Oxidative Stress Model of Lipopolysaccharide-Challenge in Piglets of Wuzhishan Miniature Pig
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Experimental Design
2.3. Sample Collection
2.4. Serum Antioxidant Capacity, Inflammation-Related Cytokines and Intestinal Permeability Indexes
2.5. Histomorphology and Scanning Electron Microscopy
2.6. Serum Metabolomics Analysis
2.6.1. Metabolites Extraction
2.6.2. LC-MS/MS Analysis
2.6.3. Data Preprocessing and Annotation
2.7. Metagenomics
2.7.1. DNA Extraction
2.7.2. DNA Library Construction
2.7.3. Data Analysis
2.8. Statistical Analysis
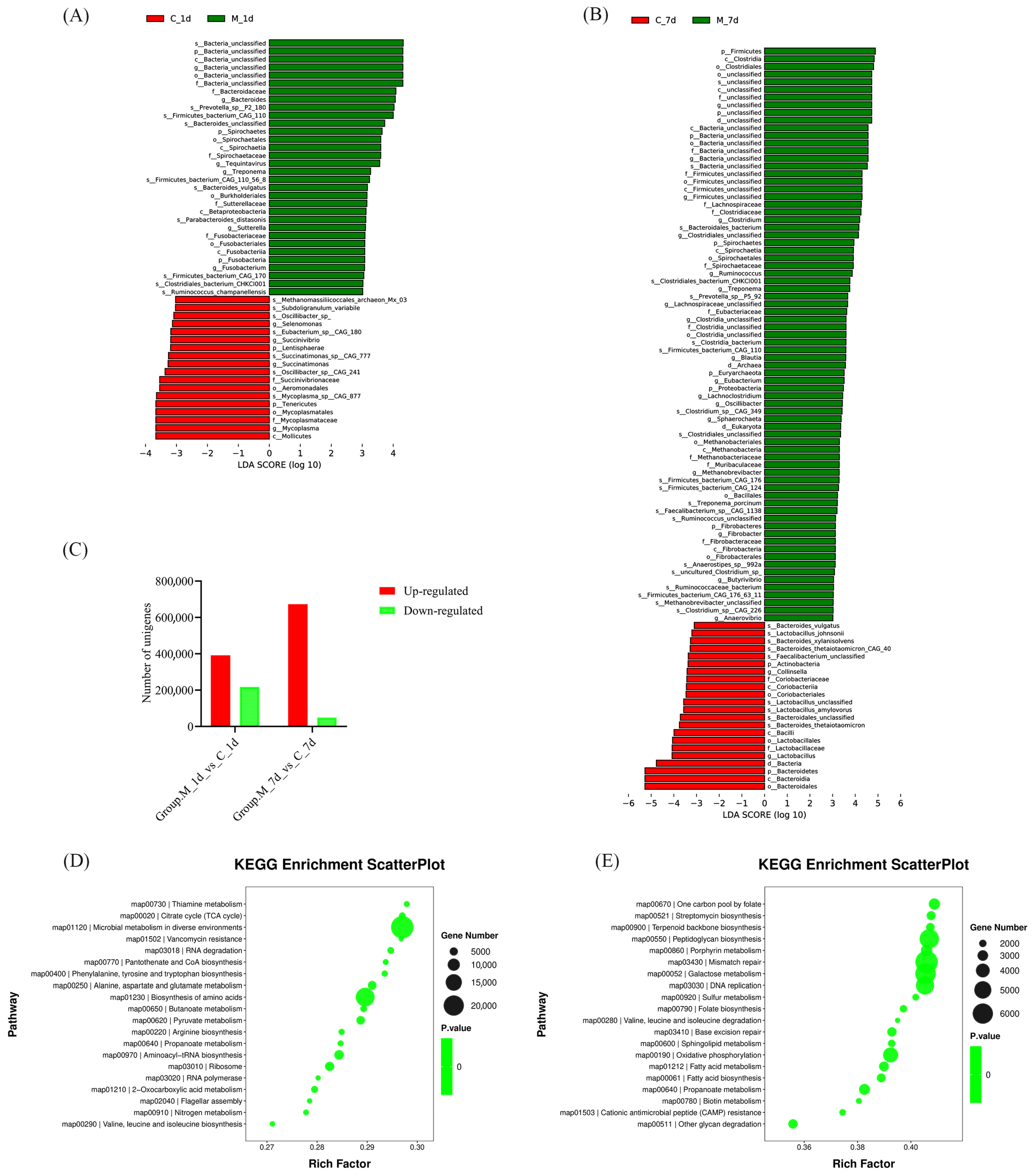
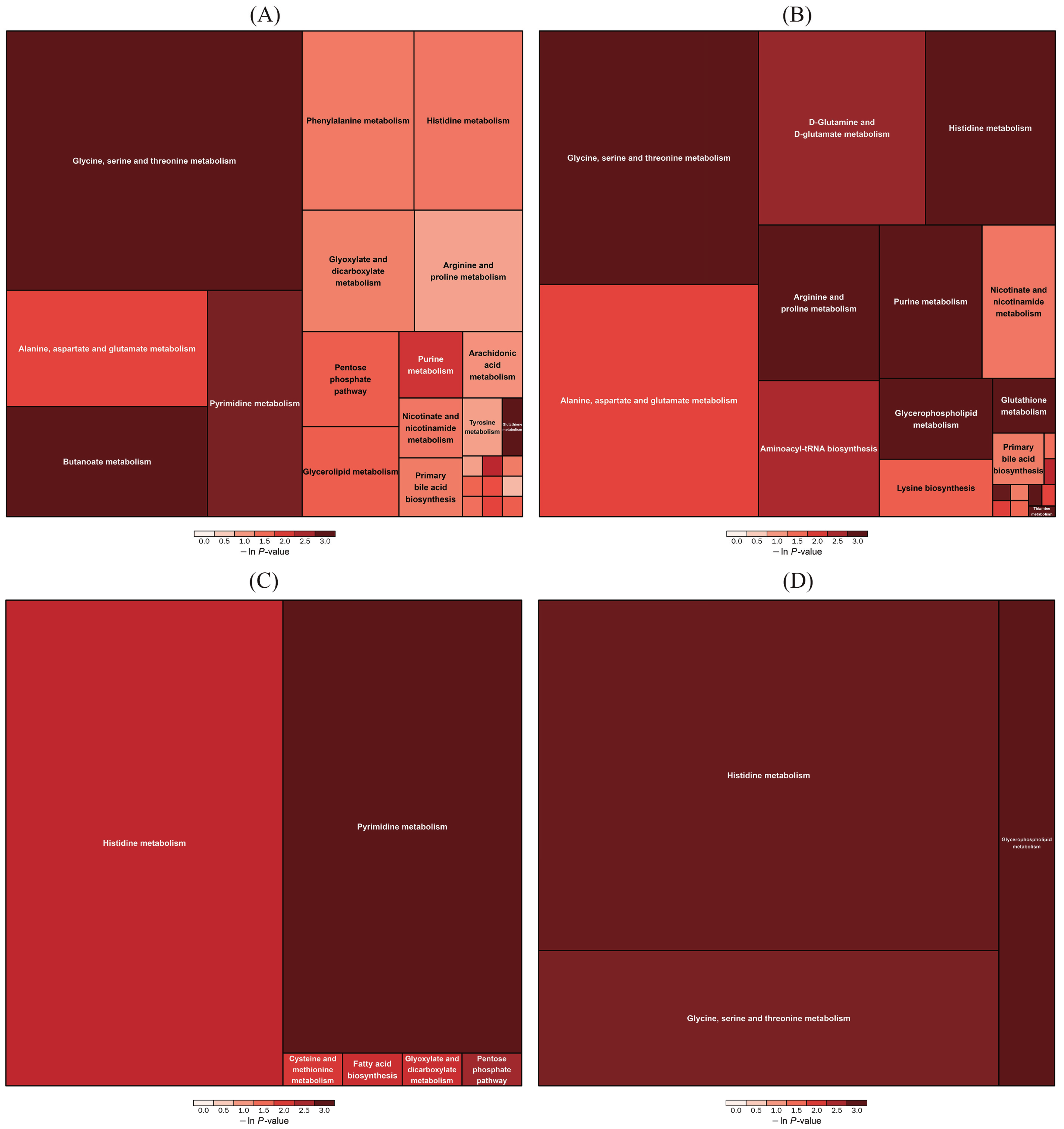
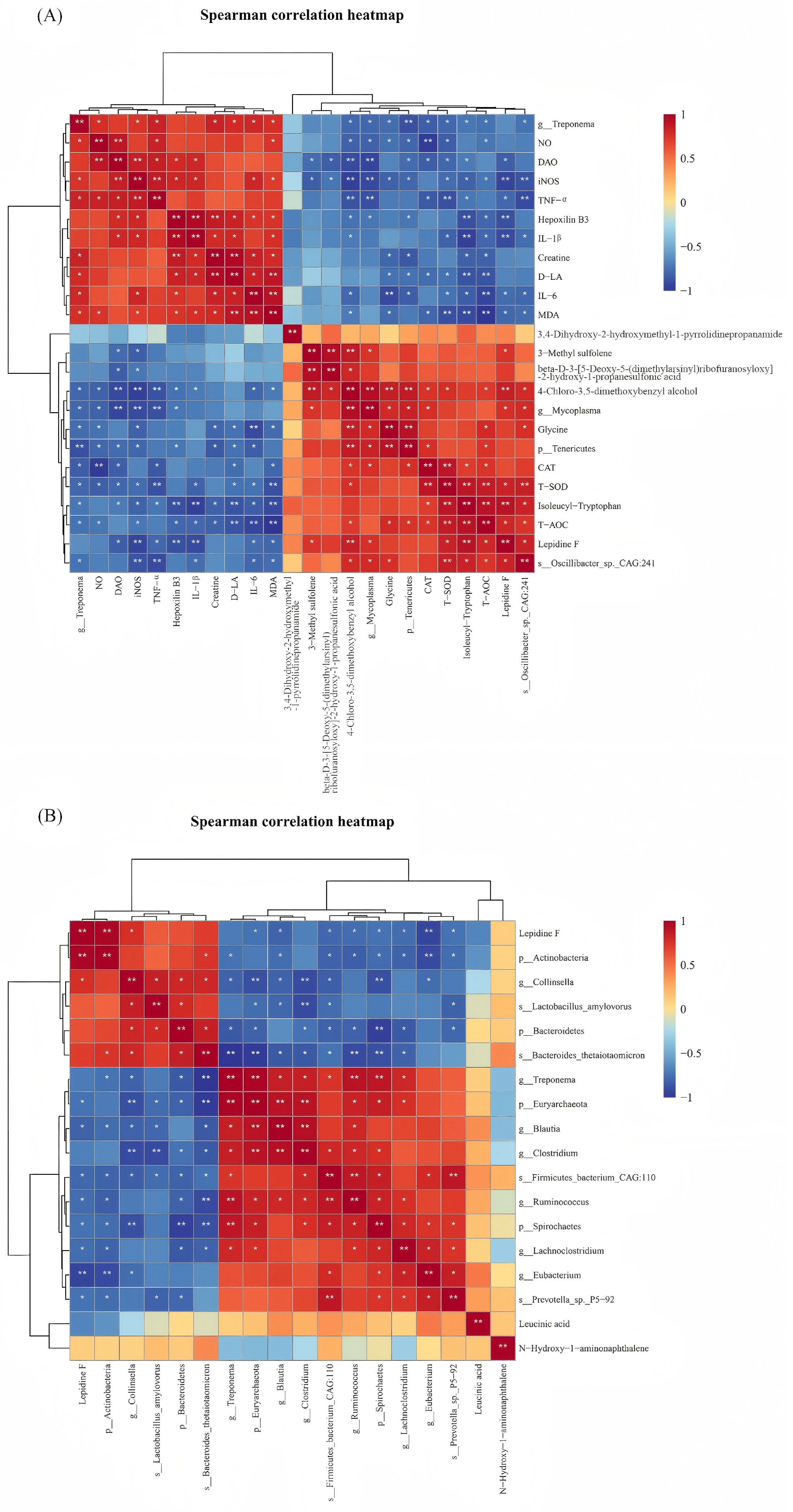
3. Results
3.1. LPS Reduces Body Weight Gain and Elevates Oxidative Stress Parameters in Piglets
3.2. LPS Increased Serum Inflammation-Related Mediators and Cytokines in Piglets
3.3. Effect of LPS on Intestinal Permeability, Morphology and Immune-Related Organs
3.4. Analysis of Piglet Gut Microbiota
3.5. Analysis of Piglet Serum Metabolite
3.6. Spearman Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WMP | Wuzhishan miniature pig |
| OS | Oxidative stress |
| ROS | Reactive oxygen species |
| LPS | Lipopolysaccharide |
| MDA | Malondialdehyde |
| T-SOD | Total superoxide dismutase |
| T-AOC | Total antioxidant capacity |
| CAT | Catalase |
| NO | Nitric oxide |
| iNOS | Inducible nitric oxide synthase |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor-α |
| DAO | Diamine oxidase |
| D-LA | D-lactic acid |
| H&E | Hematoxylin-Eosin |
