Variants in Nucleotide Sequences; Gene Expression; and Hematological, Immune, and Antioxidant Biomarkers Linked to Pneumonia Risk in Holstein Calves
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Study Design
2.2. Blood Sampling
2.3. Hematological Parameters
2.4. Total RNA Extraction, Reverse Transcription, and Quantitative Real-Time PCR
2.5. DNA Sequencing and Polymorphism Detection
2.6. Inflammatory and Antioxidant Biomarkers
2.7. Statistical Analysis
3. Results
3.1. Clinical Examination and Hematological Profile
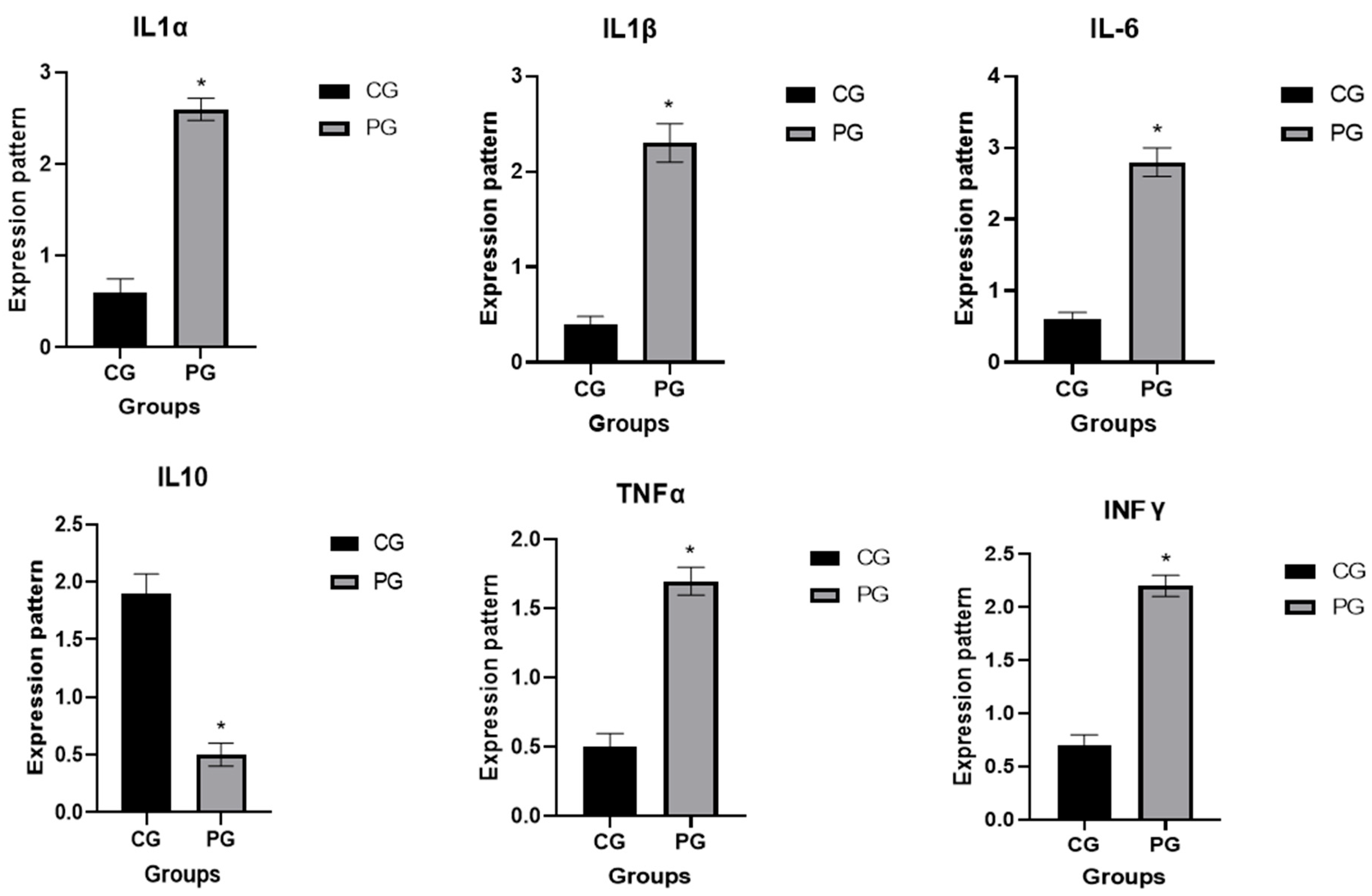
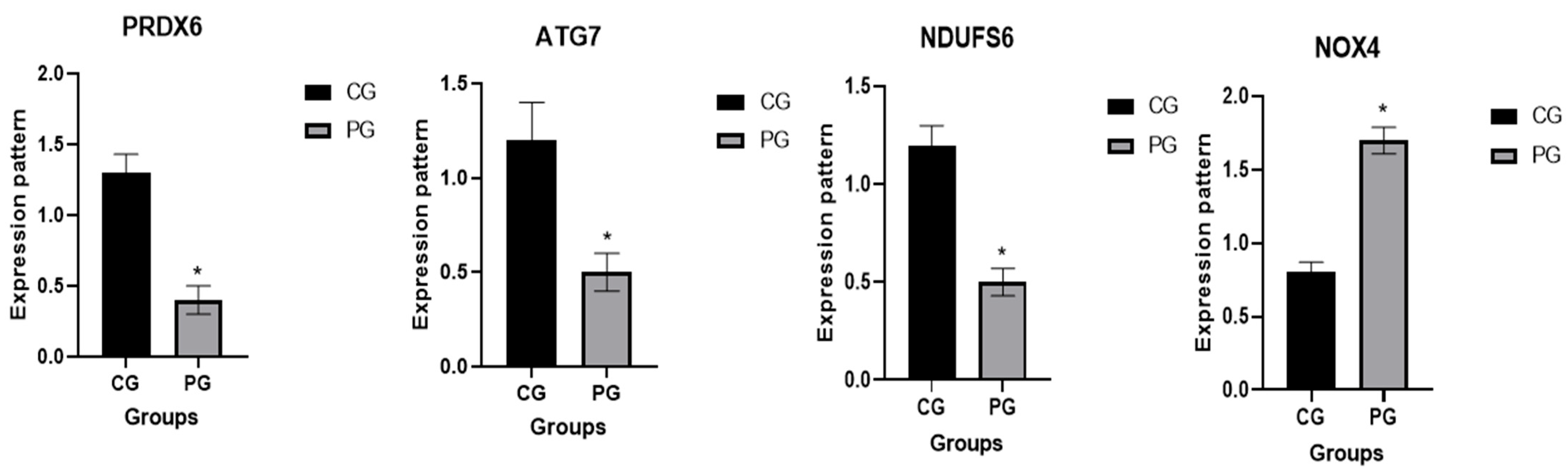
3.2. Patterns for Transcript Levels of Immune Indicators
3.3. Genetic Polymorphisms of Immune and Antioxidant Genes
3.4. Antioxidants and Immunological Profile
3.5. Association Among Gene Expression Pattern and Serum Profile of Immunological and Antioxidant Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamel, M.S.; Davidson, J.L.; Verma, M.S. Strategies for Bovine Respiratory Disease (BRD) Diagnosis and Prognosis: A Comprehensive Overview. Animals 2024, 14, 627. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.M.; More, S.J.; Clegg, T.A.; Earley, B.; O’Neill, R.G.; Johnston, D.; Gilmore, J.; Nosov, M.; McElroy, M.C.; Inzana, T.J. Risk factors associated with exposure to bovine respiratory disease pathogens during the peri-weaning period in dairy bull calves. BMC Vet. Res. 2018, 14, 53. [Google Scholar] [CrossRef]
- Buhler, V.M.; Cash, K.R.; Hurley, D.J.; Credille, B.C. Characterization and comparison of cell-mediated immune responses following ex vivo stimulation with viral and bacterial respiratory pathogens in stressed and unstressed beef calves. J. Anim. Sci. 2019, 97, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Abdallah, F.; El Damaty, H.M.; Tariq, A.; Merwad, A.M.; Alhatlani, B.Y.; Elsohaby, I. Genetic characterization of upper respiratory tract virome from nonvaccinated egyptian cow-calf operations. PLoS ONE 2022, 17, e0267036. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.K.; Pendell, D.L. Market impacts of reducing the prevalence of bovine respiratory disease in United States beef cattle feedlots. Front. Vet. Sci. 2017, 4, 189. [Google Scholar] [CrossRef]
- Donia, G.R.; El Ebissy, I.A.; Wassif, I.M. Biochemical and immunological studies on the respiratory diseases in sheep in north western coast. Eur. J. Biomed. 2018, 5, 34–41. [Google Scholar]
- Donlon, J.D.; Mee, J.F.; McAloon, C.G. Prevalence of respiratory disease in Irish preweaned dairy calves using hierarchical Bayesian latent class analysis. Front. Vet. Sci. 2023, 10, 1149929. [Google Scholar] [CrossRef]
- Teixeira, A.; McArt, J.; Bicalho, R. Efficacy of tildipirosin metaphylaxis for the prevention of respiratory disease, otitis and mortality in pre-weaned Holstein calves. Vet. J. 2017, 219, 44–48. [Google Scholar] [CrossRef]
- Zeineldin, M.; Lowe, J.; Aldridge, B. Contribution of the mucosal microbiota to bovine respiratory health. Trends Microbiol. 2019, 27, 753–770. [Google Scholar] [CrossRef]
- Constable, P.D.; Hinchcliff, K.W.; Done, S.H.; Grünberg, W. Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Dubrovsky, S.; Van Eenennaam, A.; Karle, B.; Rossitto, P.; Lehenbauer, T.; Aly, S. Epidemiology of bovine respiratory disease (BRD) in preweaned calves on California dairies: The BRD 10K study. J. Dairy Sci. 2019, 102, 7306–7319. [Google Scholar] [CrossRef]
- Bozukluhan, K.; Merhan, O.; Kiziltepe, S.; Egritag, H.E.; Akyuz, E.; Gokce, H.I. Determination of haptoglobin, some biochemical and oxidative stress parameters in calves with pneumonia. Fresenius Environ. Bull. 2021, 30, 9485–9489. [Google Scholar]
- Fulton, R.W.; Confer, A.W. Laboratory test descriptions for bovine respiratory disease diagnosis and their strengths and weaknesses: Gold standards for diagnosis, do they exist? Can. Vet. J. 2012, 53, 754–761. [Google Scholar]
