Evaluation of Cardiomegaly in Dogs Using the Manubrium Heart Score Method and Determination of Its Diagnostic Accuracy in Comparison with the Vertebral Heart Score
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- A1: Dogs with heart disease, >2 kg and ≤10 kg
- B1: Dogs with heart disease, >10 kg and ≤20 kg
- C1: Dogs with heart disease, >20 kg and ≤40 kg
- A2: Healthy dogs, >2 kg and ≤10 kg
- B2: Healthy dogs, >10 kg and ≤20 kg
- C2: Healthy dogs, >20 kg and ≤40 kg
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bagardi, M.; Zamboni, V.; Locatelli, C.; Galizzi, A.; Ghilardi, S.; Brambilla, P.G. Management of chronic congestive heart failure caused by myxomatous mitral valve disease in dogs: A narrative review from 1970 to 2020. Animals 2020, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- Freid, K.J.; Freeman, L.M.; Rush, J.E.; Cunningham, S.M.; Davis, M.S.; Karlin, E.T.; Yang, V.K. Retrospective study of dilated cardiomyopathy in dogs. J. Vet. Intern. Med. 2021, 35, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Bilgiç, B.; Tarhan, D.; Or, M.E. The Effects of Different Treatments on Serum Trace Element Levels in Dogs with Heart Failure. Animals 2024, 14, 3390. [Google Scholar] [CrossRef]
- Vatnikov, Y.A.; Rudenko, A.A.; Usha, B.V.; Kulikov, E.V.; Notina, E.A.; Bykova, I.A.; Zharov, A.N. Left ventricular myocardial remodeling in dogs with mitral valve endocardiosis. Vet. World 2020, 13, 731. [Google Scholar] [CrossRef] [PubMed]
- Ware, W.A.; Bonagura, J.D.; Scansen, B.A. Cardiomegaly. In Cardiovascular Disease in Companion Animals, 2nd ed.; Ware, W.A., Bonagura, J.D., Scansen, B.A., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 281–286. [Google Scholar]
- Saini, N.; Uppal, S.K.; Anand, A. Radiographic assessment of dogs with congestive heart failure. Indian J. Anim. Res. 2023, 57, 922–927. [Google Scholar] [CrossRef]
- Koster, L.; Vogel, J.; Springer, C.M.; Hecht, S. Radiographic lung congestion scores in dogs with acute congestive heart failure caused by myxomatous mitral valve disease. J. Vet. Intern. Med. 2023, 37, 1983–1991. [Google Scholar] [CrossRef]
- Burti, S.; Osti, V.L.; Zotti, A.; Banzato, T. Use of deep learning to detect cardiomegaly on thoracic radiographs in dogs. Vet. J. 2020, 262, 105505. [Google Scholar] [CrossRef]