| SEM | Scanning electron microscope |
| QC | Quality control |
| UHPLC | Ultra-high-performance liquid chromatography |
| IDA | Information-dependent acquisition |
| ESI | Electrospray ionization |
| CDS | Coding regions |
| SDs | Standard deviations |
| PCA | Principal component analysis |
| PCoA | Principal coordinate analysis |
| LDA | Linear discriminant analysis |
| LEfSe | Linear discriminant analysis effect size |
| KEGG | Kyoto encyclopedia of genes and genomes |
| OPLS-DA | Orthogonal partial least squares-discriminant analysis |
| NAFLD | Nonalcoholic fatty liver disease |
| AAs | Amino acids |
References
- Liu, Y.; Yang, N.; Tan, C.; Zhang, Y.; Gao, S.; Cai, Y.; Zhang, Y.; Fu, Y.; Liu, G.; Li, Y. Wuzhishan miniature pig-derived intestinal 2D monolayer organoids to investigate the enteric coronavirus infection. Front. Vet. Sci. 2024, 11, 1457719. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Liu, Y.; Xiang, L.; Zhao, Y.; Yuan, J.; Jia, Y.; Dai, X.; Chen, H. Long-term case study of a Wuzhishan miniature pig with diabetes. Anim. Model. Exp. Med. 2020, 3, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shen, L.; Zhang, D.; Wang, X.; Wang, Q.; Qin, W.; Gao, Y.; Li, X. Ammonia-induced oxidative stress triggered proinflammatory response and apoptosis in pig lungs. J. Environ. Sci. 2022, 126, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ma, J.; Xu, J.; Huangfu, W.; Zhang, Y.; Ali, Q.; Liu, B.; Li, D.; Cui, Y.; Wang, Z.; et al. Fecal microbiota transplantation alleviates intestinal inflammatory diarrhea caused by oxidative stress and pyroptosis via reducing gut microbiota-derived lipopolysaccharides. Int. J. Biol. Macromol. 