- Wolfger, B.; Timsit, E.; White, B.J.; Orsel, K. A systematic review of bovine respiratory disease diagnosis focused on diagnostic confirmation, early detection, and prediction of unfavorable outcomes in feedlot cattle. Vet. Clin. Food Anim. Pract. 2015, 31, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Blakebrough-Hall, C.; Dona, A.; D’occhio, M.; McMeniman, J.; A Gonzále, L. Diagnosi di malattia respiratoria bovina in animali allevati in feedlot mediante metabolomica 1H NMR su sangue. Summa Anim. Reddito 2020, 15, 17. [Google Scholar]
- Abebe, W.; Getinet, A.; Mekonnen, H. Study on live weight, carcass weight and dressing percentage of Issa camels in Ethiopia. Rev. Méd. Vétér. 2002, 153, 713–716. [Google Scholar]
- Sharma, N.; Singh, N.; Singh, O.; Pandey, V.; Verma, P. Oxidative stress and antioxidant status during transition period in dairy cows. Asian-Australas. J. Anim. Sci. 2011, 24, 479–484. [Google Scholar] [CrossRef]
- Surai, P.F.; Earle-Payne, K. Antioxidant defences and redox homeostasis in animals. Antioxidants 2022, 11, 1012. [Google Scholar] [CrossRef]
- McGill, J.L.; Sacco, R.E. The immunology of bovine respiratory disease: Recent advancements. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 333–348. [Google Scholar] [CrossRef]
- Ramadan, M.; Ghanem, M.; El Attar, H.E.; Abdel-Raoof, Y. Evaluation of clinical and hematobiochemical alterations in naturally occurring bovine respiratory disease in feedlot cattle calves in Egypt. Benha Vet. Med. J. 2019, 36, 305–313. [Google Scholar] [CrossRef]
- El-Deeb, W.; Fayez, M.; Elsohaby, I.; Salem, M.; Alhaider, A.; Kandeel, M. Investigation of acute-phase proteins and cytokines response in goats with contagious caprine pleuropneumonia with special reference to their diagnostic accuracy. PeerJ 2020, 8, e10394. [Google Scholar] [CrossRef]
- Chitko-McKown, C.G.; Bennett, G.L.; Kuehn, L.A.; DeDonder, K.D.; Apley, M.D.; Harhay, G.P.; Clawson, M.L.; Workman, A.M.; White, B.J.; Larson, R.L. Cytokine and haptoglobin profiles from shipping through sickness and recovery in metaphylaxis-or un-treated cattle. Front. Vet. Sci. 2021, 8, 611927. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.P.; Bermingham, M.L.; Good, M.; More, S.J. Genetics of animal health and disease in cattle. Ir. Vet. J. 2011, 64, 5. [Google Scholar] [CrossRef]
- Pierce, C.F.; Brown, V.R.; Olsen, S.C.; Boggiatto, P.; Pedersen, K.; Miller, R.S.; Speidel, S.E.; Smyser, T.J. Loci associated with antibody response in feral swine (Sus scrofa) infected with Brucella suis. Front. Vet. Sci. 2020, 7, 554674. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.M.; Abou-Souliman, I.; Reyer, H.; Wimmers, K.; Rabee, A.E. New insights into the genetic predisposition of brucellosis and its effect on the gut and vaginal microbiota in goats. Sci. Rep. 2023, 13, 20086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Nie, M.-Y.; Liu, F.; Chen, J.; Wei, L.-J.; Hua, Q. Multiple gene integration to promote erythritol production on glycerol in Yarrowia lipolytica. Biotechnol. Lett. 2021, 43, 1277–1287. [Google Scholar] [CrossRef]
- Adams, L.; Schutta, C.J. Natural resistance against brucellosis: A review. Open Vet. Sci. J. 2010, 4, 61–71. [Google Scholar] [CrossRef]
- Saco, Y.; Bassols, A. Acute phase proteins in cattle and swine: A review. Vet. Clin. Pathol. 2023, 52, 50–63. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Division on Earth, Life Studies; Committee on Nutrient Requirements of Beef Cattle. Nutrient Requirements of Beef Cattle; National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Boom, R.; Sol, C.; Salimans, M.; Jansen, C.; Wertheim-van Dillen, P.; Van der Noordaa, J. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 1990, 28, 495–503. [Google Scholar] [CrossRef]
