Plasmacytoid dendritic cell biology and its role in immune-mediated diseases. Clin. Transl. Immunol. 2020, 9, e1139. [Google Scholar] [CrossRef]
- Zhang, E.; Fang, M.; Jones, C.; Minze, L.J.; Xing, J.; Zhang, Z. Mechanisms involved in controlling RNA virus-induced intestinal inflammation. Cell. Mol. Life Sci. 2022, 79, 313. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.-C. COVID-19, SARS and Bats Coronavirus Esgenome Speculiar Homologousrna Sequences. Int. J. Res.-GRANTHAALAYAH 2020, 8, 217–263. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, J.; Zhao, S.; Castro, M.F.G.; Laurent-Rolle, M.; Dong, J.; Ran, X.; Damani-Yokota, P.; Tang, H.; Karakousi, T. SARS-CoV-2 exacerbates proinflammatory responses in myeloid cells through C-type lectin receptors and Tweety family member 2. Immunity 2021, 54, 1304–1319.e9. [Google Scholar] [CrossRef] [PubMed]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef]
- Wang, K.; Chen, W.; Zhang, Z.; Deng, Y.; Lian, J.-Q.; Du, P.; Wei, D.; Zhang, Y.; Sun, X.-X.; Gong, L. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target. Ther. 2020, 5, 283. [Google Scholar] [CrossRef]
- Woodhead, V.E.; Binks, M.H.; Chain, B.M.; Katz, D.R. From sentinel to messenger: An extended phenotypic analysis of the monocyte to dendritic cell transition. Immunology 1998, 94, 552–559. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, Y.; Li, K.; Meyerholz, D.K.; Allamargot, C.; Perlman, S. Severe Acute Respiratory Syndrome Coronavirus 2-Induced Immune Activation and Death of Monocyte-Derived Human Macrophages and Dendritic Cells. J. Infect. Dis. 2021, 223, 785–795. [Google Scholar] [CrossRef]
- Kamitani, W.; Huang, C.; Narayanan, K.; Lokugamage, K.G.; Makino, S. A two-pronged strategy to suppress host protein synthesis by SARS coronavirus Nsp1 protein. Nat. Struct. Mol. Biol. 2009, 16, 1134–1140. [Google Scholar] [CrossRef]
- Singh, M.; Bansal, V.; Feschotte, C. A Single-Cell RNA Expression Map of Human Coronavirus Entry Factors. Cell Rep. 2020, 32, 108175. [Google Scholar] [CrossRef]
- Frieman, M.; Yount, B.; Heise, M.; Kopecky-Bromberg, S.A.; Palese, P.; Baric, R.S. Severe acute respiratory syndrome coronavirus ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/Golgi membrane. J. Virol. 2007, 81, 9812–9824. [Google Scholar] [CrossRef]
- Oggenfuss, U.; Croll, D. Recent transposable element bursts are associated with the proximity to genes in a fungal plant pathogen. PLoS Pathog. 2023, 19, e1011130. [Google Scholar] [CrossRef]
- Borges, R.C.; Hohmann, M.S.; Borghi, S.M. Dendritic cells in COVID-19 immunopathogenesis: Insights for a possible role in determining disease outcome. Int. Rev. Immunol. 2021, 40, 108–125. [Google Scholar] [CrossRef] [PubMed]
- Alamri, A.; Fisk, D.; Upreti, D.; Kung, S.K.P. A Missing Link: Engagements of Dendritic Cells in the Pathogenesis of SARS-CoV-2 Infections. Int. J. Mol. Sci. 2021, 22, 1118. [Google Scholar] [CrossRef] [PubMed]