2024, 261, 129696. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.J.; Liu, W.; Li, Y.H.; Jiang, H.; Xu, Y.N.; Kim, N.H. Citrinin impairs pig oocyte maturation by inducing oxidative stress and apoptosis. Toxicon 2025, 205, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; He, Z.; Du, L.; Zhang, L.; Li, J.; Ma, Y.; Bi, S. Biomarkers of oxidative stress in broiler chickens attacked by lipopolysaccharide: A systematic review and meta-analysis. Ecotoxicol. Environ. Saf. 2023, 266, 115606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tian, X.; Wang, Y.; Yan, Y.; Wang, Y.; Su, M.; Lv, H.; Li, K.; Hao, X.; Xing, X.; et al. Application of lipopolysaccharide in establishing inflammatory models. Int. J. Biol. Macromol. 2024, 279, 135371. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Huo, R.; Wang, Y.; Ma, N.; Shi, X.; Shen, X.; Chang, G. Lentinan inhibits oxidative stress and alleviates LPS-induced inflammation and apoptosis of BMECs by activating the Nrf2 signaling pathway. Int. J. Biol. Macromol. 2022, 222, 2375–2391. [Google Scholar] [CrossRef] [PubMed]
- Sul, O.-J.; Ra, S.W. Quercetin Prevents LPS-Induced Oxidative Stress and Inflammation by Modulating NOX2/ROS/NF-kB in Lung Epithelial Cells. Molecules 2021, 26, 6949. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Han, P.; Cui, Y.; Chen, Q.; Cao, W. Quercetin attenuates inflammation in LPS-induced lung epithelial cells via the Nrf2 signaling pathway. Immunity Inflamm. Dis. 2024, 12, e1185. [Google Scholar] [CrossRef] [PubMed]