- Boesenberg-Smith, K.A.; Pessarakli, M.M.; Wolk, D.M. Assessment of DNA yield and purity: An overlooked detail of PCR troubleshooting. Clin. Microbiol. Newsl. 2012, 34, 1–6. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- El-Seedy, F.; Hassan, H.; Nabih, A.; Salem, S.; Khalifa, E.; Menshawy, A.; Abed, A. Respiratory affections in calves in upper and middle Egypt: Bacteriologic, immunologic and epidemiologic studies. Adv. Anim. Vet. Sci 2020, 8, 558–569. [Google Scholar] [CrossRef]
- Allam, T.; Said, L.; Elsayed, M.; Saleh, N. Clinical investigation of the pathogenicity of pasteurella multocida isolated from cattle in egypt regarding its effect on hematological, biochemical, and oxidant-antioxidant biomarkers as well as proinflammatory cytokines and acute phase proteins. Adv. Anim. Vet. Sci 2021, 9, 792–801. [Google Scholar] [CrossRef]
- Su, M.; She, Y.; Deng, M.; Guo, Y.; Li, Y.; Liu, G.; Sun, B.; Liu, D. Effect of Capsaicin Addition on Antioxidant Capacity, Immune Performance and Upper Respiratory Microbiota in Nursing Calves. Microorganisms 2023, 11, 1903. [Google Scholar] [CrossRef]
- El-Sheikh, M.E.-S.; El-Mekawy, M.F.; Eisa, M.I.; Abouzeid, N.Z.; Abdelmonim, M.I.; Bennour, E.M.; Yousef, S.G. Effect of two different commercial vaccines against bovine respiratory disease on cell-mediated immunity in Holstein cattle. Open Vet. J. 2024, 14, 1921–1927. [Google Scholar] [CrossRef]
- Saher, A.S.; Raza, A.; Qiu, F.; Mehmood, K.; Hussain, R.; Qayyum, A.; Idris, M.; Almutairi, M.H.; Li, K. Detection of haptoglobin and serum amyloid A as biomarkers in naturally infected Mycoplasma bovis calves. Acta Trop. 2024, 254, 107215. [Google Scholar] [CrossRef]
- Ateya, A.; Al-Sharif, M.; Abdo, M.; Fericean, L.; Essa, B. Individual genomic loci and mRNA levels of immune biomarkers associated with pneumonia susceptibility in baladi goats. Vet. Sci. 2023, 10, 185. [Google Scholar] [CrossRef]
- Abendaño Carbajo, N.; Esparza Baquer, A.; Bernales Pujana, I.; Reina, R.; De Andrés, D.; Jugo Orrantia, B.M. Gene Expression Profiling Reveals New Pathways and Genes Associated with Visna/Maedi Viral Disease. Animals 2021, 11, 1785. [Google Scholar] [CrossRef]
- Sayed, A.E.; Hafez, A.; Ateya, A.; Darwish, A.; Tahoun, A. Single nucleotide polymorphisms, gene expression and evaluation of immunological, antioxidant, and pathological parameters associated with bacterial pneumonia in Barki sheep. Ir. Vet. J. 2025, 78, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, X.; Li, Q.; Wan, K.; Gao, R.; Han, G.; Li, C.; Xu, M.; Jia, B.; Shen, X. MHC-DRB1 exon 2 polymorphism and its association with mycoplasma ovipneumonia resistance or susceptibility genotypes in sheep. J. Genet. 2020, 99, 22. [Google Scholar] [CrossRef] [PubMed]
- Leymaster, K.; Chitko-McKown, C.; Clawson, M.; Harhay, G.; Heaton, M. Effects of TMEM154 haplotypes 1 and 3 on susceptibility to ovine progressive pneumonia virus following natural exposure in sheep. J. Anim. Sci. 2013, 91, 5114–5121. [Google Scholar] [CrossRef]
- Salamaikina, S.; Karnaushkina, M.; Korchagin, V.; Litvinova, M.; Mironov, K.; Akimkin, V. TLRs Gene Polymorphisms Associated with Pneumonia before and during COVID-19 Pandemic. Diagnostics 2022, 13, 121. [Google Scholar] [CrossRef]
- Barton, N.H. Mutation and the evolution of recombination. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Kala, D.; Dhiman, G.; Yadav, V.; Krokhotin, A.; Dokholyan, N.V. Predicting the functional consequences of non-synonymous single nucleotide polymorphisms in IL8 gene. Sci. Rep. 2017, 7, 6525. [Google Scholar] [CrossRef]