- Jeong, Y.; Sung, J. An automated deep learning method and novel cardiac index to detect canine cardiomegaly from simple radiography. Sci. Rep. 2022, 12, 14494. [Google Scholar] [CrossRef]
- Puccinelli, C.; Citi, S.; Vezzosi, T.; Garibaldi, S.; Tognetti, R. A radiographic study of breed-specific vertebral heart score and vertebral left atrial size in Chihuahuas. Vet. Radiol. Ultrasound 2021, 62, 20–26. [Google Scholar] [CrossRef]
- Baisan, R.A.; Vulpe, V. Vertebral Heart Score and Vertebral Left Atrial Size as Radiographic Measurements for Cardiac Size in Dogs—A Literature Review. Animals 2025, 15, 683. [Google Scholar] [CrossRef]
- Marbella Fernández, D.; García, V.; Santana, A.J.; Montoya-Alonso, J.A. The thoracic inlet heart size, a new approach to radiographic cardiac measurement. Animals 2023, 13, 389. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, H.J.; Song, J.H.; Song, K.H. An assessment of thoracic inlet heart score in Maltese dogs with ACVIM Stage B myxomatous mitral valve disease. J. Vet. Clin. 2024, 41, 263–269. [Google Scholar] [CrossRef]
- Tangpakornsak, T.; Saisawart, P.; Sutthigran, S.; Jaturunratsamee, K.; Tachampa, K.; Thanaboonnipat, C.; Choisunirachon, N. Thoracic vertebral length-to-height ratio, a promising parameter to predict the vertebral heart score in normal Welsh Corgi Pembroke dogs. Vet. Sci. 2023, 10, 168. [Google Scholar] [CrossRef]
- Buchanan, J.W.; Bücheler, J. Vertebral scale system to measure canine heart size in radiographs. J. Am. Vet. Med. Assoc. 1995, 206, 194–199. [Google Scholar] [CrossRef]
- Saka, S.U.; Iskefli, O.; Or, M.E.; Bakırel, U. Objectivity of vertebral heart scale: Comparison of detailed radiographic findings with vertebral heart scale in symptomatic dogs with cardiac failure. Ger. J. Vet. Res. 2022, 2, 1–7. [Google Scholar] [CrossRef]
- Toombs, J.P.; Ogburn, P.N. Evaluating canine cardiovascular silhouettes: Radiographic methods and normal radiographic anatomy. Compend. Contin. Educ. Pract. Vet. 1985, 7, 579–587. [Google Scholar]
- Holmes, R.A.; Smith, F.G.; Lewis, R.E.; Kern, D.M. The effects of rotation on the radiographic appearance of the canince cardiac silhouette in dorsal recumbency. Vet. Radiol. 1985, 26, 98–101. [Google Scholar] [CrossRef]