- Netzley, A.H.; Pelled, G. The Pig as a Translational Animal Model for Biobehavioral and Neurotrauma Research. Biomedicines 2023, 11, 2165. [Google Scholar] [CrossRef] [PubMed]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Huang, Y.; Cao, S.; Wang, L.; Yang, X.; Hu, S.; Gao, K.; Jiang, Z.; Xiao, H. Accurate models and nutritional strategies for specific oxidative stress factors: Does the dose matter in swine production? J. Anim. Sci. Biotechnol. 2024, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Xu, B.; Chen, Y.; Yang, W.; Xu, Y.; Huang, J.; Duo, T.; Mao, Y.; Zhou, G.; Yan, X.; et al. Dietary ellagic acid supplementation attenuates intestinal damage and oxidative stress by regulating gut microbiota in weanling piglets. Anim. Nutr. 2022, 11, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zou, Q.; Jiang, Y.; Wang, Y.; Ding, X. A pig model exploring the postnatal hair follicle cycle. Front. Cell Dev. Biol. 2024, 12, 1361485. [Google Scholar] [CrossRef] [PubMed]
- Sorop, O.; van de Wouw, J.; Chandler, S.; Ohanyan, V.; Tune, J.D.; Chilian, W.M.; Merkus, D.; Bender, S.B.; Duncker, D.J. Experimental animal models of coronary microvascular dysfunction. Cardiovasc. Res. 2020, 116, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Science and Technology of the People’s Republic of China. Guiding Opinions on Treating Laboratory Animals Humanely (Guokefacai Zi [2006] No. 398). Available online: https://www.most.gov.cn/xxgk/xinxifenlei/fdzdgknr/fgzc/gfxwj/gfxwj2010before/201712/t20171222_137025.html (accessed on 21 July 2025).
