“My Bitch Is Empty!” an Overview of the Preconceptional Causes of Infertility in Dogs
Simple Summary
Abstract
1. Introduction
- Preconceptional causes, meaning that the bitch cannot become pregnant. These causes can be divided into three main categories: breeding management-related causes of failure and female and male causes of preconceptional failure. These are the causes of infertility on which this review will focus;
- Failure to maintain a pregnancy. These causes of infertility have been described in detail in a recent review [6].
2. Breeding Management as a Preconceptional Cause of Infertility
2.1. How to Start the Consultation
- ○
- Housing conditions and social structure of conspecifics, including the way the animals are grouped, their spatial distribution, and social interactions in the environment;
- ○
- Vaccination status (herpes virus, core and uncore vaccines, whether the bitch was regularly vaccinated);
- ○
- Deworming protocol (including specific considerations for pregnant animals and neonates);
- ○
- Feeding process (what kind of food, how is it delivered and preserved; bigger attention should be given to BARF, with any supplements);
- ○
- Travels/Exhibitions (in what countries; whether the animals had any kind of contact with other animals; how the animals are reintroduced in the breeding facility, especially respecting the quarantine period);
- ○
- Medical and surgical history and possible ongoing treatments.
- ○
- Does the male have known fertility?
- ○
- Did the male mate before?
- ○
- Did the dogs stay attached?
- ○
- How many matings were made and over what period (for example: two matings in 3 days, one protrusion per day for 10 days)?
- ○
- Did the bitch have a heat follow-up?
- ○
- When was the pregnancy diagnosis conducted (as soon as 18 to 20 days post-ovulation or later)?
2.2. Age of the Animals
2.3. Genetics and Selection
2.4. Nutrition
2.5. Ovulation Detection: Timing of Breeding
2.5.1. Breeding Soundness Examination
- ○
- Observation for symptoms of endocrinopathy (for example, dermatological issues) that may indicate hormonal imbalances;
- ○
- Examination of the oral cavity and teeth, as good oral health is essential; insufficient oral hygiene may lead to neonatal infections due to maternal licking;
- ○
- Evaluation of the mammary glands to rule out anatomical abnormalities or the presence of tumors that could impair lactation;
- ○
- Exclusion of musculoskeletal disorders, which could cause pain during mating (leading to unsuccessful reproduction) or worsen due to pregnancy.
- ○
- Evaluation of the anatomy of the vagina to ensure that mating and natural whelping are possible;
- ○
- Evaluation of the vaginal health;
- ○
- Evaluation of the uterus and ovaries to exclude diseases that could impair fertility;
- ○
- Estimation of the ovulation date using progesterone assays and ovarian ultrasound.
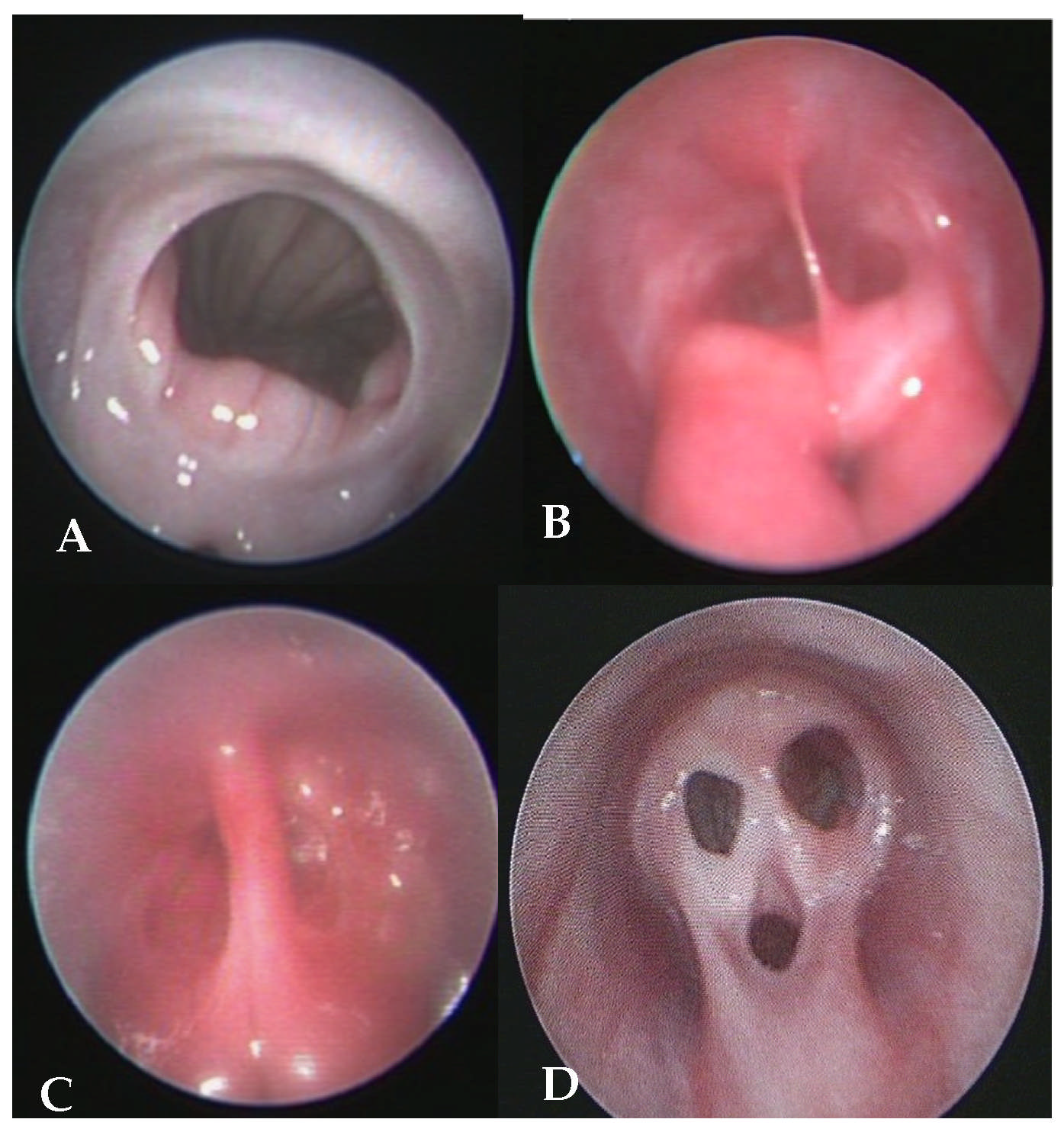
2.5.2. Evaluation of the Vagina
2.5.3. Progesterone Assays and Ovarian Ultrasound
- ○
- The same kind of tubes should be used consistently throughout the whole estrus cycle monitoring (either plasma or serum, according to the laboratory recommendations). If both options are available, the same medium should be used for the entire cycle. Tubes containing gel separators should be avoided [58];
- ○
- Lipemic plasma/serum should not be used [56];
- ○
- Blood samples should be collected at the same time (either consistently in the morning or the afternoon) to minimize variability [58].
2.6. Mating and Insemination Management
- ○
- Natural matings or intravaginal insemination: days 1 and 3 after ovulation;
- ○
- Transcervical insemination with fresh semen: days 1 and 3 after ovulation if two inseminations are possible, or day 2 or 3 if only one is possible;
- ○
- Transcervical insemination with chilled semen: days 2 and/or 3 after ovulation;
- ○
- Transcervical insemination with frozen semen: days 3–3.5 after ovulation.
2.7. Impact of Stress on Fertility
- Psychological stress, e.g., related to housing conditions (e.g., confinement, lack of environmental enrichment), social hierarchy in the group, or the relationship between owner and animal;
- Environmental stress, including thermal stress (particularly heat stress) and suboptimal nutrition;
- Metabolic or physiological stress that may be caused by underlying systemic disease or chronic inflammation.