- Bagardi, M.; Manfredi, M.; Zani, D.D.; Brambilla, P.G.; Locatelli, C. Interobserver variability of radiographic methods for the evaluation of left atrial size in dogs. Vet. Radiol. Ultrasound 2021, 62, 161–174. [Google Scholar] [CrossRef]
- Ross, E.S.; Visser, L.C.; Sbardellati, N.; Potter, B.M.; Ohlendorf, A.; Scansen, B.A. Utility of vertebral left atrial size and vertebral heart size to aid detection of congestive heart failure in dogs with respiratory signs. J. Vet. Intern. Med. 2023, 37, 2021–2029. [Google Scholar] [CrossRef]
- James, W.B. Vertebral scale system to measure heart size in radiographs. Vet. Clin. Small Anim. Pract. 2000, 30, 379–393. [Google Scholar] [CrossRef]
- Birks, R.; Fine, D.M.; Leach, S.B.; Clay, S.E.; Eason, B.D.; Britt, L.G.; Lamb, K.E. Breed-specific vertebral heart scale for the Dachshund. J. Am. Anim. Hosp. Assoc. 2017, 53, 73–79. [Google Scholar] [CrossRef]
- Wiegel, P.S.; Mach, R.; Nolte, I.; Freise, F.; Levicar, C.; Merhof, K.; Bach, J.P. Breed-specific values for vertebral heart score (VHS), vertebral left atrial size (VLAS), and radiographic left atrial dimension (RLAD) in pugs without cardiac disease, and their relationship to Brachycephalic Obstructive Airway Syndrome (BOAS). PLoS ONE 2022, 17, e0274085. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.A.; Munoz, C.; Zepeda, C.I.; Piscitelli, N.; Gentile-Solomon, J.; Szlosek, D.; Nakamura, R.K. Breed-specific vertebral heart size and vertebral left atrial size reference intervals in Yorkshire Terriers, Pomeranians, Pugs, and Boston Terriers. Am. J. Vet. Res. 2025, 86, ajvr.24.10.0320. [Google Scholar] [CrossRef] [PubMed]
- Battinelli, G.M.; Piscitelli, N.; Murphy, L.; Gentile-Solomon, J.; Szlosek, D.; Nakamura, R.K. Vertebral heart score and vertebral left atrial size reference intervals in Jack Russell Terriers, Miniature Pinschers, and Brussels Griffons. Am. J. Vet. Res. 2024, 85, ajvr.24.07.0209. [Google Scholar] [CrossRef]
- Kallassy, A.; Calendrier, E.; Bouhsina, N.; Fusellier, M. Vertebral Heart Scale for the Brittany Spaniel: Breed-Specific Range and Its Correlation with Heart Disease Assessed by Clinical and Echocardiographic Findings. Vet. Sci. 2021, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Luciani, M.G.; Withoeft, J.A.; Mondardo Cardoso Pissetti, H.; Pasini de Souza, L.; Silvestre Sombrio, M.; Bach, E.C.; Mai, W.; Müller, T.R. Vertebral heart size in healthy Australian cattle dog. Anat. Histol. Embryol. 2019, 48, 264–267. [Google Scholar] [CrossRef]