- Zou, Z.; Pan, S.; Sun, C.; Wei, J.; Xu, Y.; Xiao, K.; Zhao, J.; Gu, R. AM1241 inhibits chondrocyte inflammation and ECM degradation through the Nrf2/HO-1 and NF-κB pathways and alleviates osteoarthritis in mice. Mol. Med. 2025, 31, 9. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Pang, J.; Zhang, X.; Liu, Y.; Wu, Y.; Wang, J.; Han, D. L-arabinose Attenuates LPS-Induced Intestinal Inflammation and Injury through Reduced M1 Macrophage Polarization. J. Nutr. 2023, 153, 3327–3340. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zhang, Y.; Cai, Y.; Li, D.; Yi, B.; Xu, Q. METTL3 mediates osteoblast apoptosis by regulating endoplasmic reticulum stress during LPS-induced inflammation. Cell. Signal. 2022, 95, 110335. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Ru, H.; Chen, Y.; Xu, J.; Wang, C.; Jin, Y. Gallic acid attenuates LPS-induced inflammation in Caco-2 cells by suppressing the activation of the NF-κB/MAPK signaling pathway. Acta Biochim. Biophys. Sin. 2024, 56, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, K.; Ren, G.-M.; Wang, Y.; Wang, T.; Liu, X.; Li, D.-X.; Xiao, Y.; Chen, X.; Li, Y.-T.; et al. BRISC is required for optimal activation of NF-κB in Kupffer cells induced by LPS and contributes to acute liver injury. Cell Death Dis. 2023, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Feng, M.; Su, M.; Wang, D.; Li, Q.; Jia, S.; Luo, F.; Wang, H.; Hu, E.; Yang, X.; et al. Qihuzha granule attenuated LPS-induced acute spleen injury in mice via Src/MAPK/Stat3 signal pathway. J. Ethnopharmacol. 2021, 281, 114458. [Google Scholar] [CrossRef] [PubMed]
- Dangarembizi, R.; Erlwanger, K.; Rummel, C.; Roth, J.; Madziva, M.; Harden, L. Brewer’s yeast is a potent inducer of fever, sickness behavior and inflammation within the brain. Brain Behav. Immun. 2018, 68, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Koch, F.; Thom, U.; Albrecht, E.; Weikard, R.; Nolte, W.; Kuhla, B.; Kuehn, C. Heat stress directly impairs gut integrity and recruits distinct immune cell populations into the bovine intestine. Proc. Natl. Acad. Sci. USA 2019, 116, 10333–10338. [Google Scholar] [CrossRef] [PubMed]
- Uyanga, V.A.; Zhao, J.; Wang, X.; Jiao, H.; Onagbesan, O.M.; Lin, H. Dietary L-citrulline influences body temperature and inflammatory responses during nitric oxide synthase inhibition and endotoxin challenge in chickens. Stress 2022, 25, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Osterhout, J.A.; Kapoor, V.; Eichhorn, S.W.; Vaughn, E.; Moore, J.D.; Liu, D.; Lee, D.; DeNardo, L.A.; Luo, L.; Zhuang, X.; et al. A preoptic neuronal population controls fever and appetite during sickness. Nature 2022, 606, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, Y.; Shirawachi, S.; Mizokami, A.; Nozaki, K.; Ito, H.; Asano, S.; Oue, K.; Aizawa, H.; Yamawaki, S.; Hirata, M.; et al. Phospholipase C-related catalytically inactive protein regulates lipopolysaccharide-induced hypothalamic inflammation-mediated anorexia in mice. Neurochem. Int. 2019, 131, 104563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hou, L.; Guo, Z.; Wang, G.; Xu, J.; Zheng, Z.; Sun, K.; Guo, F. Lipid peroxidation in osteoarthritis: Focusing on 4-hydroxynonenal, malondialdehyde, and ferroptosis. Cell Death Discov. 2023, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Efremova, I.; Maslennikov, R.; Medvedev, O.; Kudryavtseva, A.; Avdeeva, A.; Krasnov, G.; Romanikhin, F.; Diatroptov, M.; Fedorova, M.; Poluektova, E.; et al. Gut Microbiota and Biomarkers of Intestinal Barrier Damage in Cirrhosis. Microorganisms 2024, 12, 463. [Google Scholar] [CrossRef] [PubMed]