- Jonny, J.; Putranto, T.A.; Irfon, R.; Sitepu, E.C. Developing dendritic cell for SARS-CoV-2 vaccine: Breakthrough in the pandemic. Front. Immunol. 2022, 13, 989685. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Zuo, J.; Dowell, A.C.; Pearce, H.; Verma, K.; Long, H.M.; Begum, J.; Aiano, F.; Amin-Chowdhury, Z.; Hoschler, K.; Brooks, T.; et al. Robust SARS-CoV-2-specific T cell immunity is maintained at 6 months following primary infection. Nat. Immunol. 2021, 22, 620–626. [Google Scholar] [CrossRef]
- Zhou, R.; To, K.K.; Wong, Y.C.; Liu, L.; Zhou, B.; Li, X.; Huang, H.; Mo, Y.; Luk, T.Y.; Lau, T.T.; et al. Acute SARS-CoV-2 Infection Impairs Dendritic Cell and T Cell Responses. Immunity 2020, 53, 864–877.e5. [Google Scholar] [CrossRef]
- Farrag, M.A.; Amer, H.M.; Bhat, R.; Hamed, M.E.; Aziz, I.M.; Mubarak, A.; Dawoud, T.M.; Almalki, S.G.; Alghofaili, F.; Alnemare, A.K.; et al. SARS-CoV-2: An Overview of Virus Genetics, Transmission, and Immunopathogenesis. Int. J. Environ. Res. Public Health 2021, 18, 6312. [Google Scholar] [CrossRef]
- Hemmat, N.; Asadzadeh, Z.; Ahangar, N.K.; Alemohammad, H.; Najafzadeh, B.; Derakhshani, A.; Baghbanzadeh, A.; Baghi, H.B.; Javadrashid, D.; Najafi, S.; et al. The roles of signaling pathways in SARS-CoV-2 infection; lessons learned from SARS-CoV and MERS-CoV. Arch. Virol. 2021, 166, 675–696. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Li, B.R.; Ning, B.T. The Comparative Immunological Characteristics of SARS-CoV, MERS-CoV, and SARS-CoV-2 Coronavirus Infections. Front. Immunol. 2020, 11, 2033. [Google Scholar] [CrossRef]
- Widagdo, W.; Sooksawasdi Na Ayudhya, S.; Hundie, G.B.; Haagmans, B.L. Host Determinants of MERS-CoV Transmission and Pathogenesis. Viruses 2019, 11, 280. [Google Scholar] [CrossRef]
- Chen, B.; Tian, E.K.; He, B.; Tian, L.; Han, R.; Wang, S.; Xiang, Q.; Zhang, S.; El Arnaout, T.; Cheng, W. Overview of lethal human coronaviruses. Signal Transduct. Target. Ther. 2020, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Sa Ribero, M.; Jouvenet, N.; Dreux, M.; Nisole, S. Interplay between SARS-CoV-2 and the type I interferon response. PLoS Pathog. 2020, 16, e1008737. [Google Scholar] [CrossRef] [PubMed]
- Bencze, D.; Fekete, T.; Pázmándi, K. Type I Interferon Production of Plasmacytoid Dendritic Cells under Control. Int. J. Mol. Sci. 2021, 22, 4190. [Google Scholar] [CrossRef]
- Desmares, M. Insights on the Mode of Actions of TLR2 Agonists Against HBV Replication Etude des Mécanismes D’action de Ligands de TLR2 Contre la Réplication du VHB. Ph.D. Thesis, Université de Lyon, Lyon, France, 2021. [Google Scholar]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef]
- Cifuentes-Rius, A.; Desai, A.; Yuen, D.; Johnston, A.P.R.; Voelcker, N.H. Inducing immune tolerance with dendritic cell-targeting nanomedicines. Nat. Nanotechnol. 2021, 16, 37–46. [Google Scholar] [CrossRef]
- Praena, B.; Wan, X.F. Influenza Virus Infections in Polarized Cells. Viruses 2022, 14, 1307. [Google Scholar] [CrossRef]
- Scheibner, D.; Salaheldin, A.H.; Bagato, O.; Zaeck, L.M.; Mostafa, A.; Blohm, U.; Müller, C.; Eweas, A.F.; Franzke, K.; Karger, A.; et al. Phenotypic effects of mutations observed in the neuraminidase of human origin H5N1 influenza A viruses. PLoS Pathog. 2023, 19, e1011135. [Google Scholar] [CrossRef]