- Salim, T.; Sershen, C.L.; May, E.E. Investigating the role of TNF-α and IFN-γ activation on the dynamics of iNOS gene expression in LPS stimulated macrophages. PLoS ONE 2016, 11, e0153289. [Google Scholar] [CrossRef]
- Martinez-Espinosa, I.; Serrato, J.A.; Ortiz-Quintero, B. Role of IL-10-producing natural killer cells in the regulatory mechanisms of inflammation during systemic infection. Biomolecules 2021, 12, 4. [Google Scholar] [CrossRef]
- Wang, Z.; Guan, D.; Huo, J.; Biswas, S.K.; Huang, Y.; Yang, Y.; Xu, S.; Lam, K.-P. IL-10 enhances human natural killer cell effector functions via metabolic reprogramming regulated by mTORC1 signaling. Front. Immunol. 2021, 12, 619195. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev.™ Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Darwish, A.; Ebissy, E.; Hafez, A.; Ateya, A.; El-Sayed, A. Nucleotide sequence variants, gene expression and serum profile of immune and antioxidant markers associated with bacterial diarrhea susceptibility in Barki lambs. BMC Vet. Res. 2024, 20, 462. [Google Scholar] [CrossRef] [PubMed]
- Al-Sharif, M.; Abdo, M.; Shabrawy, O.E.; El-Naga, E.M.A.; Fericean, L.; Banatean-Dunea, I.; Ateya, A. Investigating polymorphisms and expression profile of immune, antioxidant, and erythritol-related genes for limiting postparturient endometritis in Holstein cattle. Vet. Sci. 2023, 10, 370. [Google Scholar] [CrossRef]
- Wadley, A.J.; Aldred, S.; Coles, S.J. An unexplored role for Peroxiredoxin in exercise-induced redox signalling? Redox Biol. 2016, 8, 51–58. [Google Scholar] [CrossRef]
- Kirby, D.M.; Salemi, R.; Sugiana, C.; Ohtake, A.; Parry, L.; Bell, K.M.; Kirk, E.P.; Boneh, A.; Taylor, R.W.; Dahl, H.-H.M. NDUFS6 mutations are a novel cause of lethal neonatal mitochondrial complex I deficiency. J. Clin. Investig. 2004, 114, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Boussaha, M.; Esquerré, D.; Barbieri, J.; Djari, A.; Pinton, A.; Letaief, R.; Salin, G.; Escudié, F.; Roulet, A.; Fritz, S. Genome-wide study of structural variants in bovine Holstein, Montbéliarde and Normande dairy breeds. PLoS ONE 2015, 10, e0135931. [Google Scholar] [CrossRef]
- Durán Aguilar, M.; Román Ponce, S.; Ruiz López, F.; González Padilla, E.; Vásquez Peláez, C.; Bagnato, A.; Strillacci, M.G. Genome-wide association study for milk somatic cell score in holstein cattle using copy number variation as markers. J. Anim. Breed. Genet. 2017, 134, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Ateya, A.; Safhi, F.A.; El-Emam, H.; Marawan, M.A.; Fayed, H.; Kadah, A.; Mamdouh, M.; Hizam, M.M.; Al-Ghadi, M.Q.; Abdo, M. Combining Nucleotide Sequence Variants and Transcript Levels of Immune and Antioxidant Markers for Selection and Improvement of Mastitis Resistance in Dromedary Camels. Agriculture 2023, 13, 1909. [Google Scholar] [CrossRef]
- Ateya, A.; Al-Sharif, M.; Faraj, S.H.; Abdo, M.; Fericean, L.; Banatean-Dunea, I.; Hammad, S.J.; Mamdouh, M.; Fayed, H.; Marawan, M.A. Exploring genetic polymorphisms and transcript levels of antioxidant and metabolic markers for prediction and monitoring diarrhea in Holstein dairy calves. Acta Agric. Scand. Sect. A—Anim. Sci. 2024, 73, 86–96. [Google Scholar] [CrossRef]
- El-Sayed, A.; Faraj, S.H.; Marghani, B.H.; Safhi, F.A.; Abdo, M.; Fericean, L.; Banatean-Dunea, I.; Alexandru, C.-C.; Alhimaidi, A.R.; Ammari, A.A. The Transcript Levels and the Serum Profile of Biomarkers Associated with Clinical Endometritis Susceptibility in Buffalo Cows. Vet. Sci. 2024, 11, 340. [Google Scholar] [CrossRef]
- Khan, M.Z.; Huang, B.; Kou, X.; Chen, Y.; Liang, H.; Ullah, Q.; Khan, I.M.; Khan, A.; Chai, W.; Wang, C. Enhancing bovine immune, antioxidant and anti-inflammatory responses with vitamins, rumen-protected amino acids, and trace minerals to prevent periparturient mastitis. Front. Immunol. 2024, 14, 1290044. [Google Scholar] [CrossRef]