3. Female Preconceptional Causes of Infertility
3.1. Bitches with Irregular Cycles
3.1.1. Ovarian Causes
3.1.2. Endocrine Causes
3.2. Bitches with Regular Cycles
3.2.1. Timing of Breeding and Idiopathic Infertility
3.2.2. Uterine Environment
3.2.3. Infectious Diseases
4. Male Preconceptional Cases of Infertility
- General physical examination: assessment of general health to detect systemic diseases (e.g., endocrine disorders such as hypothyroidism) or hereditary diseases that could affect semen quality or the health of the offspring;
- Genital examination and semen analysis: assessment of reproductive anatomy (e.g., testicular size, penile structure) and performance of a semen analysis (volume, concentration, motility, morphology) to confirm functional fertility.
4.1. Mating Disorders
4.1.1. Anatomical Defects
4.1.2. Pain and Behavior Disorders
4.1.3. Ejaculatory Disorders
4.2. Semen Defects
- -
- More than 65% of the ejaculate’s sperm cells are progressive;
- -
- More than 200 million progressive motile sperm are inseminated;
- -
- The number of sperm cells in the ejaculate is high (when the quality of the ejaculate is moderate, it could be compensated by the quantity);
- -
- The percentage of morphologically normal sperm cells inseminated is over 60–70%.
4.2.1. Testicular, Epididymal, and Prostatic Causes
4.2.2. Infectious Causes
4.2.3. Hormonal Tests
4.2.4. Nutrition
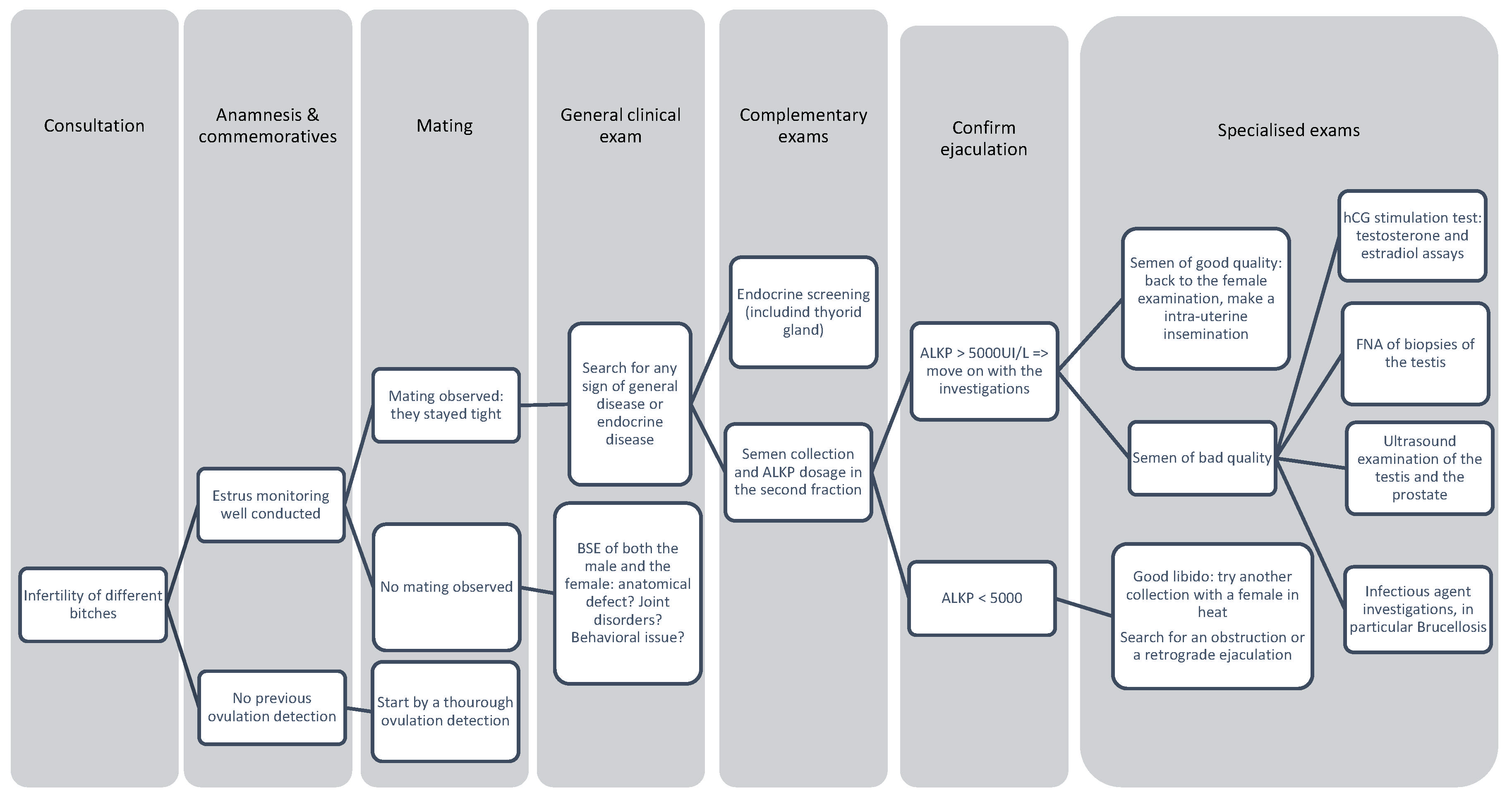
4.3. Decision Tree
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization Infertility. Available online: https://www.who.int/news-room/fact-sheets/detail/infertility (accessed on 23 May 2024).