- Neumann, G.; Chen, H.; Gao, G.F.; Shu, Y.; Kawaoka, Y. H5N1 influenza viruses: Outbreaks and biological properties. Cell Res. 2010, 20, 51–61. [Google Scholar] [CrossRef]
- Bhat, S.; James, J.; Sadeyen, J.R.; Mahmood, S.; Everest, H.J.; Chang, P.; Walsh, S.K.; Byrne, A.M.P.; Mollett, B.; Lean, F.; et al. Coinfection of Chickens with H9N2 and H7N9 Avian Influenza Viruses Leads to Emergence of Reassortant H9N9 Virus with Increased Fitness for Poultry and a Zoonotic Potential. J. Virol. 2022, 96, e0185621. [Google Scholar] [CrossRef]
- Frank, K.; Paust, S. Dynamic Natural Killer Cell and T Cell Responses to Influenza Infection. Front. Cell. Infect. Microbiol. 2020, 10, 425. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sesma, A. The influenza virus NS1 protein: Inhibitor of innate and adaptive immunity. Infect. Disord. Drug Targets 2007, 7, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Sun, C.; Ge, C.; Wang, Z.; Zhang, T.; Zhang, M.; Wang, W.; Tian, Y.; He, Y.; Yang, G.; et al. Chicken dendritic cell-targeting nanobodies mediated improved protective effects against H9N2 influenza virus challenge in a homologous sequential immunization study. Vet. Microbiol. 2023, 285, 109875. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, J.H.; Min, J.Y. A naturally truncated NS1 protein of influenza A virus impairs its interferon-antagonizing activity and thereby confers attenuation in vitro. Arch. Virol. 2017, 162, 13–21. [Google Scholar] [CrossRef]
- Mifsud, E.J.; Kuba, M.; Barr, I.G. Innate Immune Responses to Influenza Virus Infections in the Upper Respiratory Tract. Viruses 2021, 13, 2090. [Google Scholar] [CrossRef]
- Meischel, T.; Villalon-Letelier, F.; Saunders, P.M.; Reading, P.C.; Londrigan, S.L. Influenza A virus interactions with macrophages: Lessons from epithelial cells. Cell. Microbiol. 2020, 22, e13170. [Google Scholar] [CrossRef]
- Yang, Z.S.; Huang, S.W.; Wang, W.H.; Lin, C.Y.; Wang, C.F.; Urbina, A.N.; Thitithanyanont, A.; Tseng, S.P.; Lu, P.L.; Chen, Y.H.; et al. Identification of Important N-Linked Glycosylation Sites in the Hemagglutinin Protein and Their Functional Impact on DC-SIGN Mediated Avian Influenza H5N1 Infection. Int. J. Mol. Sci. 2021, 22, 743. [Google Scholar] [CrossRef]
- Lee, S.; Kang, S.; Heo, J.; Hong, Y.; Vu, T.H.; Truong, A.D.; Lillehoj, H.S.; Hong, Y.H. MicroRNA expression profiling in the lungs of genetically different Ri chicken lines against the highly pathogenic avian influenza H5N1 virus. J. Anim. Sci. Technol. 2023, 65, 838–855. [Google Scholar] [CrossRef]
- Keam, S.; Megawati, D.; Patel, S.K.; Tiwari, R.; Dhama, K.; Harapan, H. Immunopathology and immunotherapeutic strategies in severe acute respiratory syndrome coronavirus 2 infection. Rev. Med. Virol. 2020, 30, e2123. [Google Scholar] [CrossRef]
- Zou, Q.; Wu, B.; Xue, J.; Fan, X.; Feng, C.; Geng, S.; Wang, M.; Wang, B. CD8+ Treg cells suppress CD8+ T cell-responses by IL-10-dependent mechanism during H5N1 influenza virus infection. Eur. J. Immunol. 2014, 44, 103–114. [Google Scholar] [CrossRef]