- Bernabucci, U.; Ronchi, B.; Lacetera, N.; Nardone, A. Influence of body condition score on relationships between metabolic status and oxidative stress in periparturient dairy cows. J. Dairy Sci. 2005, 88, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Coleman, D.; Hu, L.; Martinez-Cortés, I.; Wang, M.; Parys, C.; Shen, X.; Loor, J. Methionine and arginine supplementation alter inflammatory and oxidative stress responses during lipopolysaccharide challenge in bovine mammary epithelial cells in vitro. J. Dairy Sci. 2020, 103, 676–689. [Google Scholar] [CrossRef]
- He, W.; Wang, Q.; Gu, L.; Zhong, L.; Liu, D. NOX4 rs11018628 polymorphism associates with a decreased risk and better short-term recovery of ischemic stroke. Exp. Ther. Med. 2018, 16, 5258–5264. [Google Scholar] [CrossRef]
- Kofler, P.A.; Pircher, H.; von Grafenstein, S.; Diener, T.; Höll, M.; Liedl, K.R.; Siems, K.; Jansen-Dürr, P. Characterisation of Nox4 inhibitors from edible plants. Planta Medica 2013, 79, 244–252. [Google Scholar] [CrossRef]
- Li, J.-Z.; Yu, S.-Y.; Wu, J.-H.; Shao, Q.-R.; Dong, X.-M. Paeoniflorin protects myocardial cell from doxorubicin-induced apoptosis through inhibition of NADPH oxidase. Can. J. Physiol. Pharmacol. 2012, 90, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Stasi, M.A.; Scioli, M.G.; Arcuri, G.; Mattera, G.G.; Lombardo, K.; Marcellini, M.; Riccioni, T.; De Falco, S.; Pisano, C.; Spagnoli, L.G. Propionyl-l-carnitine improves postischemic blood flow recovery and arteriogenetic revascularization and reduces endothelial NADPH-oxidase 4–mediated superoxide production. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 426–435. [Google Scholar] [CrossRef]
- Santos, C.X.; Hafstad, A.D.; Beretta, M.; Zhang, M.; Molenaar, C.; Kopec, J.; Fotinou, D.; Murray, T.V.; Cobb, A.M.; Martin, D. Targeted redox inhibition of protein phosphatase 1 by Nox4 regulates eIF 2α-mediated stress signaling. EMBO J. 2016, 35, 319–334. [Google Scholar] [CrossRef] [PubMed]
- de Dios, S.T.; Sobey, C.G.; Drummond, G.R. Oxidative stress and endothelial dysfunction. In Endothelial Dysfunction and Inflammation; Springer: Basel, Switzerland, 2010; pp. 37–64. [Google Scholar]
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef]
- Shimokawa, H.; Morikawa, K. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J. Mol. Cell. Cardiol. 2005, 39, 725–732. [Google Scholar] [CrossRef]
- Liu, Q.; Li, H.; Wang, N.; Chen, H.; Jin, Q.; Zhang, R.; Wang, J.; Chen, Y. Polymorphism of rs1836882 in NOX4 gene modifies associations between dietary caloric intake and ROS levels in peripheral blood mononuclear cells. PLoS ONE 2013, 8, e85660. [Google Scholar] [CrossRef]
- Pham, T.X.; Park, Y.-K.; Bae, M.; Lee, J.-Y. The potential role of an endotoxin tolerance-like mechanism for the anti-inflammatory effect of Spirulina platensis organic extract in macrophages. J. Med. Food 2017, 20, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Bannerman, D. Pathogen-dependent induction of cytokines and other soluble inflammatory mediators during intramammary infection of dairy cows. J. Anim. Sci. 2009, 87, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Feng, W.; Xin, G.; Tingting, N.; Zhanghe, Z.; Haimin, C.; Xiaojun, Y. Enhanced effect of κ-carrageenan on TNBS-induced inflammation in mice. Int. Immunopharmacol. 2016, 39, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Aslan, O.; Goksu, A.G.; Apaydin, Ν. The evaluation of oxidative stress in lambs with Pestivirus infection. J. Hell. Vet. Med. Soc. 2017, 68, 299–306. [Google Scholar] [CrossRef]