- Wilborn, R.R.; Maxwell, H.S. Clinical Approaches to Infertility in the Bitch. Vet. Clin. Small Anim. Pract. 2012, 42, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.A.; Feldman, E.; Davidson, A. Evaluation of Infertility in the Bitch. Clin. Tech. Small Anim. Pract. 2002, 17, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Renton, J.; Munro, C.; Heathcote, R.; Carmichael, S. Some Aspects of the Aetiology, Diagnosis and Treatment of Infertility in the Bitch. J. Reprod. Fertil. 1981, 61, 289–294. [Google Scholar] [CrossRef]
- Chastant-Maillard, S.; Guillemot, C.; Feugier, A.; Mariani, C.; Grellet, A.; Mila, H. Reproductive Performance and Pre-Weaning Mortality: Preliminary Analysis of 27,221 Purebred Female Dogs and 204,537 Puppies in France. Reprod. Domest. Anim. 2017, 52, 158–162. [Google Scholar] [CrossRef]
- Mantziaras, G.; Zakosek Pipan, M. “My Bitch Is Empty!” An Overview of the Reasons for Pregnancy Loss in Dogs. Vet. Sci. 2025, 12, 127. [Google Scholar] [CrossRef]
- Feldman, E.C.; Nelson, R.W. Infertility, Associated Breeding Disorders, and Disorders Os Sexual Development. In Canine and Feline Endocrinology and Reproduction, 3rd ed.; Elsevier: Maryland Heights, MO, USA, 2004; pp. 868–900. [Google Scholar]
- Johnston, S.D.; Kustritz, M.V.; Olson, P.N.S. Clinical Approach to Infertility in the Bitch. In Canine and Feline Theriogenology, 1st ed.; Saunders: Saunders Park, PA, USA, 2001; pp. 257–273. [Google Scholar]
- England, G.C.W.; Moxon, R.; Freeman, S.L. Delayed Uterine Fluid Clearance and Reduced Uterine Perfusion in Bitches with Endometrial Hyperplasia and Clinical Management with Postmating Antibiotic. Theriogenology 2012, 78, 1611–1617. [Google Scholar] [CrossRef]
- England, G.C.W.; Rijsselaere, T.; Campbell, A.; Moxon, R.; Freeman, S.L. Normal and Abnormal Response to Sperm Deposition in Female Dogs: A Review and New Hypotheses for Endometritis. Theriogenology 2021, 159, 176–183. [Google Scholar] [CrossRef]
- Mir, F.; Fontaine, E.; Albaric, O.; Greer, M.; Vannier, F.; Schlafer, D.H.; Fontbonne, A. Findings in Uterine Biopsies Obtained by Laparotomy from Bitches with Unexplained Infertility or Pregnancy Loss: An Observational Study. Theriogenology 2013, 79, 312–322. [Google Scholar] [CrossRef]
- Moxon, R.; Whiteside, H.; England, G.C.W. Prevalence of Ultrasound-Determined Cystic Endometrial Hyperplasia and the Relationship with Age in Dogs. Theriogenology 2016, 86, 976–980. [Google Scholar] [CrossRef]
- Romagnoli, S.; Concannon, P.W. Clinical Use of Progestins in Bitches and Queens: A Review; IVIS: Singapore, 2003. [Google Scholar]
- Johnston, S.D.; Root, M.V.; Olson, P.N. Disorders of the Canine Uterus and Uterine Tubes (Oviducts). In Canine and Feline Theriogenology; National Library of Medicine: Bethesda, MA, USA, 2001; pp. 206–224. [Google Scholar]
- Berky, A.V.; Townsend, W. The Relationship between the Prevalence of Uterine Lesions and the Use of Medroxyprogesterone Acetate for Canine Population Control. Aust. Vet. J. 1993, 70, 249–250. [Google Scholar] [CrossRef]
- De Bosschere, H.; Ducatelle, R.; Vermeirsch, H.; Van Den Broeck, W.; Coryn, M. Cystic Endometrial Hyperplasia- Pyometra Complex in the Bitch: Should the Two Entities Be Disconnected? Theriogenology 2001, 55, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Hagman, R. Pyometra in Small Animals 3.0. Vet. Clin. Small Anim. Pract. 2023, 53, 1223–1254. [Google Scholar] [CrossRef] [PubMed]
- Verstegen, J.; Dhaliwal, G.; Verstegen-Onclin, K. Mucometra, Cystic Endometrial Hyperplasia, and Pyometra in the Bitch: Advances in Treatment and Assessment of Future Reproductive Success. Theriogenology 2008, 70, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Borge, K.S.; Tønnessen, R.; Nødtvedt, A.; Indrebø, A. Litter Size at Birth in Purebred Dogs—A Retrospective Study of 224 Breeds; National Library of Medicine: Bethesda, MA, USA, 2011. [Google Scholar]
- Martins, A.C.L.; Vaz, M.A.; Macedo, M.M.; Santos, R.L.; Galdino, C.A.B.; Wenceslau, R.R.; Valle, G.R. Maternal Age, Paternal Age, and Litter Size Interact to Affect the Offspring Sex Ratio of German Shepherd Dogs. Theriogenology 2019, 135, 169–173. [Google Scholar] [CrossRef]
- Abah, K.O.; Fontbonne, A.; Partyka, A.; Nizanski, W. Effect of Male Age on Semen Quality in Domestic Animals: Potential for Advanced Functional and Translational Research? Vet. Res. Commun. 2023, 47, 1125–1137. [Google Scholar] [CrossRef]
- Goericke-Pesch, S.; Failing, K. Retrospective Analysis of Canine Semen Evaluations with Special Emphasis on the Use of the Hypoosmotic Swelling (HOS) Test and Acrosomal Evaluation Using Spermac®. Reprod. Domest. Anim. 2013, 48, 213–217. [Google Scholar] [CrossRef]
- Tesi, M.; Sabatini, C.; Vannozzi, I.; Di Petta, G.; Panzani, D.; Camillo, F.; Rota, A. Variables Affecting Semen Quality and Its Relation to Fertility in the Dog: A Retrospective Study. Theriogenology 2018, 118, 34–39. [Google Scholar] [CrossRef]
- Hesser, A.; Darr, C.; Gonzales, K.; Power, H.; Scanlan, T.; Thompson, J.; Love, C.; Christensen, B.; Meyers, S. Semen Evaluation and Fertility Assessment in a Purebred Dog Breeding Facility. Theriogenology 2017, 87, 115–123. [Google Scholar] [CrossRef]
- Salvado, J.; Catilina, D.; Borges, P.; Simoes, J.; Martins-Bessa, A. Influence of Two Collection Frequency Intervals on Sperm Quality of Standard and Miniature Bull Terriers during Short Breeding Periods: A Clinical Field Study. Vet. World 2024, 17, 820–828. [Google Scholar] [CrossRef]
- Bannasch, D.; Famula, T.; Donner, J.; Anderson, H.; Honkanen, L.; Batcher, K.; Safra, N.; Thomasy, S.; Rebhun, R. The Effect of Inbreeding, Body Size and Morphology on Health in Dog Breeds. Canine Genet. Epidemiol. 2021, 8, 12. [Google Scholar] [CrossRef]
- Leroy, G.; Phocas, F.; Hedan, B.; Verrier, E.; Rognon, X. Inbreeding Impact on Litter Size and Survival in Selected Canine Breeds. Vet. J. 2015, 203, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Urfer, S.R. Inbreeding and Fertility in Irish Wolfhounds in Sweden: 1976 to 2007. Acta Vet. Scand. 2009, 51, 21. [Google Scholar] [CrossRef] [PubMed]
- Chu, E.T.; Simpson, M.J.; Diehl, K.; Page, R.L.; Sams, A.J.; Boyko, A.R. Inbreeding Depression Causes Reduced Fecundity in Golden Retrievers. Mamm. Genome 2019, 30, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Marelli, S.P.; Beccaglia, M.; Bagnato, A.; Strillacci, M.G. Canine Fertility: The Consequences of Selection for Special Traits. Reprod. Domest. Anim. 2020, 55, 4–9. [Google Scholar] [CrossRef]
- Sones, J.; Balogh, O. Body Condition and Fertility in Dogs. Vet. Clin. Small Anim. Pract. 2023, 53, 1031–1045. [Google Scholar] [CrossRef]
- Martin, L.J.M.; Siliart, B.; Dumon, H.J.W.; Nguyen, P.G. Hormonal Disturbances Associated with Obesity in Dogs. Anim. Physiol. Nutr. 2006, 90, 355–360. [Google Scholar] [CrossRef]
- Barstow, C.; Wilborn, R.R.; Johnson, A.K. Breeding Soundness Examination of the Bitch. Vet. Clin. Small Anim. Pract. 2018, 48, 547–566. [Google Scholar] [CrossRef]
- Kim, H.; Wakshlag, J.J. Nutrition and Theriogenology. Vet. Clin. Small Anim. Pract. 2023, 53, 1083–1098. [Google Scholar] [CrossRef]
- Concannon, P.W. Reproductive Cycles of the Domestic Bitch. Anim. Reprod. Sci. 2011, 124, 200–210. [Google Scholar] [CrossRef]
- Kutzler, M.A. Estrus Induction and Synchronization in Canids and Felids. Theriogenology 2007, 68, 354–374. [Google Scholar] [CrossRef]
- England, G.; Concannon, P.W. Determination of the Optimal Breeding Time in the Bitch: Basic Considerations. In Recent Advances in Small Animal Reproduction; Concannon, P.W., England, G., Verstegen, J., Linde-Forsberg, C., Eds.; International Veterinary Information Service: Ithaca, NY, USA, 2002; Available online: https://www.ivis.org/library/recent-advances-small-animal-reproduction/determination-of-optimal-breeding-time-bitch (accessed on 29 June 2025).