- Marin, L.M.; Brown, J.; McBrien, C.; Baumwart, R.; Samii, V.F.; Couto, C.G. Vertebral heart size in retired racing Greyhounds. Vet. Radiol. Ultrasound 2007, 48, 332–334. [Google Scholar] [CrossRef]
- Jepsen-Grant, K.; Pollard, R.E.; Johnson, L.R. Vertebral heart scores in eight dog breeds. Vet. Radiol. Ultrasound 2013, 54, 3–8. [Google Scholar] [CrossRef]
- Bagardi, M.; Locatelli, C.; Manfredi, M.; Bassi, J.; Spediacci, C.; Ghilardi, S.; Zani, D.D.; Brambilla, P.G. Breed-specific vertebral heart score, vertebral left atrial size, and radiographic left atrial dimension in Cavalier King Charles Spaniels: Reference interval study. Vet. Radiol. Ultrasound 2022, 63, 156–163. [Google Scholar] [CrossRef]
- Lamb, C.R.; Wikeley, H.; Boswood, A.; Pfeiffer, D.U. Use of breed-specific ranges for the vertebral heart scale as an aid to the radiographic diagnosis of cardiac disease in dogs. Vet. Rec. 2001, 148, 707–711. [Google Scholar] [CrossRef]
- Prado, M.R.; Pires, S.T.; Ercolin, A.C.M.; Disselli, T.; Stanquini, C.S.; de Andrade, A.M.R.; Hage, M.C.F.N.S. Reference values for vertebral heart size and manubrium heart score for healthy Border Collies. Med. Vet. 2024, 18, 143–150. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Berry, C.R. Radiographic assessment of the cardiac silhouette in clinically normal large- and small-breed dogs. Am. J. Vet. Res. 2017, 78, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.A.; Peper, K.E.; Berry, C.R. Use of cardiac sphericity index and manubrium heart scores to assess radiographic cardiac silhouettes in large- and small-breed dogs with and without cardiac disease. J. Am. Vet. Med. Assoc. 2020, 256, 888–898. [Google Scholar] [CrossRef]
- Keene, B.W.; Atkins, C.E.; Bonagura, J.D.; Fox, P.R.; Häggström, J.; Fuentes, V.L.; Oyama, M.A.; Rush, J.E.; Stepien, R.; Uechi, M. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J. Vet. Intern. Med. 2019, 33, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B. The correlation coefficient: Its values range between+ 1/− 1, or do they? J. Mark. 2009, 17, 139–142. [Google Scholar] [CrossRef]