- Westenius, V.; Mäkelä, S.M.; Ziegler, T.; Julkunen, I.; Österlund, P. Efficient replication and strong induction of innate immune responses by H9N2 avian influenza virus in human dendritic cells. Virology 2014, 471–473, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Waithman, J.; Mintern, J.D. Dendritic cells and influenza A virus infection. Virulence 2012, 3, 603–608. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.D.; Simmons, C.P.; Thanh, T.T.; Hien, V.M.; Smith, G.J.; Chau, T.N.; Hoang, D.M.; Chau, N.V.; Khanh, T.H.; Dong, V.C.; et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 2006, 12, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Woolford, L.; Rector, A.; Van Ranst, M.; Ducki, A.; Bennett, M.D.; Nicholls, P.K.; Warren, K.S.; Swan, R.A.; Wilcox, G.E.; O’Hara, A.J. A novel virus detected in papillomas and carcinomas of the endangered western barred bandicoot (Perameles bougainville) exhibits genomic features of both the Papillomaviridae and Polyomaviridae. J. Virol. 2007, 81, 13280–13290. [Google Scholar] [CrossRef]
- Dorna, J.; Kaufmann, A.; Bockmann, V.; Raifer, H.; West, J.; Matrosovich, M.; Bauer, S. Effects of Receptor Specificity and Conformational Stability of Influenza A Virus Hemagglutinin on Infection and Activation of Different Cell Types in Human PBMCs. Front. Immunol. 2022, 13, 827760. [Google Scholar] [CrossRef]
- Chen, K.K.; Minakuchi, M.; Wuputra, K.; Ku, C.C.; Pan, J.B.; Kuo, K.K.; Lin, Y.C.; Saito, S.; Lin, C.S.; Yokoyama, K.K. Redox control in the pathophysiology of influenza virus infection. BMC Microbiol. 2020, 20, 214. [Google Scholar] [CrossRef]
- Abbas, A.; Vu Manh, T.P.; Valente, M.; Collinet, N.; Attaf, N.; Dong, C.; Naciri, K.; Chelbi, R.; Brelurut, G.; Cervera-Marzal, I.; et al. The activation trajectory of plasmacytoid dendritic cells in vivo during a viral infection. Nat. Immunol. 2020, 21, 983–997. [Google Scholar] [CrossRef]
- Qin, T.; Chen, Y.; Huangfu, D.; Miao, X.; Yin, Y.; Yin, Y.; Chen, S.; Peng, D.; Liu, X. PA-X protein of H9N2 subtype avian influenza virus suppresses the innate immunity of chicken bone marrow-derived dendritic cells. Poult. Sci. 2023, 102, 102304. [Google Scholar] [CrossRef]
- Ma, N.; Li, X.; Jiang, H.; Dai, Y.; Xu, G.; Zhang, Z. Influenza Virus Neuraminidase Engages CD83 and Promotes Pulmonary Injury. J. Virol. 2021, 95, 3. [Google Scholar] [CrossRef]
- Bates, J.M.; Flanagan, K.; Mo, L.; Ota, N.; Ding, J.; Ho, S.; Liu, S.; Roose-Girma, M.; Warming, S.; Diehl, L. Dendritic cell CD83 homotypic interactions regulate inflammation and promote mucosal homeostasis. Mucosal Immunol. 2015, 8, 414–428. [Google Scholar] [CrossRef]
- Mokhtar, D.M.; Zaccone, G.; Alesci, A.; Kuciel, M.; Hussein, M.T.; Sayed, R.K.A. Main Components of Fish Immunity: An Overview of the Fish Immune System. Fishes 2023, 8, 93. [Google Scholar] [CrossRef]