- Bizikova, P.; Pucheu-Haston, C.M.; Eisenschenk, M.N.C.; Marsella, R.; Nuttall, T.; Santoro, D. Review: Role of Genetics and the Environment in the Pathogenesis of Canine Atopic Dermatitis. Vet. Dermatol. 2015, 26, 95. [Google Scholar] [CrossRef] [PubMed]
- Løset, M.; Brown, S.J.; Saunes, M.; Hveem, K. Genetics of Atopic Dermatitis: From DNA Sequence to Clinical Relevance. Dermatology 2019, 235, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Rostaher, A.; Dolf, G.; Fischer, N.M.; Silaghi, C.; Akdis, C.; Zwickl, L.; Audergon, S.; Favrot, C. Atopic Dermatitis in a Cohort of West Highland White Terriers in Switzerland. Part II: Estimates of Early Life Factors and Heritability. Vet. Dermatol. 2020, 31, 276. [Google Scholar] [CrossRef] [PubMed]
- Lévy, X. Videovaginoscopy of the Canine Vagina. Reprod. Domest. Anim. 2016, 51, 31–36. [Google Scholar] [CrossRef]
- Schutte, A.P. Canine Vaginal Cytology—III Compilation and Evaluation of Cellular Indices. J. Small Anim. Pract. 1967, 8, 313–317. [Google Scholar] [CrossRef]
- Schutte, A.P. Canine Vaginal Cytology—I Technique and Cytological Morphology*. J. Small Anim. Pract. 1967, 8, 301–306. [Google Scholar] [CrossRef]
- Goodman, M. Demystifying Ovulation Timing. Clin. Tech. Small Anim. Pract. 2002, 17, 97–103. [Google Scholar] [CrossRef]
- Johnston, S.D.; Kustritz, M.V.; Olson, P.N.S. Vaginal Cytology. In Canine and Feline Theriogenology, 1st ed.; Saunders: Saunders Park, PA, USA, 2001; pp. 32–41. [Google Scholar]
- Schutte, A.P. Pergamon Press Ltd. Printed in Great Britain Canine Vaginal Cytology—II Cyclic Changes. J. Small Anim. Pract. 1967, 8, 307–311. [Google Scholar] [CrossRef]
- Post, K. Canine Vaginal Cytology during the Estrous Cycle. Can. Vet. J. 1985, 26, 101–104. [Google Scholar]
- Groppetti, D.; Pecile, A.; Barbero, C.; Martino, P.A. Vaginal Bacterial Flora and Cytology in Proestrous Bitches: Role on Fertility. Theriogenology 2012, 77, 1549–1556. [Google Scholar] [CrossRef]
- Leps, A.S.; Klein, B.; Schneider, M.; Meyer, C.; Šoba, A.; Simon, C.; Dyachenko, V.; Siesenop, U.; Verspohl, J.; Goericke-Pesch, S. The Canine Vaginal Flora: A Large-Cohort Retrospective Study. Vet. Sci. 2024, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Gloria, A.; Contri, A.; Carluccio, A.; Robbe, D. Blood Periovulatory Progesterone Quantification Using Different Techniques in the Dog. Anim. Reprod. Sci. 2018, 192, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Hollinshead, F.; Hanlon, D. Normal Progesterone Profiles during Estrus in the Bitch: A Prospective Analysis of 1420 Estrous Cycles. Theriogenology 2019, 125, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Lévy, X.; Fontbonne, A. Determining the Optimal Time of Mating in Bitches: Particularities. Rev. Bras. Reprodução Anim. 2007, 31, 128–134. [Google Scholar]
- Fontbonne, A.; Maenhoudt, C.; Thoumire, S.; Roos, J.; Niewiadomska, Z.; Robiteau, G.; Rousselière, E.; Buronfosse, T. Evaluation of Surface Plasmon Field-Enhanced Fluorescence Spectroscopy for Rapid Measurement of Progesterone Concentration in Bitches. Am. J. Vet. Res. 2021, 82, 417–424. [Google Scholar] [CrossRef]
- Brugger, N.; Otzdorff, C.; Walter, B.; Hoffmann, B.; Braun, J. Quantitative Determination of Progesterone (P4) in Canine Blood Serum Using an Enzyme-linked Fluorescence Assay. Reprod. Domest. Anim. 2011, 46, 870–873. [Google Scholar] [CrossRef]
- Zuercher, J.; Boes, K.M.; Balogh, O.; Helms, A.B.; Cecere, J.T. Comparison of a Point-of-Care Analyzer With a Chemiluminescent Immunoassay for Serum Progesterone Measurement in Breeding Management of the Bitch. Front. Vet. Sci. 2021, 8, 660923. [Google Scholar] [CrossRef]
- Østergård Jensen, S.; Öberg, J.; Alm, H.; Holst, B.S. Validation of a Dry-slide Immunoassay for Progesterone Analysis in Canine Plasma in a Clinical Setting. Vet. Clin. Pathol. 2022, 51, 524–532. [Google Scholar] [CrossRef]
- Tahir, M.Z.; Thoumire, S.; Raffaelli, M.; Grimard, B.; Reynaud, K.; Chastant-Maillard, S. Effect of Blood Handling Conditions on Progesterone Assay Results Obtained by Chemiluminescence in the Bitch. Domest. Anim. Endocrinol. 2013, 45, 141–144. [Google Scholar] [CrossRef]
- Thuróczy, J.; Wölfling, A.; Tibold, A.; Balogh, L.; Jánoki, G.; Solti, L. Effect of Anticoagulants and Sampling Time on Results of Progesterone Determination in Canine Blood Samples. Reprod. Domest. Anim. 2003, 38, 386–389. [Google Scholar] [CrossRef]
- Jurczak, A.; Janowski, T. Arterial Ovarian Blood Flow in the Periovulatory Period of GnRH-Induced and Spontaneous Estrous Cycles of Bitches. Theriogenology 2018, 119, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, L.H.; Nykamp, S.G.; Brisson, B.A.; Madan, P.; Gartley, C.J. An Evaluation of B-Mode and Color Doppler Ultrasonography for Detecting Periovulatory Events in the Bitch. Theriogenology 2013, 79, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.; Abdeldjelil, M.C.; Bougherara, H.; Khellaf, D.; Aissi, A. Color-Doppler Ultrasonography to Predict the Moment of Ovulation in the Bitch. Adv. Anim. Vet. Sci. 2020, 8, 782–787. [Google Scholar] [CrossRef]
- Mantziaras, G.; Luvoni, G.C. Advanced Ultrasound Techniques in Small Animal Reproduction Imaging. Reprod. Dom. Anim. 2020, 55, 17–25. [Google Scholar] [CrossRef]
- Sinagra, L.; Orlandi, R.; Caspanello, T.; Troisi, A.; Iannelli, N.M.; Vallesi, E.; Pettina, G.; Bargellini, P.; De Majo, M.; Boiti, C.; et al. Contrast-Enhanced Ultrasonography (CEUS) in Imaging of the Reproductive System in Dogs: A Literature Review. Animals 2023, 13, 1615. [Google Scholar] [CrossRef]
- Nogueira Aires, L.P.; Gasser, B.; Silva, P.; Del’Aguila-Silva, P.; Yamada, D.I.; Carneiro, R.K.; Bressianini Lima, B.; Padilha-Nakaghi, L.C.; Ramirez Uscategui, R.A.; Spada, S.; et al. Ovarian Contrast-Enhanced Ultrasonography and Doppler Fluxometry in Bitches during the Postovulatory Estrus and Corpora Lutea Formation. Theriogenology 2022, 194, 162–170. [Google Scholar] [CrossRef]