- Strohm, L.E.; Visser, L.C.; Chapel, E.H.; Drost, W.T.; Bonagura, J.D. Two-dimensional, long-axis echocardiographic ratios for assessment of left atrial and ventricular size in dogs. J. Vet. Cardiol. 2018, 20, 330–342. [Google Scholar] [CrossRef]
- Poad, M.H.; Manzi, T.J.; Oyama, M.A.; Gelzer, A.R. Utility of radiographic measurements to predict echocardiographic left heart enlargement in dogs with preclinical myxomatous mitral valve disease. J. Vet. Intern. Med. 2020, 34, 1728–1733. [Google Scholar] [CrossRef]
- Hansson, K.; Häggström, J.; Kvart, C.; Lord, P. Interobserver variability of vertebral heart size measurements in dogs with normal and enlarged hearts. Vet. Radiol. Ultrasound 2005, 46, 122–130. [Google Scholar] [CrossRef]
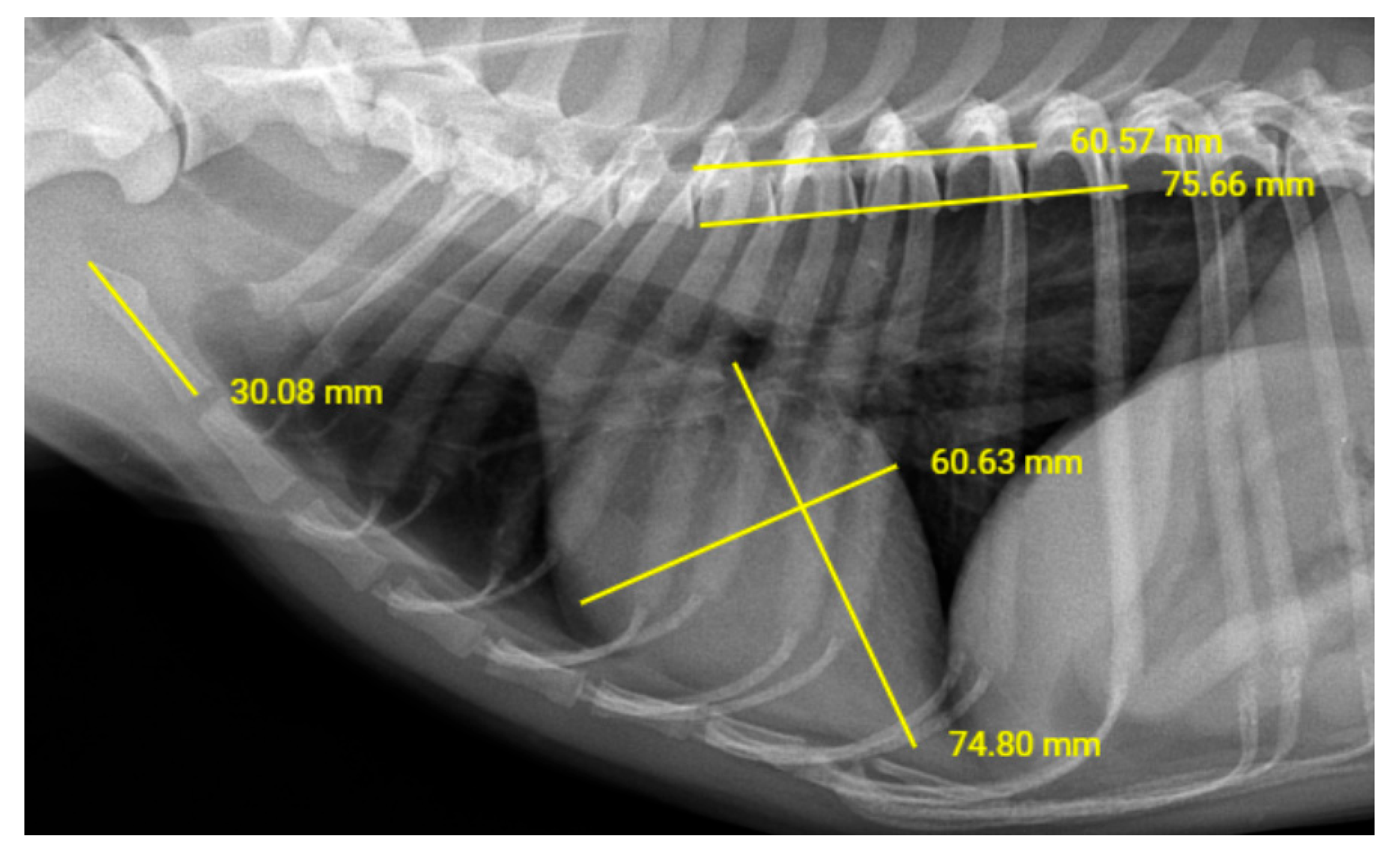

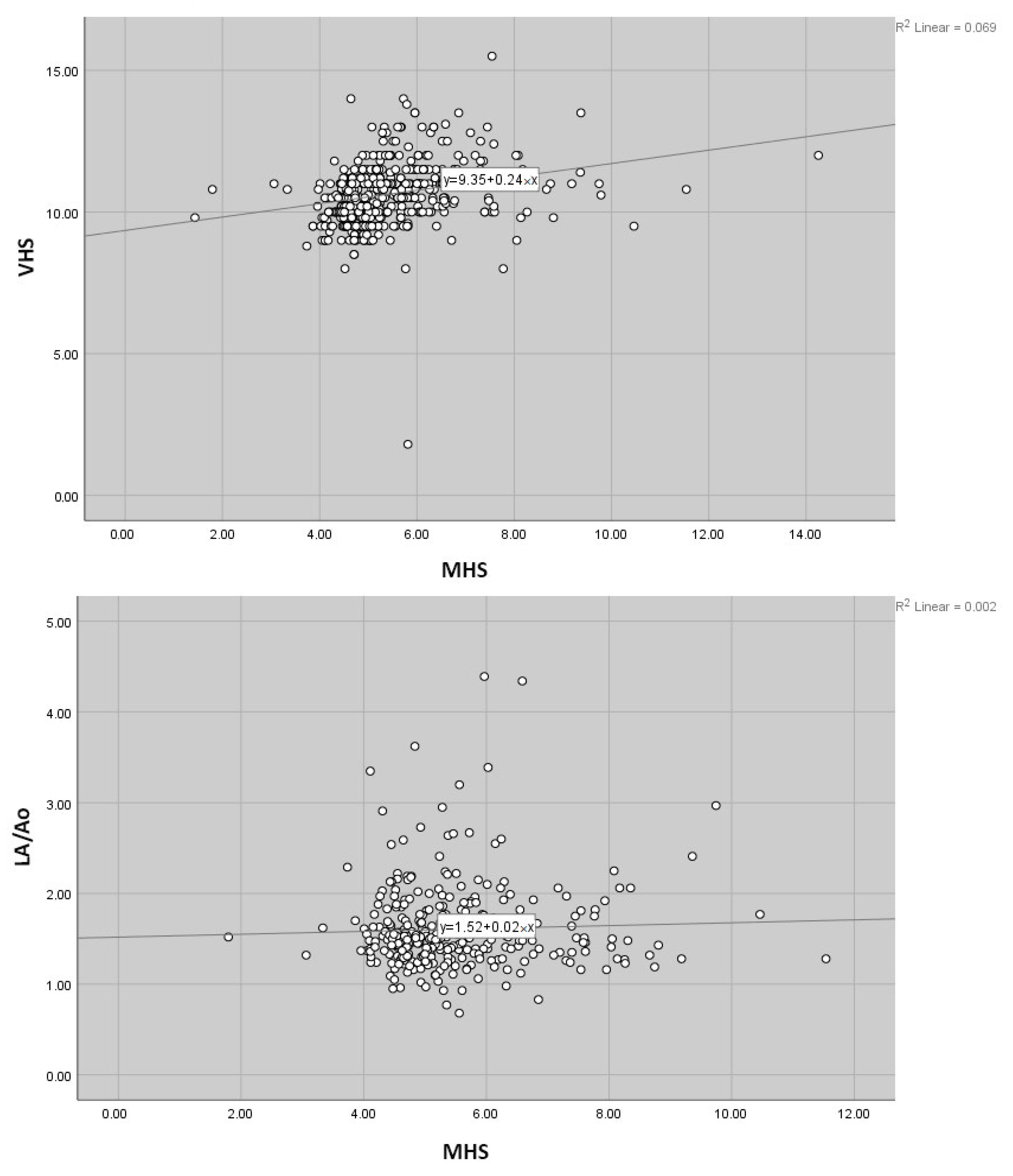
| Study Groups | Age (Years) Mean (Min–Max) | Weight (kg) Mean (Min–Max) | Sex | |
|---|---|---|---|---|
| Male | Female | |||
| A1 (n =140) | 8.95 (2–17) | 6.61 (2–10) | 72 | 68 |
| B1 (n = 100) | 9.66 (2–18) | 13.1 (10.2–19) | 55 | 45 |
| C1 (n = 50) | 10.46 (3–14) | 31.53 (25.5–45) | 31 | 19 |
| A2 (n = 100) | 6.67 (1–17) | 5.57 (1.20–9.5) | 50 | 50 |
| B2 (n = 40) | 7.41 (1–16) | 14.87 (10.5–20) | 15 | 25 |
| C2 (n = 60) | 8.48 (1–15) | 33.50 (20.5–53) | 36 | 24 |
| A1 (n = 140) | B1 (n = 100) | C1 (n = 50) | ||||
|---|---|---|---|---|---|---|
| M-Mode | Mean ± SD | Min–Max | Mean ± SD | Min–Max | Mean ± SD | Min–Max |
| IVSd (cm) | 0.75 ± 0.18 | 0.33–1.34 | 0.89 ± 0.3 | 0.5–2.51 | 1.1 ± 0.29 | 0.5–1.8 |
| LVIDd (cm) | 2.75 ± 0.88 | 0.94–5.24 | 3.31 ± 0.72 | 1.22–4.92 | 4.4 ± 1.04 | 2.32–7.83 |
| LVPWd (cm) | 0.8 ± 0.87 | 0.06–9.67 | 0.84 ± 0.18 | 0.39–1.5 | 1.1 ± 0.28 | 0.67–1.86 |
| IVSs (cm) | 1.06 ± 0.28 | 0.54–1.97 | 1.19 ± 0.28 | 0.63–2.14 | 1.41 ± 0.29 | 0.75–2.22 |
| LVIDs (cm) | 1.47 ± 0.59 | 0.29–4.25 | 1.83 ± 0.49 | 0.5–3.04 | 2.62 ± 0.82 | 1.07–5.24 |
| LVPWs (cm) | 1.03 ± 0.22 | 0.72–1.97 | 1.2 ± 0.25 | 0.62–1.68 | 1.41 ± 0.3 | 0.81–2.2 |
| EDV (mL) | 29.99 ± 25.21 | 0.83–137.5 | 46.14 ± 25.04 | 3.5–119.1 | 97.26 ± 65.52 | 18.56–328.46 |
| ESV (mL) | 6.6 ± 9.1 | 0.06–80.68 | 10.26 ± 7.32 | 0.3–36.2 | 29.3 ± 26.55 | 3.93–144.04 |
| EF (%) | 81.09 ± 9.91 | 38.89–98.58 | 78.33 ± 10.36 | 40.85–96.3 | 72.13 ± 10.95 | 30.07–86.36 |