- Blattman, A.; Kinghorn, B.; Gray, G.; Hulme, D.; JBeh, K.; Woolaston, R. A search for associations between major histocompatibility complex restriction fragment length polymorphism bands and resistance to Haemonchus contortus infection in sheep. Anim. Genet. 1993, 24, 277–282. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; McManus, D.P. Concepts in immunology and diagnosis of hydatid disease. Clin. Microbiol. Rev. 2003, 16, 18–36. [Google Scholar] [CrossRef]
- Moroz, A.; Czopowicz, M.; Sobczak-Filipiak, M.; Dolka, I.; Rzewuska, M.; Kizerwetter-Świda, M.; Chrobak-Chmiel, D.; Mickiewicz, M.; Witkowski, L.; Szaluś-Jordanow, O. The prevalence of histopathological features of pneumonia in goats with symptomatic caprine arthritis-encephalitis. Pathogens 2022, 11, 629. [Google Scholar] [CrossRef]
- Ismael, M.; El-Sayed, M.S.; Metwally, A.M.; Ibrahim, Z.K.; El-Saman, A.E.-R.M. Clinical and Haematobiochemical Evaluation of Pneumonia in Calves with Special Reference to Oxidant/Antioxidant Indices. Alex. J. Vet. Sci. 2017, 54, 40. [Google Scholar] [CrossRef]
- Kumar, P.; Jain, V.; Kumar, T.; Kumar, V.; Rana, Y. Clinical and haematobiochemical studies on respiratory disease in buffaloes. Int. J. Livest. Res. 2018, 8, 178–184. [Google Scholar] [CrossRef]
- Sayed, A.; Ali, A.; Mottelib, A.; El-Rahman, A. Bronchopneumonia in buffalo-calves in Assiut Governorate. I-Studies on bacterial causes, clinical, haematological and biochemical changes associated with the disease. Assiut Vet. Med. J. 2002, 46, 138–155. [Google Scholar]
- Smith, B.P. Large Animal Internal Medicine, 5th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Akgul, O.; Kozat, S.; Ozkan, C.; Kaya, A.; Akgul, Y. Evaluation of acute phase protein levels and some cytokine levels in pneumonic calves. Med. Weter. 2019, 75, 343–365. [Google Scholar]
- El-Naser, E.; Khamis, G. Some hematological and blood serum biochemical indices associated with respiratory affections by camels. Assiut Vet. Med. J. 2009, 55, 154–162. [Google Scholar]
- Aytekin, İ.; Mamak, N.; Ulucan, A.; Kalinbacak, A. Clinical, haematological, biochemical and pathological findings in lambs with peste des petits ruminants. Kafkas Üniversitesi Vet. Fakültesi Derg. 2011, 17, 349–355. [Google Scholar]
- Aktas, M.S.; Kandemir, F.M.; Kirbas, A.; Hanedan, B.; Aydin, M.A. Evaluation of oxidative stress in sheep infected with Psoroptes ovis using total antioxidant capacity, total oxidant status, and malondialdehyde level. J. Vet. Res. 2017, 61, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Yurdakul, İ.; Aydogdu, U. The effect of enteritis, pneumonia and omphalitis on oxidative/antioxidant balance in the calves. Turk. J. Agric.-Food Sci. Technol. 2019, 7, 539–542. [Google Scholar] [CrossRef]
- Özbek, M.; Özkan, C. Oxidative stress in calves with enzootic pneumonia. Turk. J. Vet. Anim. Sci. 2020, 44, 1299–1305. [Google Scholar] [CrossRef]
- Salci, H.; Ozdemir Salci, E.S.; Ozakin, C.; Seyrek Intas, K. A brief study on hematological, sero-biochemical and microbiological results of umbilical lesions in calves. Inter. J. Vet. Sci. 2017, 6, 86–89. [Google Scholar]
- Chirase, N.K.; Greene, L.W.; Purdy, C.W.; Loan, R.W.; Auvermann, B.W.; Parker, D.B.; Walborg, E.F.; Stevenson, D.E.; Xu, Y.; Klaunig, J.E. Effect of transport stress on respiratory disease, serum antioxidant status, and serum concentrations of lipid peroxidation biomarkers in beef cattle. Am. J. Vet. Res. 2004, 65, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, O.; Kilany, O.; Dessouki, A.; Mohmoud, N. Clinico-Pathological Studies in Some Respiratory Diseases in Newly Born Buffalo Calves. Suez Canal Vet. Med. J. SCVMJ 2015, 20, 15–26. [Google Scholar] [CrossRef]
- El-Deeb, W. Acute phase response and oxidative stress parameters in pneumonic camel calves (Camelus dromedarius). Bulg. J. Vet. Med. 2015, 18, 258–269. [Google Scholar] [CrossRef]