- Reynaud, K.; Fontbonne, A.; Marseloo, N.; Viaris De Lesegno, C.; Saint-Dizier, M.; Chastant-Maillard, S. In Vivo Canine Oocyte Maturation, Fertilization and Early Embryogenesis: A Review. Theriogenology 2006, 66, 1685–1693. [Google Scholar] [CrossRef]
- Reynaud, K.; Fontbonne, A.; Marseloo, N.; Thoumire, S.; Chebrout, M.; De Lesegno, C.V.; Chastant-Maillard, S. In Vivo Meiotic Resumption, Fertilization and Early Embryonic Development in the Bitch. Reproduction 2005, 130, 193–201. [Google Scholar] [CrossRef]
- Tsutsui, T.; Takahashi, F.; Hori, T.; Kawakami, E.; Concannon, P. Prolonged Duration of Fertility of Dog Ova. Reprod. Domest. Anim. 2009, 44, 230–233. [Google Scholar] [CrossRef]
- Thomassen, R.; Sanson, G.; Krogenæs, A.; Fougner, J.A.; Berg, K.A.; Farstad, W. Artificial Insemination with Frozen Semen in Dogs: A Retrospective Study of 10 Years Using a Non-Surgical Approach. Theriogenology 2006, 66, 1645–1650. [Google Scholar] [CrossRef]
- Hollinshead, F.K.; Hanlon, D.W. Factors Affecting the Reproductive Performance of Bitches: A Prospective Cohort Study Involving 1203 Inseminations with Fresh and Frozen Semen. Theriogenology 2017, 101, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Linde-Forsberg, C.; Ström Holst, B.; Govette, G. Comparison of Fertility Data from Vaginal vs Intrauterine Insemination of Frozen-Thawed Dog Semen: A Retrospective Study. Theriogenology 1999, 52, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.J.; Rous, N.R. Comparison of Endoscopic-Assisted Transcervical and Laparotomy Insemination with Frozen-Thawed Dog Semen: A Retrospective Clinical Study. Theriogenology 2014, 82, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Joonè, C. Evidence-based Practice in Canine Artificial Insemination. Aust. Vet. J 2024, 102, 377–384. [Google Scholar] [CrossRef]
- Daşkin, A.; Tekin, N.; Akçay, E. The Effect of Transcervical Intrauterine and Intravaginal Insemination Methods on Fertility in Dogs. Turk. J. Vet. Anim. Sci. 2003, 27, 235–239. [Google Scholar]
- Mason, S.J. Current Review of Artificial Insemination in Dogs. Vet. Clin. Small Anim. Pract. 2018, 48, 567–580. [Google Scholar] [CrossRef]
- Von Borell, E.; Dobson, H.; Prunier, A. Stress, Behaviour and Reproductive Performance in Female Cattle and Pigs. Horm. Behav. 2007, 52, 130–138. [Google Scholar] [CrossRef]
- Sominsky, L.; Hodgson, D.M.; McLaughlin, E.A.; Smith, R.; Wall, H.M.; Spencer, S.J. Linking Stress and Infertility: A Novel Role for Ghrelin. Endocr. Rev. 2017, 38, 432–467. [Google Scholar] [CrossRef]
- Divyashree, S.; Yajurvedi, H.N. Chronic Stress Effects and Their Reversibility on the Fallopian Tubes and Uterus in Rats. Reprod. Fertil. Dev. 2018, 30, 380. [Google Scholar] [CrossRef]
- Johnston, S.D.; Kustritz, M.V.; Olson, P.N.S. The Canine Estrus Cycle. In Canine and Feline Theriogenology, 1st ed.; Saunders: Saunders Park, PA, USA, 2001; pp. 16–31. [Google Scholar]
- Meyers-Wallen, V.N. Unusual and Abnormal Canine Estrous Cycles. Theriogenology 2007, 68, 1205–1210. [Google Scholar] [CrossRef]
- Johnston, S.D.; Kustritz, M.V.; Olson, P.N.S. Disorders of the Canine Ovary. In Canine and Feline Theriogenology, 1st ed.; Saunders: Saunders Park, PA, USA, 2001; pp. 193–205. [Google Scholar]
- Dow, C. Ovarian Abnormalities in the Bitch. J. Comp. Pathol. Ther. 1960, 70, 59-IN2. [Google Scholar] [CrossRef] [PubMed]
- Arlt, S.; Haimerl, P. Cystic Ovaries and Ovarian Neoplasia in the Female Dog—A Systematic Review. Reprod. Domest. Anim. 2016, 51, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, J.K.; Patra, M.K.; Singh, L.K.; Saxena, A.C.; De, U.K.; Singh, V.; Mathesh, K.; Kumar, H.; Krishnaswamy, N. Ovarian Cysts in the Bitch: An Update. Top. Companion Anim. Med. 2021, 43, 100511. [Google Scholar] [CrossRef] [PubMed]
- Knauf, Y.; Köhler, K.; Knauf, S.; Wehrend, A. Histological Classification of Canine Ovarian Cyst Types with Reference to Medical History. J. Vet. Sci. 2018, 19, 725. [Google Scholar] [CrossRef]
- Arlt, S.; Spankowsky, S.; Heuwieser, W. Follicular Cysts and Prolonged Oestrus in a Female Dog after Administration of a Deslorelin Implant. N. Z. Vet. J. 2011, 59, 87–91. [Google Scholar] [CrossRef]
- Knauf, Y.; Failing, K.; Knauf, S.; Wehrend, A. Treatment of bitches with ovarian cysts using human chorionic gonadotropin-releasing hormone analogue. A case series of 30 bitches. Tierarztl Prax Ausg K Kleintiere Heimtiere 2013, 41, 93–100. [Google Scholar]
- Nilesen, S.W.; Misdorp, W.; McEntee, K. Tumours of the Ovary. Bull. World Health Organ. 1976, 53, 203–215. [Google Scholar]
- Russo, M.; England, G.C.W.; Catone, G.; Marino, G. Imaging of Canine Neoplastic Reproductive Disorders. Animals 2021, 11, 1213. [Google Scholar] [CrossRef]
- Goto, S.; Iwasaki, R.; Sakai, H.; Mori, T. A Retrospective Analysis on the Outcome of 18 Dogs with Malignant Ovarian Tumours. Vet. Comp. Oncol. 2021, 19, 442–450. [Google Scholar] [CrossRef]
- Meyers-Wallen, V.N. Gonadal and Sex Differentiation Abnormalities of Dogs and Cats. Sex. Dev. 2012, 6, 46–60. [Google Scholar] [CrossRef]
- Roos, J.; Fontbonne, A. Le Syndrome de Rémanence Ovarienne Chez La Chienne: Mécanismes, Clinique et Diagnostic. Le Point Vétérinaire 2017, 48, 28–33. [Google Scholar]
- Cortese, L.; Ouva, G.; Verstegen, J.; Ciaramella, P.; Persechino, A. Hyperprolactinaemia and Galactorrhoea Associated with Primary Hypothyroidism in a Bitch. J. Small Anim. Pract. 1997, 38, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Buckrell, B.C.; Johnson, W.H. Anestrus and Spontaneous Galactorrhea in a Hypothyroid Bitch. Can. Vet. J. 1986, 27, 204–205. [Google Scholar]
- Peter, A.T.; Gaines, J.D.; Smith, C.L. Association of Weak Estrual Signs and Irregular Estrous Cycles with Hypothyroidism in a Bitch. Can. Vet. J. 1989, 30, 957–958. [Google Scholar]