| FS (%) | 47.3 ± 9.95 | 19–78.38 | 44.23 ± 10.68 | 4.76–66.67 | 39.48 ± 8.69 | 13.64–53.57 |
| 2D | Mean ± SD | Min–Max | Mean ± SD | Min–Max | Mean ± SD | Min–Max |
| LA (cm) | 2.12 ± 0.89 | 0.99–7.13 | 2.39 ± 0.564 | 1.29–4.35 | 2.96 ± 0.67 | 1.52–5.52 |
| Ao (cm) | 1.19 ± 0.33 | 0.6–2.61 | 1.41 ± 0.25 | 0.88–2.32 | 1.92 ± 0.37 | 1.16–2.61 |
| LA/Ao | 1.88 ± 0.65 | 0.83–4.39 | 1.73 ± 0.408 | 0.93–3.47 | 1.57 ± 0.33 | 0.77–2.21 |
| Doppler | Mean ± SD | Min–Max | Mean ± SD | Min–Max | Mean ± SD | Min–Max |
| MVEmax (cm/s) | 72.31 ± 31.38 | 26.93–200.14 | 73.71 ± 23.98 | 32.51–150.52 | 61.59 ± 15.27 | 36.2–119.53 |
| MVAmax (cm/s) | 60.26 ± 22.44 | 21.86–161.21 | 59.6 ± 18.33 | 23.97–104.45 | 57.6 ± 16.4 | 31.58–111.88 |
| MVE/A | 1.26 ± 0.46 | 0.41–2.64 | 1.3 ± 0.42 | 0.62–2.68 | 1.12 ± 0.29 | 0.48–1.92 |
| TVEmax (cm/s) | 64.03 ± 22.97 | 17.76–120.68 | 60.42 ± 15.69 | 28.53–126.61 | 59.81 ± 14.95 | 37.22–89.34 |
| TVAmax (cm/s) | 50.2 ± 17.59 | 19.66–105.60 | 48.64 ± 14.08 | 20.92–86.18 | 51.62 ± 13.29 | 31.7–81.2 |
| TVE/A | 1.31 ± 0.37 | 0.69–2.53 | 1.29 ± 0.32 | 0.75–2.29 | 1.2 ± 0.29 | 0.72–1.74 |
| MRVmax (m/s) | 4.48 ± 1.66 | 1.21–7.09 | 4.7 ± 1.43 | 1.12–6.78 | 4.25 ± 1.17 | 2.47–6.36 |
| TRVmax (m/s) | 2.77 ± 0.84 | 0.93–4.30 | 2.58 ± 0.54 | 1.58–3.38 | 2.29 ± 0.24 | 1.95–2.45 |
| Avmax (cm/s) | 98.26 ± 26.58 | 42–178 | 108.42 ± 27.54 | 58–224 | 123.23 ± 54.2 | 75–364 |
| Pvmax (cm/s) | 83.96 ± 25.57 | 30.68–204 | 94.11 ± 24.94 | 49–189 | 110.19 ± 70.95 | 56–505 |
| A2 (n = 100) | B2 (n = 40) | C2 (n = 60) | ||||
|---|---|---|---|---|---|---|
| M-Mode | Mean ± SD | Min–Max | Mean ± SD | Min–Max | Mean ± SD | Min–Max |
| IVSd (cm) | 0.7 ± 0.17 | 0.35–1.41 | 0.97 ± 0.24 | 0.67–1.64 | 1.17 ± 0.36 | 0.51–2.55 |
| LVIDd (cm) | 2.27 ± 0.47 | 1.23–4.16 | 3.07 ± 0.57 | 1.31–4.16 | 3.97 ± 0.67 | 1.45–5.69 |
| LVPWd (cm) | 0.68 ± 0.22 | 0.41–1.9 | 0.91 ± 0.15 | 0.57–1.17 | 1.13 ± 0.24 | 0.45–1.74 |
| IVSs (cm) | 0.93 ± 0.18 | 0.47–1.7 | 1.23 ± 0.25 | 0.79–1.77 | 1.47 ± 0.34 | 0.72–2.49 |
| LVIDs (cm) | 1.26 ± 0.37 | 0.39–2.31 | 1.7 ± 0.4 | 0.45–2.31 | 2.17 ± 0.59 | 0.59–3.63 |
| LVPWs (cm) | 0.93 ± 0.2 | 0.54–1.85 | 1.28 ± 0.18 | 0.94–1.63 | 1.51 ± 0.31 | 0.94–2.42 |
| EDV (mL) | 17.48 ± 10.57 | 2.57–76.71 | 36.85 ± 16.47 | 4.25–76.71 | 70.52 ± 27.18 | 5.53–159.58 |