- Fernández, S.; Galapero, J.; Rey, J.; Pérez, C.; Ramos, A. Cytokines study in lungs infected with Pasteurellaceae and Mycoplasma spp. from fattening lambs. J. Med. Microbiol. Immunol. Res 2018, 2, 1–7. [Google Scholar]
- Rizk, M.A.; El-Sayed, S.A.E.-S.; Salman, D.; Marghani, B.H.; Gadalla, H.E.; Sayed-Ahmed, M.Z. Immunomodulatory effect of vitamin C on proinflammatory cytokines production in Ossimi Lambs (Ovis aries) with Pneumonic Pasteurellosis. Animals 2021, 11, 3374. [Google Scholar] [CrossRef] [PubMed]
- Arbaga, A.; Hassan, H.; Anis, A.; Osthman, N. Hematological changes and serum minerals concentrations in pneumonic sheep. Benha Vet. Med. J. 2022, 42, 143–146. [Google Scholar] [CrossRef]
- Van Reeth, K.; Nauwynck, H. Proinflammatory cytokines and viral respiratory disease in pigs. Vet. Res. 2000, 31, 187–213. [Google Scholar] [CrossRef]
- Moore, T.A.; Standiford, T.J. The role of cytokines in bacterial pneumonia: An inflammatory balancing act. Proc. Assoc. Am. Physicians 1998, 110, 297–305. [Google Scholar]
| Examined Indicator | Primer | Product Size (bp) | Annealing Temperature (°C) | GenBank Isolate |
|---|---|---|---|---|
| IL-1α | F5′-GTCCCTGACCTCTTTGAAGACCT-3 R5′-ACGTTACTCTGGAAGCTGTAATG-3′ | 356 | 60 | NM_174092.1 |
| IL-1β | F5′-GAGAATGAGCTGTTATTTGAG-3 R5′-GCTCATGCAGAACACCACTTC-3′ | 423 | 58 | NM_174093.1 |
| IL-6 | F5′-AGCGCCTTCACTCCATTCGCT-3′ R5′-CTCATACTCGTTCTGGAGGTAG-3′ | 384 | 58 | NM_173923.2 |
| IL10 | F5′-TACCTGGGTTGCCAAGCCTTGTC-3′ R5′-CTTTTGCATCTTCGTTGTCAT-3′ | 306 | 58 | NM_174088.1 |
| TNFα | F5′-TCCTTCCTCCTGGTTGCAGGAGC-3′ R5′-CTCCTCCCTGGTAGATGGGTTC-3′ | 490 | 60 | NM_173966.3 |
| IFN-γ | F5′-TATACAAGCTATTTCTTAGCT-3′ R5′-AGAGCTGCCATTCAAGAACTTC-3′ | 321 | 58 | NM_174086.1 |
| PRDX6 | F5′-ACGGAGCTCGGCAGAGCAGCA-3′ R5′-ATGGTTGGAAGGACCATCACGC-3′ | 434 | 60 | NM_174643.1 |
| ATG7 | F5′-GGTGTCAGACACATCACGTTCGT-3′ R5′-CTGGGCTTCTTCAGGCCGTGTC-3′ | 416 | 60 | NM_001142967.1 |
| NDUFS6 | F5′-ACGCGCGGCCTGCCCGGGGGC-3′ R5′-TCCTGTCCTGGAAGCTCTGTGCG-3′ | 405 | 58 | XM_005221658.4 |
| NOX4 | F5′-CACCTCTGCCTGCTTATCTGGC-3′ R5′-TCGGTATCTTGCTGCATTCAGT-3′ | 396 | 60 | NM_001304775.1 |
| GAPDH | F5′-CCTGCCCGTTCGACAGATAG-3′ R5′-ATGGCGACGATGTCCACTTT-3′ | 153 | 58 | NM_001034034.2 |
| Parameters | Healthy Calves | BRD Calves | p-Values |
|---|---|---|---|
| Temperature (°C) | 38.8 ± 0.05 | 41.3 ± 0.3 * | 0.005 |
| Pulse rate (beat/min) | 93.8 ± 0.4 | 145.4 ± 4.5 * | 0.002 |
| Respiration rate (breath/min) | 29.4 ± 0.3 | 51.9 ± 2.3 * | 0.003 |
| Parameters | Healthy Calves | Pneumonic Calves | p-Values |
|---|---|---|---|
| RBCs (×106/µL) | 6.3 ± 0.6 | 4.2 ± 0.5 * | 0.001 |
| Hb (g/dL) | 11.9 ± 1.5 | 8.1 ± 1.01 * | 0.001 |
| PCV (%) | 32.1 ± 0.5 | 22 ± 0.3 * | 0.001 |
| MCV (fl) | 34.4 ± 1.2 | 32.9 ± 0.5 | 0.1 |
| MCH (pg) | 22.1 ± 1.9 | 19.1 ± 0.88 | 0.09 |
| MCHC (%) | 36.1 ± 1.4 | 33.4 ± 0.8 | 0.07 |
| WBC (×103/µL) | 11.22 ± 1.4 | 18.11 ± 3.32 * | 0.001 |
| Neutrophils (×103/µL) | 4.02 ± 0.08 | 8.99 ± 0.29 * | 0.004 |
| Lymphocyte (×103/µL) | 4.1 ± 0.08 | 2.11 ± 0.55 * | 0.03 |
| Genes | SNPs | Healthy n = 45 | Pneumonia n = 180 | Total n = 225 | kind of Inherited Change | Amino Acid Order and Sort | Chi Squar Value X2 | p-Value |
|---|---|---|---|---|---|---|---|---|
| IL-1α | G56C | 21/45 | -/180 | 21/225 | Non-synonymous | 19 S to R | 92.6 | <0.001 |
| A182G | 34/45 | -/180 | 34/225 | Non-synonymous | 61 K to R | 149.2 | <0.001 | |
| T300C | -/45 | 67/180 | 67/225 | Synonymous | 100 N | 23.8 | <0.001 | |
| IL-1β | C153T | 19/45 | -/180 | 18/225 | Synonymous | 51 I | 37.5 | <0.001 |
| IL-6 | G221A | -/45 | 131/180 | 131/225 | Non-synonymous | 74 S to N | 78.3 | <0.001 |
| G323A | 28/45 | -/180 | 28/225 | Non-synonymous | 108 R to K | 127.9 | <0.001 | |
| IL-10 | A105T | 39/45 | -/180 | 39/225 | Synonymous | 35 S | 188.7 | <0.001 |
| G129C | -/45 | 51/180 | 51/225 | Synonymous | 43 L | 16.4 | <0.001 | |