- Remund, B.; Yilmaz, B.; Sokollik, C. D-Lactate: Implications for Gastrointestinal Diseases. Children 2023, 10, 945. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Ramesh, G.; Wu, M.; Jensen, E.T.; Crago, O.; Bertoni, A.G.; Gao, C.; Hoffman, K.L.; Sheridan, P.A.; Wong, K.E.; et al. Butyrate-Producing Bacteria and Insulin Homeostasis: The Microbiome and Insulin Longitudinal Evaluation Study (MILES). Diabetes 2022, 71, 2438–2446. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Fan, C.; Liu, F.; Li, Y.; Hou, H.; Ma, Y.; Tan, Y.; Li, Y.; Hai, Y.; Wu, T.; et al. Structural and functional dysbiosis of gut microbiota in Tibetan subjects with coronary heart disease. Genomics 2022, 114, 110483. [Google Scholar] [CrossRef] [PubMed]
- Huelsmann, M.; Schubert, O.T.; Ackermann, M. A framework for understanding collective microbiome metabolism. Nat. Microbiol. 2024, 9, 3097–3109. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.K.; Tang, M.; Lei, L.; Li, J.R.; Sun, H.; Jiang, J.; Dong, B.; Li, H.Y.; Jiang, J.D.; et al. Bacteroides thetaiotaomicron ameliorates mouse hepatic steatosis through regulating gut microbial composition, gut-liver folate and unsaturated fatty acids metabolism. Gut Microbes 2024, 16, 2304159. [Google Scholar] [CrossRef] [PubMed]
- Gul, L.; Modos, D.; Fonseca, S.; Madgwick, M.; Thomas, J.P.; Sudhakar, P.; Booth, C.; Stentz, R.; Carding, S.R.; Korcsmaros, T. Extracellular vesicles produced by the human commensal gut bacterium Bacteroides thetaiotaomicron affect host immune pathways in a celltype specific manner that are altered in inflammatory bowel disease. J. Extracell. Vesicles 2022, 11, e12189. [Google Scholar] [CrossRef] [PubMed]
- Xerri, N.L.; Payne, S.M. Bacteroides thetaiotaomicron Outer Membrane Vesicles Modulate Virulence of Shigella flexneri. mBio 2022, 13, e0236022. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Mayer, M.J.; Juge, N.; Narbad, A.; Sayavedra, L. Bacteroides thetaiotaomicron enhances H(2)S production in Bilophila wadsworthia. Gut Microbes 2024, 16, 2431644. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Bhardwaj, K.; Valko, M.; Alomar, S.Y.; Alwasel, S.H.; Cruz-Martins, N.; Dhanjal, D.S.; Singh, R.; Kuča, K.; Verma, R.; et al. Antioxidative potential of Lactobacillus sp. in ameliorating D-galactose-induced aging. Appl. Microbiol. Biotechnol. 2022, 106, 4831–4843. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, J.; Zhao, Y.; Lin, Z.; Ji, L.; Ma, X. Tibetan Pig-Derived Probiotic Lactobacillus amylovorus SLZX20-1 Improved Intestinal Function via Producing Enzymes and Regulating Intestinal Microflora. Front. Nutr. 2022, 9, 846991. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, X.; Zou, Y.; Zhang, X.; Wang, Z.; Hu, J.; Han, D.; Zhao, J.; Dai, Z.; Wang, J. Lactobacillus amylovorus Promotes Lactose Utilization in Small Intestine and Enhances Intestinal Barrier Function in Intrauterine Growth Restricted Piglets. J. Nutr. 2023, 154, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Mu, C.; Wang, H.; Shen, J.; Zoetendal, E.G.; Zhu, W. Amino acid utilization allows intestinal dominance of Lactobacillus amylovorus. ISME J. 2022, 16, 2491–2502. [Google Scholar] [CrossRef] [PubMed]
- Pruksaphon, K.; Amsri, A.; Jeenkeawpieam, J.; Thammasit, P.; Nosanchuk, J.D.; Youngchim, S. The microbial damage and host response framework: Lesson learned from pathogenic survival trajectories and immunoinflammatory responses of Talaromyces marneffei infection. Front. Immunol. 2024, 15, 1448729. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Wang, Z.; Cai, M.; Guo, T.; Mao, S.; Yang, Y. A multi-omics investigation of the lung injury induced by PM(2.5) at environmental levels via the lung-gut axis. Sci. Total. Environ. 2024, 926, 172027. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Shen, F.; Liao, T.; Qian, H.; Liu, Y. Sporidiobolus pararoseus polysaccharides relieve rheumatoid arthritis by regulating arachidonic acid metabolism and bone remodeling signaling pathway. Int. J. Biol. Macromol. 2024, 281, 136272. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, X.; Huang, D.; Zhao, Y.; Chen, J.; Dong, Z.; Chen, W.; Zhang, F.; Sun, L. Unveiling the Pharmacological Mechanisms of Davidiin’s Anti-Diabetic Efficacy in Streptozotocin-Treated Rats: A Comprehensive Analysis of Serum Metabolome. Drug Des. Dev. Ther. 2024, 18, 1981–1996. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-T.; Chen, X.; Huo, D.; Arifuzzaman, M.; Qiao, S.; Jin, W.-B.; Shi, H.; Li, X.V.; Iliev, I.D.; Artis, D.; et al. Microbiota metabolism of intestinal amino acids impacts host nutrient homeostasis and physiology. Cell Host Microbe 2024, 32, 661–675.e10. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhu, Y.; Zhao, Z.; Chu, Y.; Yang, W. The role of amino acid metabolism in inflammatory bowel disease and other inflammatory diseases. Front. Immunol. 2023, 14, 1284133. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Stout, J.R. Creatine in Health and Disease. Nutrients 2021, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wei, S.; Liu, B.; Wang, F.; Lu, Z.; Jin, M.; Wang, Y. Maternal consumption of a fermented diet protects offspring against intestinal inflammation by regulating the gut microbiota. Gut Microbes 2022, 14, 2057779. [Google Scholar] [CrossRef] [PubMed]


| Items | Groups | ||||
|---|---|---|---|---|---|
| Control | L-LPS | M-LPS | H-LPS | ||
| T-AOC (U/mol) | Day 0 | 3.05 ± 0.60 | 3.08 ± 1.36 | 3.00 ± 1.25 A | 3.30 ± 1.50 A |
| Day 1 | 3.13 ± 0.70 a | 2.40 ± 1.22 b | 1.29 ± 0.40 Cc | 0.89 ± 0.37 Cc | |
| Day 3 | 2.66 ± 0.67 a | 2.30 ± 0.77 a | 1.53 ± 0.60 Cb | 1.69 ± 0.24 BCb | |
| Day 5 | 2.77 ± 0.88 a | 2.22 ± 0.75 ab | 1.92 ± 0.60 BCb | 1.97 ± 0.99 BCb | |
| Day 7 | 2.67 ± 0.61 | 2.34 ± 0.63 | 2.40 ± 0.88 AB | 2.07 ± 1.58 B | |
| T-SOD (U/mL) | Day 0 | 107.20 ± 7.50 | 105.10 ± 16.83 | 103.34 ± 7.59 A | 103.88 ± 26.58 A |
| Day 1 | 106.60 ± 12.60 a | 98.50 ± 24.95 ab | 90.44 ± 8.33 Bb | 88.02 ± 10.15 Bb | |
| Day 3 | 109.89 ± 12.69 a | 107.74 ± 11.41 a | 102.47 ± 10.37 Aab | 96.34 ± 15.36 ABb | |
| Day 5 | 109.25 ± 3.22 a | 109.51 ± 5.23 ab | 101.84 ± 13.50 Ab | 104.94 ± 17.65 Ab | |
| Day 7 | 115.51 ± 19.98 | 107.77 ± 13.92 | 105.01 ± 9.26 A | 109.33 ± 20.09 A | |
| CAT (U/mol) | Day 0 | 23.59 ± 1.35 | 23.21 ± 2.25 A | 22.89 ± 1.40 A | 22.99 ± 1.70 A |
| Day 1 | 22.00 ± 5.32 a | 16.46 ± 6.68 Bab | 14.62 ± 8.91 Bb | 11.73 ± 7.28 Cb | |
| Day 3 | 23.78 ± 1.67 a | 21.56 ± 3.63 Ab | 20.68 ± 3.29 Ab | 19.37 ± 2.95 Bb | |
| Day 5 | 23.28 ± 2.27 | 22.22 ± 3.08 A | 22.34 ± 1.91 A | 22.22 ± 2.04 AB | |
| Day 7 | 23.15 ± 1.18 | 22.27 ± 2.98 A | 22.44 ± 1.18 A | 22.50 ± 1.37 A | |
| MDA (mmol/mL) | Day 0 | 7.63 ± 0.42 B | 7.53 ± 0.27 B | 7.44 ± 0.51 B | 7.52 ± 0.71 B |
| Day 1 | 7.98 ± 0.30 Ab | 8.52 ± 0.28 Aa | 8.64 ± 1.01 Aa | 8.67 ± 0.70 Aa | |
| Day 3 | 7.80 ± 0.39 AB | 7.78 ± 0.41 B | 7.75 ± 0.40 B | 7.70 ± 0.40 B | |
| Day 5 | 7.71 ± 0.48 AB | 7.55 ± 0.56 B | 7.61 ± 0.89 B | 7.95 ± 0.89 B | |
| Day 7 | 7.71 ± 0.35 AB | 7.54 ± 0.32 B | 7.46 ± 0.46 B | 7.65 ± 0.60 B | |
| Items | Groups | ||||
|---|---|---|---|---|---|
| Control | L-LPS | M-LPS | H-LPS | ||
| NO (μmol/L) | Day 0 | 16.24 ± 5.05 | 17.97 ± 9.10 | 16.32 ± 11.46 | 17.39 ± 9.91 C |
| Day 1 | 16.35 ± 11.99 c | 25.64 ± 13.68 bc | 28.85 ± 8.73 b | 39.86 ± 11.14 Aa | |
| Day 3 | 22.30 ± 8.52 b | 21.40 ± 9.83 b | 21.21 ± 9.61 b | 32.72 ± 9.63 ABa | |
| Day 5 | 24.37 ± 16.68 | 25.30 ± 15.96 | 25.91 ± 17.98 | 23.55 ± 14.8 BC | |
| Day 7 | 18.05 ± 15.56 | 23.62 ± 16.68 | 20.30 ± 12.98 | 22.11 ± 12.17 BC | |
| iNOS (μmol/mL) | Day 0 | 10.77 ± 1.75 | 10.99 ± 1.81 | 11.04 ± 1.08 B | 12.44 ± 2.02 |