| ESV (mL) | 4.11 ± 3.46 | 0.15–18.44 | 8.25 ± 4.92 | 0.22–18.44 | 16.31 ± 10.64 | 0.47–55.5 |
| EF (%) | 79.24 ± 9.97 | 38.89–96.57 | 78.27 ± 9.69 | 50.94–94.75 | 78.45 ± 8.61 | 62.5–97.12 |
| FS (%) | 45.78 ± 9.33 | 17.54–70.97 | 45.11 ± 8.78 | 25–65.71 | 46.08 ± 9.05 | 33.33–74.67 |
| 2D | Mean ± SD | Min–Max | Mean ± SD | Min–Max | Mean ± SD | Min–Max |
| LA (cm) | 1.56 ± 0.37 | 0.69–2.76 | 2.03 ± 0.29 | 1.57–2.91 | 2.75 ± 0.54 | 1.5–4.44 |
| Ao (cm) | 1.12 ± 0.26 | 0.46–1.94 | 1.45 ± 0.22 | 1.12–1.84 | 1.96 ± 0.41 | 1–3.27 |
| LA/Ao | 1.4 ± 0.18 | 0.68–1.52 | 1.42 ± 0.16 | 0.97–1.56 | 1.41 ± 0.18 | 0.95–1.63 |
| Doppler | Mean ± SD | Min–Max | Mean ± SD | Min–Max | Mean ± SD | Min–Max |
| MVEmax (cm/s) | 59.98 ± 14.50 | 34.4–93.99 | 70 ± 17.96 | 41.34–108.43 | 69.4 ± 15 | 36.5–106.8 |
| MVAmax (cm/s) | 49.73 ± 17.96 | 1.68–127.8 | 57.82 ± 16.28 | 31.54–97.08 | 55.3 ± 14.1 | 30.6–91.6 |
| MVE/A | 1.28 ± 0.43 | 0.64–2.64 | 1.25 ± 0.29 | 0.8–1.99 | 1.3 ± 0.3 | 0.4–2.1 |
| TVEmax (cm/s) | 54.95 ± 16.85 | 0.67–103.29 | 59.84 ± 15.04 | 34.78–90.34 | 63.6 ± 16 | 37.7–126.8 |
| TVAmax (cm/s) | 48.78 ± 13.46 | 15.02–88.67 | 50.85 ± 14.46 | 24.7–78.67 | 52.2 ± 17.7 | 27–101.6 |
| TVE/A | 1.21 ± 0.34 | 0.65–2.26 | 1.22 ± 0.28 | 0.73–1.7 | 1.3 ± 0.3 | 0.8–2.3 |
| Avmax (cm/s) | 94.1 ± 24.79 | 48–161 | 122.07 ± 28.4 | 69–180 | 116.6 ± 28.4 | 69–179 |
| Pvmax (cm/s) | 85.6 ± 20.21 | 42–126 | 104.65 ± 24.22 | 68–165 | 101.5 ± 24.8 | 45–153 |
| cLAL (cm) | cSAL (cm) | Manubrium (cm) | VHS (cm) | MHS (cm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Groups | Mean (Min–Max) | SD | Mean (Min–Max) | SD | Mean (Min–Max) | SD | Mean (Min–Max) | SD | Mean (Min–Max) | SD |
| A1 (n = 140) | 8.17 (4.85–13.76) | 1.62 | 6.91 (4.09–13.24) | 1.43 | 2.68 (1.15–4.58) | 0.73 | 11.05 (8.5–15.50) | 1.12 | 5.85 (3.06–10.46) | 1.38 |
| A2 (n = 100) | 7.30 (4.75–9.84) | 1.09 | 6.02 (3.83–8.91) | 0.99 | 2.54 (1.18–6.23) | 0.75 | 9.96 (8–11.50) | 0.61 | 5.54 (1.43–11.54) | 1.28 |
| B1 (n = 100) | 10.15 (6.95–16.03) | 1.50 | 8.36 (5.36–12.83) | 1.16 | 3.57 (1.69–5.01) | 0.59 | 11.07 (9–13.80) | 0.91 | 5.29 (3.97–14.25) | 1.17 |
| B2 (n = 40) | 10.09 (7.89–14.07) | 1.29 | 8.11 (6.84–10.78) | 0.84 | 3.61 (2.29–4.64) | 0.44 | 10.21 (8.50–14) | 0.89 | 5.07 (4.12–6.59) | 0.56 |