- de Winde, C.M.; Munday, C.; Acton, S.E. Molecular mechanisms of dendritic cell migration in immunity and cancer. Med. Microbiol. Immunol. 2020, 209, 515–529. [Google Scholar] [CrossRef]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; El Zowalaty, M.E.; Rahman, A.T.; Ashour, H.M. Zoonotic Diseases: Etiology, Impact, and Control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Kim, K.D.; Lee, H.K. The role of dendritic cells in tumor microenvironments and their uses as therapeutic targets. BMB Rep. 2021, 54, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Hasheminasab, S.S.; Conejeros, I.; Gärtner, U.; Kamena, F.; Krueger, A.; Taubert, A.; Hermosilla, C. Human dendritic cell interactions with the zoonotic parasite Cryptosporidium parvum result in activation and maturation. Front. Immunol. 2024, 15, 1388366. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Kang, C.; Zhang, J.; Liu, Y.; Liu, J.; Hu, T.; Zeng, X.; Qiu, S. The role of dendritic cells in allergic diseases. Int. Immunopharmacol. 2022, 113 Pt B, 109449. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Li, Y.; Huang, F.; Luo, B.; Yuan, Y.; Xia, B.; Ma, X.; Yang, T.; Yu, F.; et al. The ORF8 protein of SARS-CoV-2 mediates immune evasion through down-regulating MHC-I. Proc. Natl. Acad. Sci. USA 2021, 118, e2024202118. [Google Scholar] [CrossRef]
- Hillaire, M.L.; Nieuwkoop, N.J.; Boon, A.C.; de Mutsert, G.; Vogelzang-van Trierum, S.E.; Fouchier, R.A.; Osterhaus, A.D.; Rimmelzwaan, G.F. Binding of DC-SIGN to the hemagglutinin of influenza A viruses supports virus replication in DC-SIGN expressing cells. PLoS ONE 2013, 8, e56164. [Google Scholar] [CrossRef][Green Version]
- Cai, Z.; Qiao, Y.; Wuri, Q.; Zhang, K.; Qu, X.; Zhang, S.; Wu, H.; Wu, J.; Wang, C.; Yu, X.; et al. Flt3 ligand augments immune responses to soluble PD1-based DNA vaccine via expansion of type 1 conventional DCs. Int. Immunopharmacol. 2024, 141, 112956. [Google Scholar] [CrossRef]
- Bardi, G. Challenges in the Therapeutic Exploitation of Chemokine Receptor-Mediated Internalization of Nanocarriers. Front. Biosci. (Landmark Ed.) 2024, 29, 350. [Google Scholar] [CrossRef]
- Széles, L.; Meissner, F.; Dunand-Sauthier, I.; Thelemann, C.; Hersch, M.; Singovski, S.; Haller, S.; Gobet, F.; Fuertes Marraco, S.A.; Mann, M.; et al. TLR3-Mediated CD8+ Dendritic Cell Activation Is Coupled with Establishment of a Cell-Intrinsic Antiviral State. J. Immunol. 2015, 195, 1025–1033. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, X.; Han, Y.; Yin, Y.; Yang, Y.; Chen, S.; Jiao, X.; Qin, T.; Peng, D. Immune Responses of Dendritic Cells to Zoonotic DNA and RNA Viruses. Vet. Sci. 2025, 12, 692. https://doi.org/10.3390/vetsci12080692
Miao X, Han Y, Yin Y, Yang Y, Chen S, Jiao X, Qin T, Peng D. Immune Responses of Dendritic Cells to Zoonotic DNA and RNA Viruses. Veterinary Sciences. 2025; 12(8):692. https://doi.org/10.3390/vetsci12080692
Chicago/Turabian StyleMiao, Xinyu, Yixuan Han, Yinyan Yin, Yang Yang, Sujuan Chen, Xinan Jiao, Tao Qin, and Daxin Peng. 2025. "Immune Responses of Dendritic Cells to Zoonotic DNA and RNA Viruses" Veterinary Sciences 12, no. 8: 692. https://doi.org/10.3390/vetsci12080692
APA StyleMiao, X., Han, Y., Yin, Y., Yang, Y., Chen, S., Jiao, X., Qin, T., & Peng, D. (2025). Immune Responses of Dendritic Cells to Zoonotic DNA and RNA Viruses. Veterinary Sciences, 12(8), 692. https://doi.org/10.3390/vetsci12080692