- Panciera, D.L.; Purswell, B.J.; Kolster, K.A. Effect of Short-Term Hypothyroidism on Reproduction in the Bitch. Theriogenology 2007, 68, 316–321. [Google Scholar] [CrossRef]
- Segalini, V.; Hericher, T.; Grellet, A.; Rosenberg, D.; Garnier, F.; Fontbonne, A. Thyroid Function and Infertility in the Dog: A Survey in Five Breeds. Reprod. Domest. Anim. 2009, 44, 211–213. [Google Scholar] [CrossRef]
- Sontas, B.H.; Schwendenwein, I.; Schäfer-Somi, S. Primary Anestrus Due to Dietary Hyperthyroidism in a Miniature Pinscher Bitch. Can. Vet. J. 2014, 55, 781–785. [Google Scholar]
- McIntyre, R.L.; Levy, J.K.; Roberts, J.F.; Reep, R.L. Developmental Uterine Anomalies in Cats and Dogs Undergoing Elective Ovariohysterectomy. J. Am. Vet. Med. Assoc. 2010, 237, 542–546. [Google Scholar] [CrossRef]
- Fontaine, E.; Levy, X.; Grellet, A.; Luc, A.; Bernex, F.; Boulouis, H.; Fontbonne, A. Diagnosis of Endometritis in the Bitch: A New Approach. Reprod. Domest. Anim. 2009, 44, 196–199. [Google Scholar] [CrossRef]
- Schlafer, D. Diseases of the Canine Uterus. Reprod. Domest. Anim. 2012, 47, 318–322. [Google Scholar] [CrossRef]
- Christensen, B.; Schlafer, D.; Agnew, D.; Wang, C.; Kozlowski, C.; Asa, C. Diagnostic Value of Transcervical Endometrial Biopsies in Domestic Dogs Compared with Full-Thickness Uterine Sections. Reprod. Domest. Anim. 2012, 47, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Pascottini, O.B.; Aurich, C.; England, G.; Grahofer, A. General and Comparative Aspects of Endometritis in Domestic Species: A Review. Reprod. Domest. Anim. 2023, 58, 49–71. [Google Scholar] [CrossRef]
- Orfanou, D.; Ververidis, H.; Pourlis, A.; Fragkou, I.; Kokoli, A.; Boscos, C.; Taitzoglou, I.; Tzora, A.; Nerou, C.; Athanasiou, L.; et al. Post-Partum Involution of the Canine Uterus—Gross Anatomical and Histological Features. Reprod. Domest. Anim. 2009, 44, 152–155. [Google Scholar] [CrossRef]
- Banchi, P.; Colitti, B.; Del Carro, A.; Corrò, M.; Bertero, A.; Ala, U.; Del Carro, A.; Van Soom, A.; Bertolotti, L.; Rota, A. Challenging the Hypothesis of in Utero Microbiota Acquisition in Healthy Canine and Feline Pregnancies at Term: Preliminary Data. Vet. Sci. 2023, 10, 331. [Google Scholar] [CrossRef] [PubMed]
- Gil-Miranda, A.; Macnicol, J.; Orellana-Guerrero, D.; Samper, J.C.; Gomez, D.E. Reproductive Tract Microbiota of Mares. Vet. Sci. 2024, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Vomstein, K.; Krog, M.C.; Wrønding, T.; Nielsen, H.S. The Microbiome in Recurrent Pregnancy Loss—A Scoping Review. J. Reprod. Immunol. 2024, 163, 104251. [Google Scholar] [CrossRef]
- Vitale, S.G.; Ferrari, F.; Ciebiera, M.; Zgliczyńska, M.; Rapisarda, A.M.C.; Vecchio, G.M.; Pino, A.; Angelico, G.; Knafel, A.; Riemma, G.; et al. The Role of Genital Tract Microbiome in Fertility: A Systematic Review. Int. J. Mol. Sci. 2021, 23, 180. [Google Scholar] [CrossRef]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence That the Endometrial Microbiota Has an Effect on Implantation Success or Failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef]
- Toson, B.; Simon, C.; Moreno, I. The Endometrial Microbiome and Its Impact on Human Conception. Int. J. Mol. Sci. 2022, 23, 485. [Google Scholar] [CrossRef]
- Pretzer, S.D. Bacterial and Protozoal Causes of Pregnancy Loss in the Bitch and Queen. Theriogenology 2008, 70, 320–326. [Google Scholar] [CrossRef]
- Verstegen, J.; Dhaliwal, G.; Verstegen-Onclin, K. Canine and Feline Pregnancy Loss Due to Viral and Non-Infectious Causes: A Review. Theriogenology 2008, 70, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Sebzda, M.K.; Kauffman, L.K. Update on Brucella canis. Vet. Clin. Small Anim. Pract. 2023, 53, 1047–1062. [Google Scholar] [CrossRef] [PubMed]
- Djokic, V.; Freddi, L.; De Massis, F.; Lahti, E.; Van Den Esker, M.H.; Whatmore, A.; Haughey, A.; Ferreira, A.C.; Garofolo, G.; Melzer, F.; et al. The Emergence of Brucella canis as a Public Health Threat in Europe: What We Know and What We Need to Learn. Emerg. Microbes Infect. 2023, 12, 2249126. [Google Scholar] [CrossRef]
- Lopate, C. The Problem Stud Dog. Vet. Clin. Small Anim. Pract. 2012, 42, 469–488. [Google Scholar] [CrossRef]
- Mason, S.J. An Update on Male Canine Infertility. Vet. Clin. Small Anim. Pract. 2023, 53, 1063–1081. [Google Scholar] [CrossRef]
- Arlt, S.P.; Reichler, I.M.; Herbel, J.; Schäfer-Somi, S.; Riege, L.; Leber, J.; Frehner, B. Diagnostic Tests in Canine Andrology—What Do They Really Tell Us about Fertility? Theriogenology 2023, 196, 150–156. [Google Scholar] [CrossRef]
- Roos, J.; Fontbonne, A. Infécondité Du Chien Mâle. EMC—Vétérinaire 2019, 16, 1–13. [Google Scholar]
- Christensen, B.W.; Meyers, S. Canine Semen Evaluation and Processing. Vet. Clin. Small Anim. Pract. 2023, 53, 921–930. [Google Scholar] [CrossRef]
- Johnston, S.D.; Kustritz, M.V.; Olson, P.N.S. Clinical Approach to Infertility in the Male Dog. In Canine and Feline Theriogenology, 1st ed.; Saunders: Saunders Park, PA, USA, 2001; pp. 370–406. [Google Scholar]
- Beaufays, F.; Onclin, K.; Verstegen, J. Retrograde Ejaculation Occurs in the Dog, but Can Be Prevented by Pre-Treatment with Phenylpropanolamine: A Urodynamic Study. Theriogenology 2008, 70, 1057–1064. [Google Scholar] [CrossRef]
- Kawakami, E.; Koga, H.; Hori, T.; Tsutsui, T. Sperm Granuloma and Sperm Agglutination in a Dog with Asthenozoospermia. J. Vet. Med. Sci. 2003, 65, 409–412. [Google Scholar] [CrossRef]
- Hesser, A.C.; Davidson, A.P. Spermatocele in a South African Boerboel Dog. Top. Companion Anim. Med. 2015, 30, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.M.M.; Fernandez, P.G.; Muela, M.S. Sperm Granuloma in the Dog: Complication of Vasectomy. J. Small Anim. Pract. 1996, 37, 392–393. [Google Scholar] [CrossRef] [PubMed]