| C1 (n = 50) | 14.02 (8.41–21.57) | 2.18 | 11.38 (6.91–17.54) | 1.80 | 4.94 (2.60–6.98) | 0.82 | 11 (9.50–13.00) | 0.68 | 5.19 (4.23–8.17) | 0.69 |
| C2 (n = 60) | 13.59 (10.04–17.15) | 1.35 | 10.82 (8.75–13.54) | 1.08 | 4.91 (2.64–6.64) | 0.79 | 10.18 (9–11.20) | 0.59 | 5.08 (3.96–8.66) | 0.90 |
| A (2–10 kg) | Mean (Min–Max) | Median | SD | p | |
| VHS | HD | 11.05 (8.5–15.50) | 11 | 1.12 | <0.001 |
| Healthy | 9.96 (8–11.50) | 10 | 0.61 | ||
| MHS | HD | 5.85 (3.06–10.46) | 5.60 | 1.38 | 0.071 |
| Healthy | 5.54 (1.43–11.54) | 5.23 | 1.28 | ||
| B (10–20 kg) | Mean (Min–Max) | Median | SD | p | |
| VHS | HD | 11.07 (9–13.80) | 11 | 0.91 | <0.001 |
| Healthy | 10.21 (8.50–14) | 10.20 | 0.89 | ||
| MHS | HD | 5.29 (3.97–14.25) | 5.06 | 1.17 | 0.527 |
| Healthy | 5.07 (4.12–6.59) | 4.99 | 0.56 | ||
| C (20–40 kg) | Mean (Min–Max) | Median | SD | p | |
| VHS | HD | 11 (9.50–13.00) | 11 | 0.68 | <0.001 |
| Healthy | 10.18 (9–11.20) | 10 | 0.59 | ||
| MHS | HD | 5.19 (4.23–8.17) | 5.11 | 0.69 | 0.065 |
| Healthy | 5.08 (3.96–8.66) | 4.82 | 0.90 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilgiç, B.; İskefli, O.; Pugliese, M.; Or, M.E. Evaluation of Cardiomegaly in Dogs Using the Manubrium Heart Score Method and Determination of Its Diagnostic Accuracy in Comparison with the Vertebral Heart Score. Vet. Sci. 2025, 12, 619. https://doi.org/10.3390/vetsci12070619
Bilgiç B, İskefli O, Pugliese M, Or ME. Evaluation of Cardiomegaly in Dogs Using the Manubrium Heart Score Method and Determination of Its Diagnostic Accuracy in Comparison with the Vertebral Heart Score. Veterinary Sciences. 2025; 12(7):619. https://doi.org/10.3390/vetsci12070619
Chicago/Turabian StyleBilgiç, Bengü, Onur İskefli, Michela Pugliese, and Mehmet Erman Or. 2025. "Evaluation of Cardiomegaly in Dogs Using the Manubrium Heart Score Method and Determination of Its Diagnostic Accuracy in Comparison with the Vertebral Heart Score" Veterinary Sciences 12, no. 7: 619. https://doi.org/10.3390/vetsci12070619
APA StyleBilgiç, B., İskefli, O., Pugliese, M., & Or, M. E. (2025). Evaluation of Cardiomegaly in Dogs Using the Manubrium Heart Score Method and Determination of Its Diagnostic Accuracy in Comparison with the Vertebral Heart Score. Veterinary Sciences, 12(7), 619. https://doi.org/10.3390/vetsci12070619