| C168T | -/45 | 76/180 | 76/225 | Synonymous | 56 C | 28.6 | <0.001 | |
| G203A | 37/45 | -/180 | 37/225 | Non-synonymous | 68 R to K | 177.1 | <0.001 | |
| TNFα | A120G | 26/45 | -/180 | 26/225 | Synonymous | 40 Q | 117.5 | <0.001 |
| C213T | -/45 | 39/180 | 39/225 | Synonymous | 71 D | 11.7 | <0.001 | |
| T468C | 18/45 | -/180 | 18/225 | Synonymous | 156 Y | 78.2 | <0.001 | |
| IFN-γ | G35T | -/45 | 58/180 | 58/225 | Non-synonymous | 12 G to V | 19.5 | <0.001 |
| PRDX6 | T117C | -/45 | 82/180 | 82/225 | Synonymous | 39 N | 20.4 | <0.001 |
| T225C | -/45 | 126/180 | 126/225 | Synonymous | 75 D | 71.5 | <0.001 | |
| AT7G | T48C | 38/45 | -/180 | 38/225 | Synonymous | 16 S | 182.8 | <0.001 |
| G214A | 31/45 | -/180 | 31/225 | Non-synonymous | 72 V to I | 143.8 | <0.001 | |
| C333T | 19/45 | -/180 | 19/225 | Synonymous | 111 A | 83 | <0.001 | |
| NDUFS6 | G354A | -/45 | 103/180 | 103/180 | Synonymous | 118 S | 47.9 | <0.001 |
| NOX4 | T200A | 28/45 | -/180 | 28/225 | Non-synonymous | 67 F to Y | 127.9 | <0.001 |
| G213A | 21/45 | -/180 | 21/225 | Synonymous | 71 S | 92.6 | <0.001 |
| Parameters | Healthy Calves | BRD Calves | p-Values |
|---|---|---|---|
| SOD (U/mL) | 55 ± 0.5 | 40 ± 0.5 * | 0.001 |
| GSH (mg/dl) | 37.6 ± 0.8 | 26.3 ± 0.8 * | 0.001 |
| TAC (mM/L) | 49 ± 0.5 | 35 ± 0.5 * | 0.001 |
| MDA (nmol/mL) | 5.8 ± 0.05 | 10 ± 0.1 * | 0.002 |
| IL1α (pg/mL) | 32.6 ± 2.7 | 155.1 ± 3.9 * | 0.002 |
| IL1β (pg/mL) | 25.4 ± 1.6 | 141.3 ± 5.3 * | 0.001 |
| IL 6 (pg/mL) | 26 ± 1.2 | 140.5 ± 1.2 * | 0.001 |
| IL 10 (pg/mL) | 119.4 ± 2.5 | 27.5 ± 1.7 * | 0.002 |
| TNFα (pg/mL) | 29.3 ± 1.9 | 117.1 ± 3 * | 0.001 |
| IFNγ (Pg/mL) | 46.7 ± 0.9 | 143.8 ± 8.2 * | 0.007 |
| Gene Expression | Serum Profile |
|---|---|
| PRDX6 | SOD (r = 0.877 and p = 0.024) GSH (r = 0.924 and p = 0.008) TAC (r = 0.961 and p = 0.0.002) |
| NDUFS6 | SOD (r = 0.917 and p = 0.01) GSH (r = 0.925 and p = 0.008) TAC (r = 0.904 and p = 0.0.01) MDA (r = −0.887 and p = 0.01) |
| NOX4 | SOD (r = −0.975 and p = 0.001) GSH (r = −0.973 and p = 0.001) TAC (r = −0.9634 and p = 0.003) MDA (r = 0.951 and p = 0.003) |
| ATG7 | GSH (r = 0.853 and p = 0.013) TAC (r = 0.849 and p = 0.03) |
| IL1α | INFγ (r = −0.997 and p = 0.04) |
| IL10 | TNFα (r = −0.998 and p = 0.04) |
| INFγ | TNFα (r = 1 and p = 0.005) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sayed, A.; Eissa, A.; Ebrahim, D.; Ateya, A.; Gadalla, H.; Alharbi, H.M.; Alwutayd, K.M.; Babaker, M.A.; Aly Elzeer, A. Variants in Nucleotide Sequences; Gene Expression; and Hematological, Immune, and Antioxidant Biomarkers Linked to Pneumonia Risk in Holstein Calves. Vet. Sci. 2025, 12, 620. https://doi.org/10.3390/vetsci12070620
El-Sayed A, Eissa A, Ebrahim D, Ateya A, Gadalla H, Alharbi HM, Alwutayd KM, Babaker MA, Aly Elzeer A. Variants in Nucleotide Sequences; Gene Expression; and Hematological, Immune, and Antioxidant Biomarkers Linked to Pneumonia Risk in Holstein Calves. Veterinary Sciences. 2025; 12(7):620. https://doi.org/10.3390/vetsci12070620
Chicago/Turabian StyleEl-Sayed, Ahmed, Attia Eissa, Doaa Ebrahim, Ahmed Ateya, Hossam Gadalla, Hanan M. Alharbi, Khairiah M. Alwutayd, Manal A. Babaker, and Aya Aly Elzeer. 2025. "Variants in Nucleotide Sequences; Gene Expression; and Hematological, Immune, and Antioxidant Biomarkers Linked to Pneumonia Risk in Holstein Calves" Veterinary Sciences 12, no. 7: 620. https://doi.org/10.3390/vetsci12070620
APA StyleEl-Sayed, A., Eissa, A., Ebrahim, D., Ateya, A., Gadalla, H., Alharbi, H. M., Alwutayd, K. M., Babaker, M. A., & Aly Elzeer, A. (2025). Variants in Nucleotide Sequences; Gene Expression; and Hematological, Immune, and Antioxidant Biomarkers Linked to Pneumonia Risk in Holstein Calves. Veterinary Sciences, 12(7), 620. https://doi.org/10.3390/vetsci12070620