- Batista-Arteaga, M.; Santana, M.; Lozano, O.; Niño, T.; Alamo, D.; Rodríguez, F. Bilateral Epididymal Sperm Granulomas Following Urethrostomy in a German Shepherd Dog. Reprod. Domest. Anim. 2011, 46, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Kutzler, M.A.; Solter, P.F.; Hoffman, W.E.; Volkmann, D.H. Characterization and Localization of Alkaline Phosphatase in Canine Seminal Plasma and Gonadal Tissues. Theriogenology 2003, 60, 299–306. [Google Scholar] [CrossRef]
- Schäfer-Somi, S.; Fröhlich, T.; Schwendenwein, I. Measurement of Alkaline Phosphatase in Canine Seminal Plasma—An Update. Reprod. Domest. Anim. 2013, 48, e10–e12. [Google Scholar] [CrossRef]
- Burgess, D.; Mitchell, K.; Thomas, P. Coeliotomy-assisted Intrauterine Insemination in Dogs: A Study of 238 Inseminations. Aust. Vet. J. 2012, 90, 283–290. [Google Scholar] [CrossRef]
- Krakowski, L.; Wąchocka, A.; Brodzki, P.; Wrona, Z.; Piech, T.; Wawron, W.; Chałabis-Mazurek, A. Sperm Quality and Selected Biochemical Parameters of Seminal Fluid in Dogs with Benign Prostatic Hyperplasia. Anim. Reprod. Sci. 2015, 160, 120–125. [Google Scholar] [CrossRef]
- Flores, R.; Angrimani, D.; Rui, B.; Brito, M.; Abreu, R.; Vannucchi, C. The Influence of Benign Prostatic Hyperplasia on Sperm Morphological Features and Sperm DNA Integrity in Dogs. Reprod. Domest. Anim. 2017, 52, 310–315. [Google Scholar] [CrossRef]
- Aquino-Cortez, A.; Pinheiro, B.; Silva, H.; Lima, D.; Silva, T.; Souza, M.; Viana, D.; Xavier Júnior, F.; Evangelista, J.; Brandão, F.; et al. Serum Testosterone, Sperm Quality, Cytological, Physicochemical and Biochemical Characteristics of the Prostatic Fraction of Dogs with Prostatomegaly. Reprod. Domest. Anim. 2017, 52, 998–1003. [Google Scholar] [CrossRef]
- Cunto, M.; Mariani, E.; Anicito Guido, E.; Ballotta, G.; Zambelli, D. Clinical Approach to Prostatic Diseases in the Dog. Reprod. Domest. Anim. 2019, 54, 815–822. [Google Scholar] [CrossRef]
- Cunto, M.; Ballotta, G.; Zambelli, D. Benign Prostatic Hyperplasia in the Dog. Anim. Reprod. Sci. 2022, 247, 107096. [Google Scholar] [CrossRef] [PubMed]
- Alonge, S.; Melandri, M.; Leoci, R.; Lacalandra, G.; Aiudi, G. Canine Prostate Specific Esterase (CPSE) as an Useful Biomarker in Preventive Screening Programme of Canine Prostate: CPSE Threshold Value Assessment and Its Correlation with Ultrasonographic Prostatic Abnormalities in Asymptomatic Dogs. Reprod. Domest. Anim. 2018, 53, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Melandri, M.; Alonge, S. Highlights on the Canine Prostatic Specific Esterase (CPSE): A Diagnostic and Screening Tool in Veterinary Andrology. Vet. Med. Sci 2021, 7, 35–40. [Google Scholar] [CrossRef]
- Christensen, B.W. Canine Prostate Disease. Vet. Clin. Small Anim. Pract. 2018, 48, 701–719. [Google Scholar] [CrossRef]
- Alonge, S.; Melandri, M.; Fanciullo, L.; Lacalandra, G.; Aiudi, G. Prostate Vascular Flow: The Effect of the Ejaculation on the Power Doppler Ultrasonographic Examination. Reprod. Domest. Anim. 2018, 53, 110–115. [Google Scholar] [CrossRef]
- Ferré-Dolcet, L.; Frigotto, L.; Contiero, B.; Bedin, S.; Romagnoli, S. Prostatic Fluid Composition and Semen Quality in Dogs with Benign Prostatic Hyperplasia Undergoing Treatment with Osaterone Acetate. Reprod. Domest. Anim. 2022, 57, 72–79. [Google Scholar] [CrossRef]
- Romagnoli, S.; Bonaccini, P.; Stelletta, C.; Garolla, A.; Menegazzo, M.; Foresta, C.; Mollo, A.; Milani, C.; Gelli, D. Clinical Use of Testicular Fine Needle Aspiration Cytology in Oligozoospermic and Azoospermic Dogs. Reprod. Domest. Anim. 2009, 44, 329–333. [Google Scholar] [CrossRef]
- Gouletsou, P.; Galatos, A.; Leontides, L.; Sideri, A. Impact of Fine- or Large-Needle Aspiration on Canine Testes: Clinical, In Vivo Ultrasonographic and Seminological Assessment. Reprod. Domest. Anim. 2011, 46, 712–719. [Google Scholar] [CrossRef]
- Attia, K.A.; Zaki, A.A.; Eilts, B.E.; Paccamonti, D.L.; Hosgood, G.; Dietrich, M.A.; Horohov, D.W.; Blouin, D.C. Anti-Sperm Antibodies and Seminal Characteristics after Testicular Biopsy or Epididymal Aspiration in Dogs. Theriogenology 2000, 53, 1355–1363. [Google Scholar] [CrossRef]
- Kustritz, M.V.R.; Johnston, S.D.; Olson, P.N.; Lindeman, C.J. Relationship between Inflammatory Cytology of Canine Seminal Fluid and Significant Aerobic Bacterial, Anaerobic Bacterial or Mycoplasma Cultures of Canine Seminal Fluid: 95 Cases (1987–2000). Theriogenology 2005, 64, 1333–1339. [Google Scholar] [CrossRef]
- Goericke-Pesch, S.; Weiss, R.; Wehrend, A. Bacteriological Findings in Different Fractions of Canine Ejaculates Showing Normospermia, Teratozoospermia or Azoospermia: SMALL ANIMALS. Aust. Vet. J. 2011, 89, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Banchi, P.; Bertolotti, L.; Spanoghe, L.; Ali Hassan, H.; Lannoo, J.; Domain, G.; Henzel, K.S.; Gaillard, V.; Rota, A.; Van Soom, A. Characterization of the Semen Microbiota of Healthy Stud Dogs Using 16S RNA Sequencing. Theriogenology 2024, 216, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Farahani, L.; Tharakan, T.; Yap, T.; Ramsay, J.W.; Jayasena, C.N.; Minhas, S. The Semen Microbiome and Its Impact on Sperm Function and Male Fertility: A Systematic Review and Meta-analysis. Andrology 2021, 9, 115–144. [Google Scholar] [CrossRef] [PubMed]
- Domrazek, K.; Konieczny, P.; Majka, M.; Czopowicz, M.; Cywińska, A.; Jurka, P. The Lack of the Influence of Various Species of Mycoplasma spp. on Canine Semen Quality. Theriogenology 2024, 219, 86–93. [Google Scholar] [CrossRef]
- Domrazek, K.; Konieczny, P.; Majka, M.; Czopowicz, M.; Jurka, P. The Impact of Microorganisms on Canine Semen Quality. Animals 2024, 14, 1267. [Google Scholar] [CrossRef]
- Souza, F.F.D.; Leme, D.P.; Uechi, E.; Trinca, L.A.; Lopes, M.D. Evaluation Testicular Fine Needle Aspiration Cytology and Serum Testosterone Levels in Dogs. Braz. J. Vet. Res. Anim. Sci. 2004, 41, 98–105. [Google Scholar] [CrossRef]
- Fukuda, S. Circadian Rhythm of Serum Testosterone Levels in Male Beagle Dogs—Effects of Lighting Time Zone—. Exp. Anim. 1990, 39, 65–68. [Google Scholar] [CrossRef]