| Day 1 | 9.70 ± 1.76 b | 10.93 ± 2.60 ab | 12.95 ± 3.45 Aa | 12.96 ± 3.44 a | |
| Day 3 | 10.08 ± 2.74 | 11.95 ± 4.75 | 11.72 ± 2.85 AB | 11.38 ± 4.62 | |
| Day 5 | 9.79 ± 2.63 | 10.57 ± 4.39 | 11.78 ± 2.87 AB | 11.76 ± 3.19 | |
| Day 7 | 9.65 ± 3.79 | 9.57 ± 5.16 | 11.28 ± 2.72 AB | 10.17 ± 4.47 | |
| IL-6 (pg/mol) | Day 0 | 21.42 ± 9.41 | 18.52 ± 10.91 B | 21.79 ± 12.86 B | 24.98 ± 12.51 C |
| Day 1 | 22.10 ± 8.89 b | 40.52 ± 7.09 Aa | 49.88 ± 14.75 Aa | 52.65 ± 5.69 Aa | |
| Day 3 | 20.93 ± 5.51 b | 30.29 ± 14.65 ABab | 32.87 ± 12.44 Bab | 36.10 ± 4.97 Ba | |
| Day 5 | 22.53 ± 4.40 | 24.40 ± 8.75 B | 25.27 ± 9.48 B | 27.93 ± 6.39 BC | |
| Day 7 | 15.69 ± 7.19 | 15.64 ± 12.92 B | 25.45 ± 13.25 B | 27.03 ± 5.23 BC | |
| IL-1β (pg/mol) | Day 0 | 8.84 ± 6.02 | 9.56 ± 8.73 B | 10.04 ± 5.22 B | 12.73 ± 5.92 C |
| Day 1 | 11.26 ± 5.40 c | 23.45 ± 5.76 Ab | 31.20 ± 9.20 Aab | 37.93 ± 10.61 Aa | |
| Day 3 | 12.52 ± 8.24 b | 11.91 ± 2.84 Bb | 16.87 ± 7.72 Bab | 24.28 ± 10.87 Ba | |
| Day 5 | 12.14 ± 8.48 | 8.65 ± 6.84 B | 15.85 ± 5.37 B | 10.32 ± 7.14 C | |
| Day 7 | 14.49 ± 12.83 | 16.82 ± 14.1 AB | 17.97 ± 7.85 B | 11.28 ± 8.40 C | |
| TNF-α (pg/mol) | Day 0 | 40.12 ± 6.78 | 38.42 ± 11.09 | 43.91 ± 11.79 B | 42.89 ± 6.53 B |
| Day 1 | 38.08 ± 12.21 b | 43.43 ± 12.13 b | 67.70 ± 12.41 Aa | 73.43 ± 14.38 Aa | |
| Day 3 | 33.53 ± 6.08 b | 36.68 ± 10.09 ab | 41.81 ± 13.67 Bab | 47.59 ± 7.80 Ba | |
| Day 5 | 35.38 ± 11.44 | 31.88 ± 6.96 | 34.21 ± 14.35 B | 46.01 ± 13.19 B | |
| Day 7 | 38.74 ± 8.54 | 36.12 ± 13.35 | 38.07 ± 12.35 B | 39.88 ± 14.79 B | |
| Items | Groups | ||||
|---|---|---|---|---|---|
| Control | L-LPS | M-LPS | H-LPS | ||
| DAO (U/mL) | Day 0 | 34.22 ± 1.52 | 34.77 ± 1.94 AB | 32.59 ± 1.72 B | 32.93 ± 3.23 E |
| Day 1 | 35.77 ± 6.74 b | 40.93 ± 4.05 Ab | 58.04 ± 10.36 Aa | 58.88 ± 3.57 Aa | |
| Day 3 | 32.56 ± 4.04 c | 34.26 ± 1.04 Bc | 40.07 ± 3.89 Bb | 54.78 ± 2.35 Ba | |
| Day 5 | 30.69 ± 3.76 b | 33.35 ± 7.65 ABb | 35.16 ± 3.02 Bb | 36.75 ± 1.51 Ca | |
| Day 7 | 31.99 ± 1.01 | 34.82 ± 6.47 AB | 36.50 ± 4.77 B | 37.89 ± 1.77 D | |
| D-LA (μmol/L) | Day 0 | 235.72 ± 14.19 | 244.05 ± 18.03 AB | 244.87 ± 26.51 B | 242.06 ± 24.34 B |
| Day 1 | 222.06 ± 19.16 c | 274.04 ± 17.67 Ab | 297.91 ± 27.62 Ab | 344.90 ± 21.41 Aa | |
| Day 3 | 246.60 ± 13.16 b | 243.18 ± 8.24 ABb | 262.95 ± 21.01 Bb | 333.78 ± 23.18 Aa | |
| Day 5 | 244.24 ± 31.45 ab | 238.69 ± 18.57 Bb | 241.77 ± 11.53 Bab | 250.70 ± 11.06 Ba | |
| Day 7 | 237.60 ± 29.30 | 235.55 ± 24.90 B | 236.04 ± 27.88 B | 245.12 ± 29.05 B | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, R.; Qiu, P.; Hu, Y.; Chen, J.; Li, X.; Wang, Q.; Li, Y.; Shi, H.; Zhang, H.; Wang, X. Oxidative Stress Model of Lipopolysaccharide-Challenge in Piglets of Wuzhishan Miniature Pig. Vet. Sci. 2025, 12, 694. https://doi.org/10.3390/vetsci12080694
Bao R, Qiu P, Hu Y, Chen J, Li X, Wang Q, Li Y, Shi H, Zhang H, Wang X. Oxidative Stress Model of Lipopolysaccharide-Challenge in Piglets of Wuzhishan Miniature Pig. Veterinary Sciences. 2025; 12(8):694. https://doi.org/10.3390/vetsci12080694
Chicago/Turabian StyleBao, Ruiying, Pingfei Qiu, Yanrong Hu, Junpu Chen, Xiaochun Li, Qin Wang, Yongqiang Li, Huiyu Shi, Haiwen Zhang, and Xuemei Wang. 2025. "Oxidative Stress Model of Lipopolysaccharide-Challenge in Piglets of Wuzhishan Miniature Pig" Veterinary Sciences 12, no. 8: 694. https://doi.org/10.3390/vetsci12080694
APA StyleBao, R., Qiu, P., Hu, Y., Chen, J., Li, X., Wang, Q., Li, Y., Shi, H., Zhang, H., & Wang, X. (2025). Oxidative Stress Model of Lipopolysaccharide-Challenge in Piglets of Wuzhishan Miniature Pig. Veterinary Sciences, 12(8), 694. https://doi.org/10.3390/vetsci12080694