- De Souza, M.B.; England, G.C.W.; Mota Filho, A.C.; Ackermann, C.L.; Sousa, C.V.S.; De Carvalho, G.G.; Silva, H.V.R.; Pinto, J.N.; Linhares, J.C.S.; Oba, E.; et al. Semen Quality, Testicular B-Mode and Doppler Ultrasound, and Serum Testosterone Concentrations in Dogs with Established Infertility. Theriogenology 2015, 84, 805–810. [Google Scholar] [CrossRef]
- Hess, R.A.; Fernandes, S.A.F.; Gomes, G.R.O.; Oliveira, C.A.; Lazari, M.F.M.; Porto, C.S. Estrogen and Its Receptors in Efferent Ductules and Epididymis. J. Androl. 2011, 32, 600–613. [Google Scholar] [CrossRef]
- Buvat, J. Hyperprolactinemia and Sexual Function in Men: A Short Review. Int. J. Impot. Res. 2003, 15, 373–377. [Google Scholar] [CrossRef]
- Mogheiseh, A.; Vara, N.; Ayaseh, M.; Jalali, P. Effects of Cabergoline on Thyroid Hormones and Semen Quality of Dog. Top. Companion Anim. Med. 2017, 32, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Domain, G.; Buczkowska, J.; Kalak, P.; Wydooghe, E.; Banchi, P.; Pascottini, O.B.; Niżański, W.; Van Soom, A. Serum Anti-Müllerian Hormone: A Potential Semen Quality Biomarker in Stud Dogs? Animals 2022, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.; Batista, M.; Alamo, D.; Cabrera, F.; González, F.; Gracia, A. Influence of Sexual Stimulation and the Administration of Human Chorionic Gonadotrophin on Plasma Testosterone Levels in Dogs. Reprod. Domest. Anim. 2012, 47, e43–e46. [Google Scholar] [CrossRef]
- Perez-Rivero, J.-J.; Martinez-Maya, J.-J.; Perez-Martinez, M.; Aguilar-Setien, A.; Garcia-Suarez, M.-D.; Serrano, H. Phytoestrogen Treatment Induces Testis Alterations in Dogs. Potential Use in Population Control. Vet. Res. Commun. 2009, 33, 87–95. [Google Scholar] [CrossRef]
- Główny, D.; Sowińska, N.; Cieślak, A.; Gogulski, M.; Konieczny, K.; Szumacher-Strabel, M. Raw diets for dogs and cats: Potential health benefits and threats. Pol. J. Vet. Sci. 2024, 27, 151–159. [Google Scholar] [CrossRef]
- Alonge, S.; Melandri, M.; Leoci, R.; Lacalandra, G.; Caira, M.; Aiudi, G. The Effect of Dietary Supplementation of Vitamin E, Selenium, Zinc, Folic Acid, and N-3 Polyunsaturated Fatty Acids on Sperm Motility and Membrane Properties in Dogs. Animals 2019, 9, 34. [Google Scholar] [CrossRef]
- Santos, M.C.; Milani, C.; Zucchini, P.; Quirino, C.R.; Romagnoli, S.; Da Cunha, I.C.N. Residual Effect after Salmon Oil Supplementation on Semen Quality and Serum Levels of Testosterone in Dogs. Reprod. Domest. Anim. 2019, 54, 1393–1399. [Google Scholar] [CrossRef]
- Santos, M.C.; Milani, C.; Zucchini, P.; Quirino, C.R.; Romagnoli, S.; Da Cunha, I.C.N. Salmon Oil Supplementation in Dogs Affects the Blood Flow of Testicular Arteries. Reprod. Domest. Anim. 2021, 56, 476–483. [Google Scholar] [CrossRef]
- Domosławska, A.; Zduńczyk, S.; Janowski, T. Improvement of Sperm Motility within One Month under Selenium and Vitamin E Supplementation in Four Infertile Dogs with Low Selenium Status. J. Vet. Res. 2019, 63, 293–297. [Google Scholar] [CrossRef]



| Elecsys® (Roche, Basel, Switzerland) | Minividas® (Biomérieux, Marcy-l’Étoile, France) | Speedreader® (Virbac, Carros, France) | AU10V® (Fujifilm, Tokyo, Japan) | Catalyst® (IDEXX, Westbrook, ME, USA) | AIA 360® (Kitvia, South San Francisco, CA, USA) | |
|---|---|---|---|---|---|---|
| Basal values | <2 ng/mL | <3 ng/mL | <2 ng/mL | <2 ng/mL | <1.9 ng/mL | <2 ng/mL |
| LH surge | 2 ng/mL | 3–6 ng/mL | 2–4 ng/mL | 2–4 ng/mL | 2–3 ng/mL | 2–4 ng/mL |
| Ovulation | 5 ng/mL | 10–12 ng/mL | 5–10 ng/mL | 4–8 ng/mL | 5–12 ng/mL | 5–10 ng/mL |
| Reference | Type of Semen Used | Timing (Days Post-Ovulation) | Number of Bitches Inseminated | Pregnancy Rate |
|---|---|---|---|---|
| Hollinshead & Hallon, 2017 [69] | Frozen | 3rd and/or 4th | 645 | 71% (and smaller litter size) |
| Fresh | 2nd, 3rd or 4th | 543 | 80% | |
| Chilled | 15 | / | ||
| Thomassen et al., 2006 [68] | Frozen intra-uterine | 2nd and/or 3rd | 665 | 75% |
| Frozen intra-vaginal | 20 | 10% | ||
| Linde-Forsberg et al., 1999 [70] | Frozen intra-uterine | Between the 2nd and 5th | 167 | 84.4% |
| Frozen intra-vaginal | 141 | 58.9% |
| Results | Possible Interpretations |
|---|---|
| Low testosterone and estrogen T0 and T1 |
|
| High estrogen T0 and T1 |
|
| Estrogens evolving like testosterone or low testosterone with high estrogens at T1 |
|
| Prolactin elevated |
|
| Molecule | Effects | Dose | References |
|---|---|---|---|
| Zinc | Prevention of chromatin decondensation. Positive effects on sperm motility. Antioxidant activity on the semen. | 3 mg/kg/d | [115,157] |
| Carnitine | Antioxidant | 50 mg/kg/d | [115] |
| Fish oil (omega-3 and omega-6 supplementation) | Improvement in sperm counts, motility, sperm viability, and fertility. Increase in testicular blood flow. | 25% docosahexaenoic acid and 10% eicosapentaenoic acid 54 mg/kg/d | [74,158,159] |
| Vitamin E and Selenium | Antioxidant: protects the testis from oxidative damage and stabilizes sperm membranes. Improvement in motility and associated fertility. | Vitamin E: 5 mg/kg/d Selenium: 0.6 mg/kg | [160] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roos-Pichenot, J.; Zakošek Pipan, M. “My Bitch Is Empty!” an Overview of the Preconceptional Causes of Infertility in Dogs. Vet. Sci. 2025, 12, 663. https://doi.org/10.3390/vetsci12070663
Roos-Pichenot J, Zakošek Pipan M. “My Bitch Is Empty!” an Overview of the Preconceptional Causes of Infertility in Dogs. Veterinary Sciences. 2025; 12(7):663. https://doi.org/10.3390/vetsci12070663
Chicago/Turabian StyleRoos-Pichenot, Juliette, and Maja Zakošek Pipan. 2025. "“My Bitch Is Empty!” an Overview of the Preconceptional Causes of Infertility in Dogs" Veterinary Sciences 12, no. 7: 663. https://doi.org/10.3390/vetsci12070663
APA StyleRoos-Pichenot, J., & Zakošek Pipan, M. (2025). “My Bitch Is Empty!” an Overview of the Preconceptional Causes of Infertility in Dogs. Veterinary Sciences, 12(7), 663. https://doi.org/10.3390/vetsci12070663







